Abstract
Recent studies have described malignant stem cells as central to the initiation, growth, and potential relapse of acute and chronic myelogenous leukemia (AML and CML). Because of their important role in pathogenesis, rare and biologically distinct leukemia stem cells (LSCs) represent a critical target for therapeutic intervention. However, to date, very few agents have been shown to directly target the LSC population. The present studies demonstrate that parthenolide (PTL), a naturally occurring small molecule, induces robust apoptosis in primary human AML cells and blast crisis CML (bcCML) cells while sparing normal hematopoietic cells. Furthermore, analysis of progenitor cells using in vitro colony assays, as well as stem cells using the nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenograft model, show that PTL also preferentially targets AML progenitor and stem cell populations. Notably, in comparison to the standard chemotherapy drug cytosine arabinoside (Ara-C), PTL is much more specific to leukemia cells. The molecular mechanism of PTL-mediated apoptosis is strongly associated with inhibition of nuclear factor κ B (NF-κB), proapoptotic activation of p53, and increased reactive oxygen species (ROS). On the basis of these findings, we propose that the activity of PTL triggers LSC-specific apoptosis and as such represents a potentially important new class of drugs for LSC-targeted therapy.
Introduction
Acute myelogenous leukemia (AML) is a malignant disease characterized by an aberrant accumulation of immature myeloid hematopoietic cells. Although remission can be achieved in most patients, relapse is common and long-term survival is poor for most cases. Studies using a variety of experimental systems have shown that AML arises from a rare population of leukemic stem cells (LSCs).1-3 LSCs share some antigenic features with normal hematopoietic stem cells (HSCs), such as CD34+, CD38-, CD71-, and HLA-DR-, but can be phenotypically distinguished from HSCs by virtue of several disparate markers.1-6 In addition, LSCs, like HSCs, are largely quiescent and display heterogeneity within the stem cell compartment.7-9 As a consequence of these common features, it has been relatively difficult to define strategies to differentially target the LSC population. However, recent studies have demonstrated that LSCs do display certain unique molecular properties such as constitutive activation of nuclear factor κB (NF-κB), expression of CD123, and potentially elevated levels of interferon regulatory factor 1 (IRF-1) and death-associated protein (DAP) kinase.6,8,10,11 Features such as these suggest that LSC-specific targeted therapy should be feasible using a variety of strategies.
Current chemotherapy regimens for AML commonly use drugs such as nucleoside analogs (eg, cytosine arabinoside [Ara-C]) and anthracyclines (eg, idarubicin, daunorubicin) that interfere with DNA replication and induce apoptosis primarily in replicating cells. Since LSCs are mostly quiescent, it is likely that at least some malignant stem cells are refractory to standard chemotherapy and may thereby contribute to relapse. Moreover, current regimens may not effectively discriminate between normal and malignant cells. Consequently, substantial damage to normal tissues is likely to occur in the course of standard treatments. For this reason, it is important to identify therapies that can specifically target the LSC population without affecting normal cells.
We have previously shown that a combination of the proteasome inhibitor MG-132 and the anthracycline idarubicin was sufficient to preferentially ablate human LSCs in vitro11 while sparing normal hematopoietic cells. These studies demonstrate that LSC-specific targeting can be achieved. Interestingly, leukemia cell death was associated with inhibition of NF-κB and proapoptotic activation of p53. However, the overall toxicity of a proteasome inhibitor and an anthracycline to other tissues remains unknown. In addition, the cardiac toxicity of anthracyclines limits their use particularly in older individuals. Consequently, we have investigated other agents that may specifically ablate the LSC population.
Parthenolide (PTL) is a sesquiterpene lactone found as the major active component in Feverfew (Tanacetum parthenium), an herbal medicine that has been used to treat migraine and rheumatoid arthritis for centuries.12 More recently, PTL has been found to have several other properties, including antitumor activity, inhibition of DNA synthesis, and inhibition of cell proliferation in different cancer cell lines.13-16 In addition, PTL sensitizes cancer cells to other antitumor agents17-20 and acts as a chemopreventive agent in a UVB-induced skin cancer animal model.21 PTL is a potent inhibitor of NF-κB activation and has been shown to directly bind IκB-kinase (IKK)22,23 and to modify the p50 and p65 NF-κB subunits.24,25 PTL can also block signal transducers and activators of transcription 3 (STAT3) phosphorylation on Tyr705,26 sustain c-Jun N-terminal kinase (JNK) activation,17,18 and increase intracellular reactive oxygen species (ROS).13,27 In this study, we analyzed the effect of PTL on survival of primary AML, blast crisis chronic myelogenous leukemia (bcCML), and normal hematopoietic cells. Our data demonstrate that PTL can selectively ablate primitive leukemia cells without affecting normal stem and progenitor cells. These findings indicate PTL and similar sesquiterpene lactones may represent a novel class of agents for targeting myeloid LSCs.
Materials and methods
Cell isolation and culture
AML cells, bcCML cells, normal bone marrow (BM), and umbilical cord blood (CB) cells were obtained from volunteer donors with informed consent or from the National Disease Research Interchange (NDRI). The cells were isolated and processed as described.6 Briefly, samples were subjected to Ficoll-Paque (Pharmacia Biotech, Piscataway, NY) density gradient separation to isolate mononuclear cells. In some cases cells were cryopreserved in freezing medium consisting of Iscoves modified Dulbecco medium (IMDM), 40% fetal bovine serum (FBS), and 10% dimethylsulfoxide (DMSO). The viability of the leukemic cells after thawing was 50% to 95%. All the AML samples were 100% CD123+. The percent CD34 in the samples analyzed ranged from 20% to 80%. Fresh or thawed cells were cultured in serum-free medium (SFM)28 for 1 hour before the addition of drugs. For PGJ2 (15-deoxy-delta12,14-prostaglandin J2; Cayman Chemical, Ann Arbor, MI) treatment, cells were cultured in the absence of bovine serum albumin (BSA). All the drug treatments were performed in triplicate. PTL (Biomol, Plymouth Meeting, PA) was reconstituted in DMSO to a stock concentration of 0.2 M and subsequently diluted in phosphate buffer saline (PBS). Ara-C was obtained from Sigma (St Louis, MO). Total cell numbers were determined before and after culture for several specimens. No significant nonspecific loss of cells was observed as a consequence of culture (data not shown).
Methylcellulose colony-forming assay
AML or normal cells (BM or CB) were cultured in SFM as described in “Cell isolation and culture” for 18 hours in the presence or absence of drugs. Cells were then plated at 50 000 cells/mL in Methocult GF H4534 (1% methylcellulose in IMDM, 30% FBS, 1% BSA, 10-4 M 2-mercaptoethanol, 2 mM l-glutamine, 50 ng/mL recombinant human [rh] stem cell factor, 10 ng/mL rh granulocyte-macrophage colony-stimulating factor [GM-CSF], 10 ng/mL rh interleukin 3 [IL-3]; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 3 units/mL of erythropoietin and 50 ng/mL G-CSF (R&D Systems, Minneapolis, MN). Colonies were scored after 10 to 14 days of culture.
NOD/SCID mouse assays
Nonobese diabetic/severe combined immunodeficient (NOD/SCID; NOD.CB17-prdkdc scid/J) mice (Jackson Laboratories, Bar Harbor, ME) were sublethally irradiated with 2.7 Gy (270 rad) using a RadSource X-ray irradiator (RadSource, Boca Raton, FL) the day before transplantation. Cells to be assayed were injected via tail vein (5-10 million cells) in a final volume of 0.2 mL of PBS with 0.5% FBS. After 6 to 8 weeks, animals were killed and BM was analyzed for the presence of human cells by flow cytometry.
EMSA and immunoblot analysis
Electrophoretic mobility shift assay (EMSA) was performed as described.8 Briefly, nuclear extracts equivalent to 200 000 cells were incubated with 2 μg of poly-d(I-C) (Roche Molecular Biochemicals, Indianapolis, IN) and 10-14 mol 32P-labeled NF-κB probe in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 5 mM Tris (tris(hydroxymethyl)aminomethane), 50 mM KCl, 1.2 mM EDTA (ethylenediaminetetraacetic acid), and 10% glycerol for 15 minutes at room temperature. Protein/DNA complexes were resolved on a native polyacrylamide gel in 0.25 × Tris-borate-EDTA (TBE). For immunoblots, cells were prepared and analyzed as previously described.6 Blots were probed with anti–phospho-p53 (ser15), anti–phospho-PTEN (anti–phospho–phosphatase with tensin homology), anti-PTEN from Cell Signaling (Beverly, MA); anti-p53 (DO-1) from Santa Cruz Biotechnology (Santa Cruz, CA); and antiactin (AC-15) from Sigma.
Flow cytometry
Apoptosis assays were performed as described.8 Briefly, after 18 hours of treatment, specimens were labeled with anti–CD38-APC (anti–CD38-allophycocyanin) and anti–CD34-PE (anti–CD34-phycoerythrin; Becton Dickinson, San Jose, CA) for 20 minutes. Cells were then washed in cold PBS and resuspended in 200 μL of annexin-V buffer. Annexin-V–fluorescein isothiocyanate (FITC) and 7-aminoactinomycin (7-AAD; Molecular Probes, Eugene, OR) were added and the samples were incubated at room temperature for 15 minutes followed by analysis using a Becton Dickinson LSRII flow cytometer. The total number of events collected ranged from 100 000 to 1 000 000, depending on the CD34+/CD38- content on the sample. The percent viable cells was defined as annexin-V-/7-AAD- cells on total (ungated) cells and on gates set for CD34+ and CD34+/CD38- populations. To analyze human cell engraftment in the NOD/SCID xenogeneic model, BM cells were isolated, blocked with anti-Fc receptor antibody 2.4G2 and 25% human serum, and labeled with antihuman CD45, CD33, or CD19 antibodies (Becton Dickinson).
Statistical analysis
We analyzed the data from CD34+ and total cells separately by fitting separate linear mixed effects models to each dataset. We first log-transformed the percent viability measurements in order to reduce the influence of outliers and symmetrize the error distributions. Linear mixed effects models were then used to model the log (viability) as a function of the fixed effects for group (normal, AML, CML), dose (5.0, 7.5), and their interaction, along with a random intercept and dose effect for each subject to account for the correlation structure of the data. In addition, the residual variance was allowed to differ for each of the 3 groups (normal, AML, CML). Restricted maximum likelihood was used to estimate the parameters, and P values and 95% symmetric confidence intervals were computed using the robust “sandwich” estimator for standard errors on the logarithmic scale. Finally, the point estimates and confidence bounds were exponentiated back to the original scale of percent viability to obtain the estimated geometric means and fold changes along with their associated 95% asymmetric confidence intervals.
Results
PTL preferentially targets leukemia cells while sparing normal hematopoietic cells
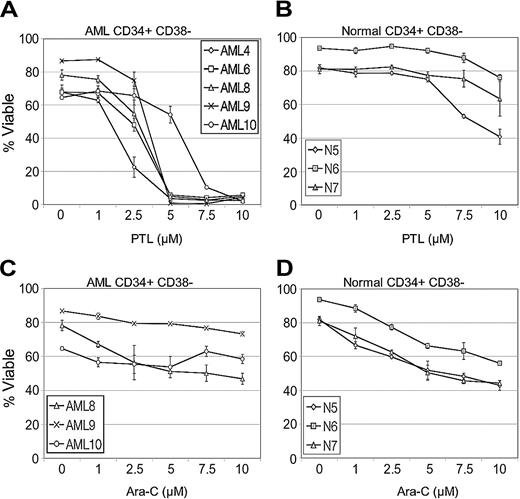
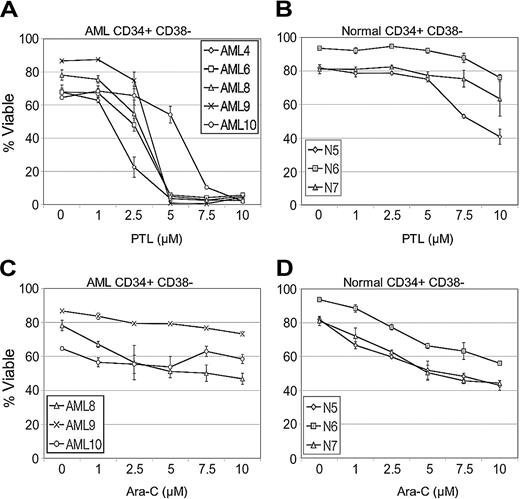
Initial studies were performed to compare the effects of PTL on primary leukemia versus normal specimens during short-term suspension culture. The AML specimens represented different French-American-British (FAB) subtypes (Table 1) from both de novo and relapsed cases. Table 1 shows the effects of PTL treatment on AML, bcCML, and normal hematopoietic cells in both total and more primitive CD34+ populations. Viability was determined by annexin labeling after 18 hours of culture. At a 5-μM concentration, most of the AML specimens demonstrated very low viability after 18 hours in culture (mean, 9.3% viable CD34+ cells; 95% confidence interval [CI], 5.3, 16.2), with the exception of specimens 10 and 13 (68% and 57% viability, respectively). However, the viability of these 2 specimens was greatly reduced by increasing PTL to a concentration of 7.5 μM (7.3% and 6.8%, respectively). Overall, the viability of AML CD34+ cells was more than 10-fold less than normal CD34+ controls (P < .001). Similarly, bcCML cells also showed a strong cytotoxic response to PTL in the 5 to 7.5 μM concentration range. In contrast, both the total and CD34+ cells from normal specimens showed almost no decrease in viability when treated with 5 μM PTL (mean, 93.4% viable CD34+ cells; 95% CI, 85.4, 102.2) and a very modest decrease at 7.5 μM PTL (mean, 86.5% viable CD34+ cells; 95% CI, 74.0, 101.2). Thus, PTL is much more toxic to AML cells than normal, even within the CD34+ population, which is relatively enriched for progenitors. In order to look more specifically at primitive stem cell populations, the same suspension culture assays were analyzed with respect to cells bearing a CD34+/CD38- immunophenotype, which has previously been shown to be characteristic of both normal and AML stem cells.1 PTL was tested at 0 to 10 μM concentrations on all the AML and normal specimens described in Table 1. A representative graph (Figure 1A) using 5 AML specimens demonstrates that the CD34+CD38- leukemic population is very sensitive to PTL treatment and shows a strong apoptotic response in the 5 to 7.5 μM range. Control experiments with normal CD34+CD38- cells (Figure 1B) show no appreciable toxicity at 5 μM PTL and only begin to demonstrate modest effects at 10 μM PTL. Thus, phenotypically primitive cells also demonstrate strong AML-specific toxicity in response to PTL.
As a means to further assess the relative efficacy of PTL, we also performed side-by-side comparison studies with the standard chemotherapy drug Ara-C. Analysis of CD34+/CD38- cells showed Ara-C was more toxic than PTL for normal cells (Figure 1D) while demonstrating very little toxicity to AML CD34+/CD38- cells (Figure 1C). This observation is consistent with our previous report that showed reduced Ara-C toxicity to AML CD34+/CD38- cells in comparison to the overall AML cell population.8 Furthermore, Ara-C effects on LSC viability appear to plateau at levels above 7.5 μM, even at concentrations as high as 200 μM (data not shown). These experiments indicate that PTL has greater toxicity to AML cells than Ara-C and has less nonspecific toxicity to normal cells.
PTL induces apoptosis in CD34+CD38- AML cells but not in normal cells in a dose-dependent manner. In vitro cultures were maintained for 18 hours followed by analysis of viability using annexin-V labeling. Each plot shows the average percent cell viability for CD34+CD38- AML (A,C) and normal (N) cells (B,D) treated with increasing concentrations of PTL (A-B) or Ara-C (C-D). Each error bar represents the SD. All assays were performed in triplicate.
PTL induces apoptosis in CD34+CD38- AML cells but not in normal cells in a dose-dependent manner. In vitro cultures were maintained for 18 hours followed by analysis of viability using annexin-V labeling. Each plot shows the average percent cell viability for CD34+CD38- AML (A,C) and normal (N) cells (B,D) treated with increasing concentrations of PTL (A-B) or Ara-C (C-D). Each error bar represents the SD. All assays were performed in triplicate.
PTL treatment affects leukemic but not normal progenitor and stem cell activity
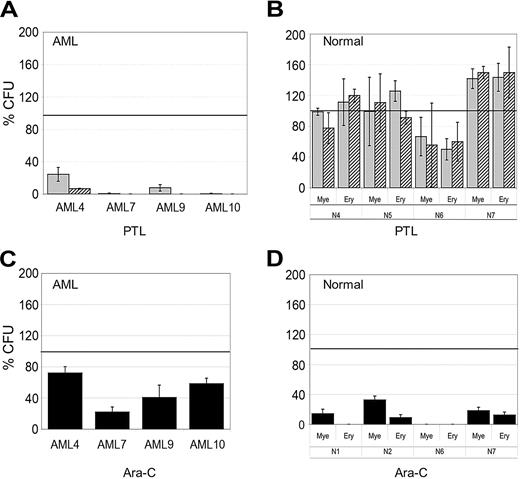
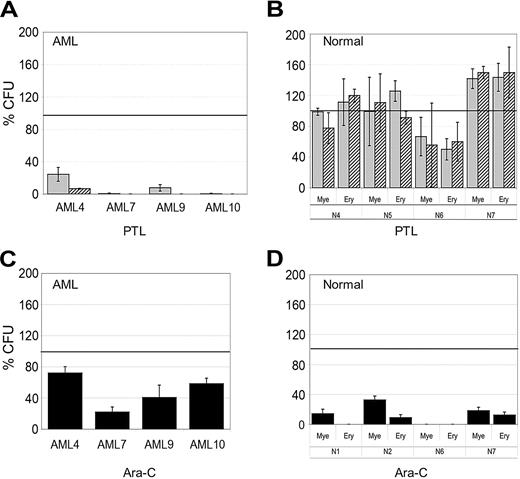
To functionally assess the effects of PTL treatment, in vitro colony assays and the NOD/SCID mouse xenotransplant model were used to determine whether PTL affected the potential of primitive cells. Different AML and normal specimens were first treated with 5.0 or 7.5 μM PTL for 18 hours followed by analysis using methylcellulose colony assays. Figure 2A shows that AML colony-forming units (CFUs) were dramatically reduced by PTL treatment. In contrast, normal CFUs show little to no effect (Figure 2B) and in some cases are even slightly increased by PTL treatment. Further, from a qualitative perspective, neither the size/morphology of normal colonies nor the frequency of myeloid and erythroid colonies was affected by PTL treatment. The CFU assays demonstrate that preferential targeting of AML cells by PTL is also evident at the progenitor cell level. For comparison, we also treated AML and normal specimens with 5.0 Ara-C μM for 18 hours and then analyzed CFU potential. As shown in Figure 2C, AML CFUs were only reduced by approximately 50%, however; both myeloid and erythroid colonies from normal donors were strongly reduced as a consequence of Ara-C treatment (average ∼85% inhibition).
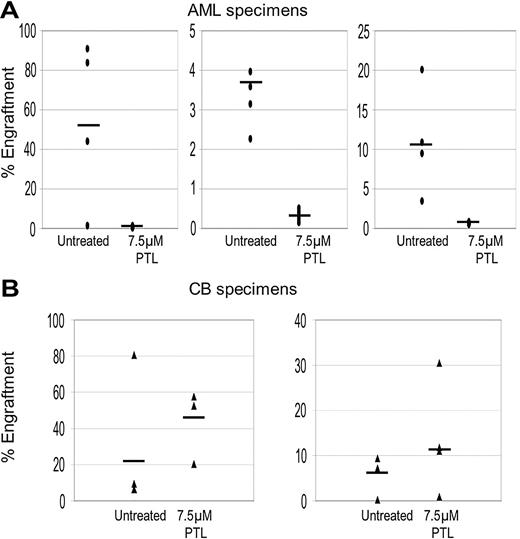
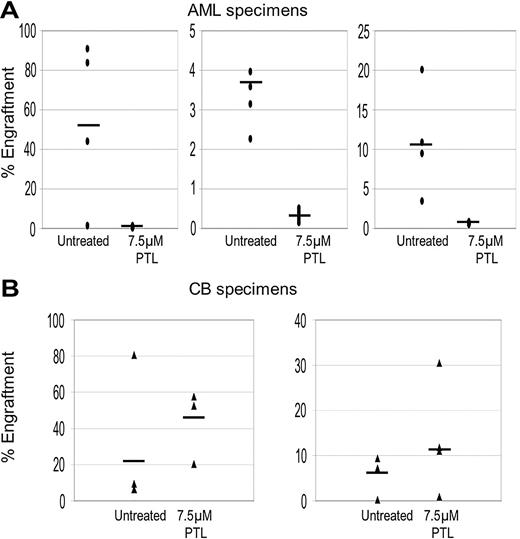
To further validate ablation of primitive cells, the NOD/SCID xenogeneic model was used to assess LSC and HSC potential after PTL treatment. Primary cells were treated with 7.5 μM PTL for 18 hours and then transplanted into sublethally irradiated mice. After 6 to 8 weeks, BM cells were harvested and labeled with antihuman CD45 antibody to determine the percentage of human cells engrafted in each animal. As shown in Figure 3, PTL treatment strongly reduces the ability of LSCs to engraft in the NOD/SCID mouse but did not affect the activity of normal HSCs. In addition, lineage analysis of mice that received a transplant of normal cells show that PTL does not effect in vivo differentiation of myeloid or lymphoid lineages (data not shown). Taken together, these results indicate that PTL is able to induce LSC-specific apoptosis while sparing normal stem and progenitor cells.
In vitro colony assays for AML and normal cells treated with PTL and Ara-C. AML versus normal cells in panels A and B were treated with 5 μM (▦) or 7.5 μM PTL (▨). AML versus normal cells in panels C and D were treated with 5 μM Ara-C (▪). All treatments were performed for 18 hours in suspension culture, followed by plating in methylcellulose culture. Error bars represent the SD. Average percent of colony-forming units (CFU) are normalized to untreated control (horizontal bar). All assays were performed in triplicate. Mye represents myeloid; Ery, erythroid.
In vitro colony assays for AML and normal cells treated with PTL and Ara-C. AML versus normal cells in panels A and B were treated with 5 μM (▦) or 7.5 μM PTL (▨). AML versus normal cells in panels C and D were treated with 5 μM Ara-C (▪). All treatments were performed for 18 hours in suspension culture, followed by plating in methylcellulose culture. Error bars represent the SD. Average percent of colony-forming units (CFU) are normalized to untreated control (horizontal bar). All assays were performed in triplicate. Mye represents myeloid; Ery, erythroid.
PTL inhibits NOD/SCID repopulating ability for AML but not normal cells. Percentage of engraftment for NOD/SCID mice that received a transplant with AML (A) or normal CB (B) cells after 18 hours of culture with or without 7.5 μM PTL. Each • or ▴ represents a single animal analyzed at 6 to 8 weeks after transplantation. Each plot represents an AML/CB specimen. Mean engraftment is indicated by the horizontal bars.
PTL inhibits NOD/SCID repopulating ability for AML but not normal cells. Percentage of engraftment for NOD/SCID mice that received a transplant with AML (A) or normal CB (B) cells after 18 hours of culture with or without 7.5 μM PTL. Each • or ▴ represents a single animal analyzed at 6 to 8 weeks after transplantation. Each plot represents an AML/CB specimen. Mean engraftment is indicated by the horizontal bars.
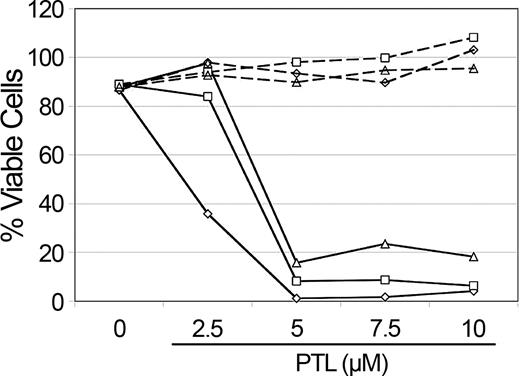
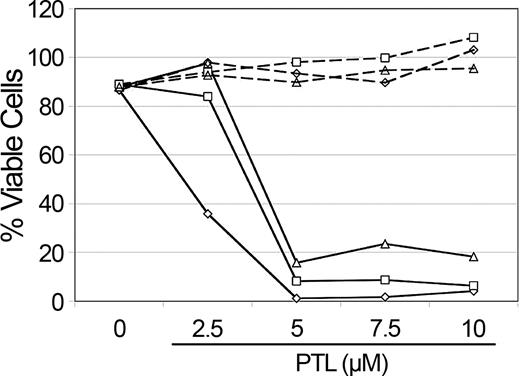
NAC treatment abolishes PTL apoptosis induction in AML cells. Percent viability of CD34+ cells from 3 different AML specimens treated with increasing concentrations of PTL. Cells were precultured with 800 μM NAC (- - -) versus untreated controls (—) for 1 hour and immediately washed and treated with PTL for 18 hours. Specimens shown correspond to AML5 (□), AML10 (⋄) and AML15 (▵).
NAC treatment abolishes PTL apoptosis induction in AML cells. Percent viability of CD34+ cells from 3 different AML specimens treated with increasing concentrations of PTL. Cells were precultured with 800 μM NAC (- - -) versus untreated controls (—) for 1 hour and immediately washed and treated with PTL for 18 hours. Specimens shown correspond to AML5 (□), AML10 (⋄) and AML15 (▵).
Parthenolide effects on cell survival are mediated by changes in oxidative state
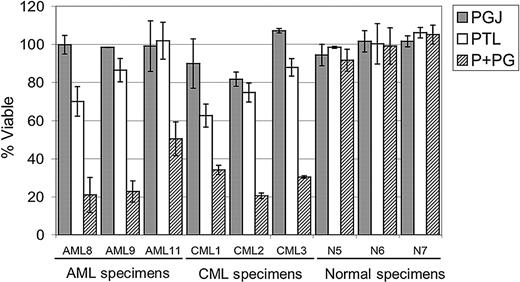
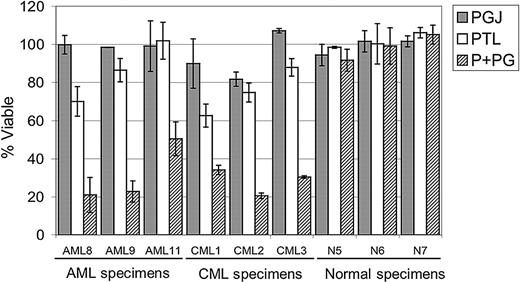
PTL has been reported to increase intracellular reactive oxygen species (ROS).11,23 Therefore, to determine whether the rapid apoptosis induced by PTL in AML cells involved redox changes, we precultured cells for 1 hour in 800 μM NAC (N-acetyl-l-cysteine), a potent antioxidant. Cells were then washed and cultured with different concentrations of PTL. Pretreatment of AML cells with NAC completely abolished the effects of PTL, even at 10-μM concentration of drug (Figure 4). These data suggest that an increased oxidative state may be a component of the leukemia cell death mechanism. To further address this possibility, we stained primary AML cells, in the presence or absence of PTL, with DCF (2,7-dichlorodihydrofluorescein diacetate; H2DCF-DA), a commonly used redox-sensitive dye. PTL-treated cells showed an increase in DCF fluorescence when compared with untreated cells, consistent with a more oxidized state (data not shown). To test whether increasing ROS alone was sufficient to induce AML apoptosis, we also treated AML cells with buthionine sulfoximine (BSO; data not shown). This treatment had no effect on AML viability. Since the data suggested an increase in ROS as part of the leukemia-specific apoptosis mechanism, we hypothesized that agents that increase ROS might enhance the activity of PTL. This theory was investigated by combining PTL with PGJ2, a natural ligand of peroxisome proliferator-activated receptor-gamma (PPAR-γ) that has been reported to induce apoptosis of cancer cells by generation of ROS.29-32 As illustrated in Figure 5, 2.5 μM PTL and 0.5 μM PGJ2 alone have little to no effect on CD34+CD38- AML or bcCML cells after 18 hours of treatment. However, when 2.5 μM PTL is combined with 0.5 μM PGJ2, a substantial decrease is observed for both AML and bcCML cells. Moreover, normal CD34+CD38- hematopoietic cells were not affected by the same treatment. These data suggest a role for redox changes in leukemia-specific apoptosis induction and indicate that PGJ2 can act to sensitize myeloid leukemia cells to PTL.
PGJ2 increases the sensitivity of leukemia cells to PTL. Average percent viability for CD34+CD38- cells normalized to untreated controls. Three AML, 3 bcCML, and 3 normal specimens were treated for 18 hours with 0.5 μM PGJ2 (▦), 2.5 μM PTL (□), or both (▨). Each error bar represents the SD. All assays were performed in triplicate.
PGJ2 increases the sensitivity of leukemia cells to PTL. Average percent viability for CD34+CD38- cells normalized to untreated controls. Three AML, 3 bcCML, and 3 normal specimens were treated for 18 hours with 0.5 μM PGJ2 (▦), 2.5 μM PTL (□), or both (▨). Each error bar represents the SD. All assays were performed in triplicate.
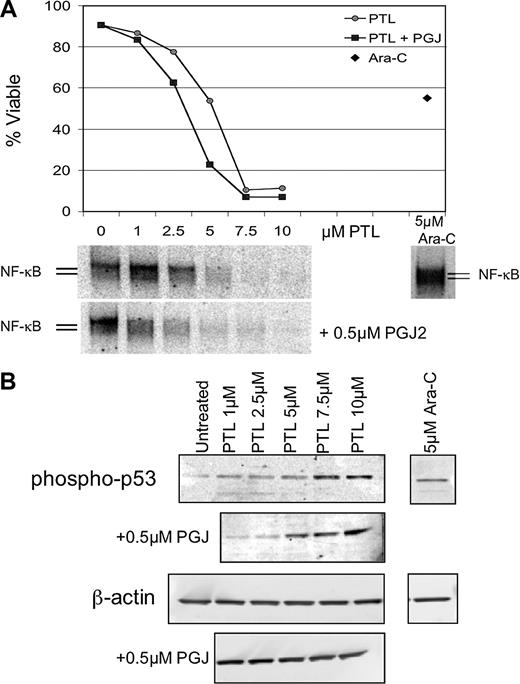
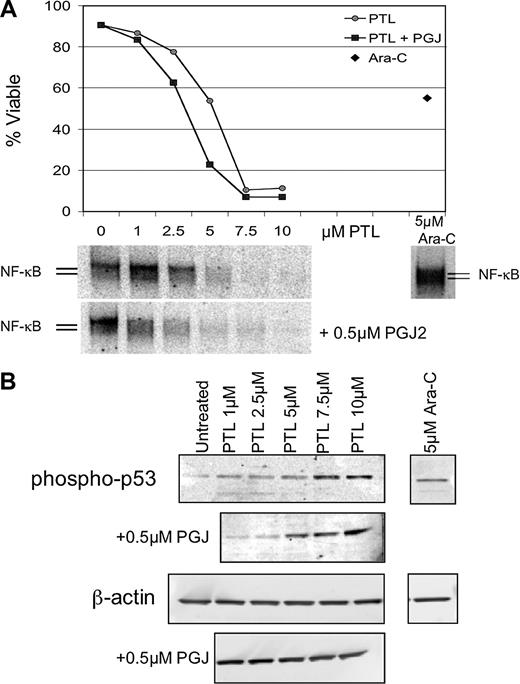
Apoptosis induction by PTL or PTL/PGJ2 correlates to inhibition of NF-κB and increased phosphorylation of p53(ser15). (A) Percent viability and NF-κB electrophoretic mobility shift assay (EMSA) of a representative AML specimen treated with increasing concentrations of PTL alone (•) or in combination with 0.5 μM PGJ2 (▪). The viability is compared with and Ara-C (5μM; ♦) treatments. (B) Immunoblot analysis of phospho-p53(ser15) and actin for the same representative AML specimen treated with increasing dose of PTL 0.5 μM PGJ2.
Apoptosis induction by PTL or PTL/PGJ2 correlates to inhibition of NF-κB and increased phosphorylation of p53(ser15). (A) Percent viability and NF-κB electrophoretic mobility shift assay (EMSA) of a representative AML specimen treated with increasing concentrations of PTL alone (•) or in combination with 0.5 μM PGJ2 (▪). The viability is compared with and Ara-C (5μM; ♦) treatments. (B) Immunoblot analysis of phospho-p53(ser15) and actin for the same representative AML specimen treated with increasing dose of PTL 0.5 μM PGJ2.
NF-κB inhibition and p53 activation are associated with the AML-specific apoptosis mechanism
NF-κB is constitutively active in AML cells but not in normal hematopoietic cells and its inhibition is correlated with leukemia-specific cell death.8 In addition, we have reported that p53 is activated when AML cells are treated with the proteasome inhibitor MG-132 in combination with idarubicin.11 Therefore, we sought to investigate whether common underlying pathways are invoked when leukemia-specific cell death is induced by PTL. To address how NF-κB inhibition correlates with cell death upon PTL or PTL + PGJ2 treatment, DNA-binding assays were performed after 6 hours of treatment. Figure 6A, shows a representative example of EMSA data from 5 independent experiments. The AML viability curve (Figure 6A top panel) illustrates the PTL and PTL + PGJ2 dose response and is compared with the degree of NF-κB inhibition for each concentration of PTL (Figure 6A bottom panel). For comparison, the figure also shows the level of NF-κB activity for Ara-C (5 μM) treatment. Clearly, as the inhibition of NF-κB is increased, the survival of the malignant cells is decreased. Moreover, in the case of PTL + PGJ2 treatment, the cells are more sensitive and displayed a lower NF-κB activity when compared with the same concentration of PTL alone. In contrast, treatment with 5 μM Ara-C showed an increase in NF-κB activity, as has been reported for many chemotherapeutic drugs. This suggests that there exists a direct correlation between NF-κB inhibition and the sensitivity of the AML cells to undergo apoptosis. To determine the degree of proapoptotic p53 activation, immunoblots were performed using phospho-ser15–specific anti-p53 antibody. Figure 6B (top panel) shows an increase in p53-ser15 phosphorylation upon PTL and PTL + PJG2 treatments. As cell death is increased, p53-ser15 phosphorylation increases as well. In addition, PTL in combination with PGJ2 shows even higher levels of phospho-p53-ser15. In the case of Ara-C treatment, we observed some increase in p53-ser15 phosphorylation; however, in the absence of NF-κB inhibition, we propose that any p53-mediated proapoptotic activity is abrogated by NF-κB–mediated survival signals.
Discussion
These studies demonstrate that PTL is able to induce rapid and robust death of myeloid leukemia cells. Our experiments show that an 18-hour treatment with PTL at 5 to 7.5 μM is highly toxic to total AML and bcCML populations, phenotypically primitive (CD34+/CD38-) AML cells, AML colony-forming cells, and AML stem cells as assayed by engraftment of NOD/SCID mice. In contrast, normal hematopoietic cells were almost completely unaffected by the same conditions. Thus, not only is PTL a potent antileukemia agent, it has no significant toxicity to normal cells at the concentrations tested. By comparison, analysis of the commonly used leukemia drug Ara-C showed modest toxicity to AML cells and relatively high toxicity to normal cells. Increased concentrations of Ara-C can be achieved in clinical studies (∼50-100 μM), which may result in greater AML cell killing; however, we did not generally test higher levels because of the strong cytotoxicity observed for normal cells at 5 to 10 μM. Taken together, these data suggest that PTL has intriguing potential as an antileukemia agent, particularly with regard to primitive AML stem and progenitor cells. These data also indicate that PTL may be useful for bcCML, but due to limited specimen availability we have only performed the preliminary studies shown in Table 1 and Figure 5.
Previous studies have described several characteristics of PTL in other cell systems. Perhaps most importantly, PTL is known to be a potent inhibitor of NF-κB.33 The mechanism of NF-κB down-regulation appears to occur via inhibition of the IKK complex. We also observed strong inhibition of NF-κB in primary AML cells and speculate that this activity contributes to the efficacy of PTL. However, our previous genetic studies using a dominant-negative repressor of NF-κB activity have shown that inhibition of NF-κB alone is not sufficient to mediate the robust cell death observed with PTL. Rather, blockade of the NF-κB pathway appears to sensitize primary AML cells to death and induces a relatively slow spontaneous apoptosis (∼50% cell death in 36 hours).11 Similarly, studies by Romano et al34 showed that treatment of primary AML blasts with NF-κB decoy oligonucleotides was not sufficient to induce a strong apoptotic response. Consequently, PTL must be affecting other pathways relevant to AML-specific survival. One such pathway appears to be mediated by the activity of p53. PTL induced rapid up-regulation of p53 protein with concomitant phosphorylation on serine 15. We have previously shown that activation of this pathway in AML cells up-regulates p53 proapoptotic target genes such as Bcl-2 associated X protein (Bax), p21, and growth arrest and DNA damage inducible protein (GADD45). Thus, we speculate that p53 activation is one component of the AML-specific cell death mechanism.
Another activity described for PTL is the induction of ROS. Indeed, antitumor activity of PTL has been strongly linked to the increased oxidative state observed during PTL treatment of other tumor types (eg, colorectal and hepatic cancers).13,27 We observed that pretreatment of AML cells with the free radical scavenger N-acetylcysteine completely abrogated PTL-mediated cell death. Notably, the NF-κB inhibitory activity of PTL was also completely blocked by NAC (data not shown). These data indicate that primitive AML cells may be more sensitive to changes in oxidative state than normal cells and that increased ROS contributes to AML-specific cell death. Interestingly, many chemotherapeutic agents are known to increase ROS, but such agents also typically up-regulate NF-κB activity. Our data indicate that inhibition of NF-κB may sensitize AML cells to simultaneous increases in ROS. If true, then one interesting future use of PTL may be a chemosensitizing agent in combination with several common antileukemia drugs. Indeed, a recent study by Nakshatri et al18 showed that PTL reverses resistance of cancer cells to TRAIL (tumor necrosis factor [TNF]–related apoptosis-inducing ligand). Previous reports with other drugs such as bortezomib also suggest NF-κB inhibition can be used to augment the response of cancer cells to chemotherapy agents.35,36
The naturally occurring prostaglandin PGJ2 has antiproliferative and proapoptotic effects in different types of cancer cells.29-32,37,38 PGJ2 is an inhibitor of NF-κB activation39 and can also induce intracellular oxidative stress.40 When low concentrations (0.5 μM) of PGJ2 were combined with low concentrations of PTL (2.5 μM), a cooperative effect was observed with respect to both cell death and modulation of NF-κB and p53. Thus, PGJ2 appears to enhance the activity of PTL and/or affect other pathways relevant to leukemia cell survival.
Taken together, the results of the present study confirm and extend data for a model we have previously described to explain unique aspects of LSC survival.41 From a general perspective, when AML stem and progenitor cells are deprived of survival signals and simultaneously exposed to cellular stress, they appear to be preferentially sensitized to cell death. We propose that inhibition of NF-κB, proapoptotic activation of p53, and increased oxidative stress are among the molecular changes that occur to mediate this event. Importantly, these specific events were noted in the present studies with PTL and in earlier studies using MG-132 and idarubicin. Thus, 2 completely disparate chemical approaches to LSC-specific apoptosis display these common molecular changes. Further, we observed that while Ara-C treatment produced p53 (ser-15) phosphorylation at levels similar to PTL, NF-κB was not inhibited. Therefore, the cellular response to Ara-C does not meet the criteria observed for PTL-induced LSC apoptosis.
Although PTL very effectively induces LSC-specific cell death and appears to be a good candidate for AML therapy, its pharmacologic properties may be limiting. In a dose escalation study for Feverfew, PTL plasma levels were well below the concentrations that showed an effect on AML survival.42 In vitro studies using purified material indicate PTL is only soluble to approximately 1.0 mg/mL in aqueous solutions and therefore may be difficult to deliver at concentrations sufficient for leukemia therapy. However, recent studies have shown that the PTL molecule can be chemically modified to improve water solubility by 100- to 1000-fold (P. Crooks, University of Kentucky, personal written communication, January 2004). Several such analogs appear to retain their antitumor properties and are currently being tested for in vivo efficacy.
In summary, this study demonstrates that selective targeting of LSCs is possible by exploiting unique molecular characteristics of leukemic cells. PTL as a single agent induces cooperating molecular events that are sufficient to cause LSC-specific cell death in vitro. This biologic activity can be abrogated by changes in redox state or enhanced by the added effects of PGJ2 treatment. Going forward, the main challenge will be to translate these findings to a clinically relevant setting and demonstrate that LSCs can be targeted in vivo.
Prepublished online as Blood First Edition Paper, February 1, 2005; DOI 10.1182/blood-2004-10-4135.
Supported by grants from the National Institutes of Health (NIH; R01-CA90446) and the US Department of Defense (DAMD17-03-1-0263). C.T.J. is a scholar of the Leukemia and Lymphoma Society.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Drs Fay Young and James Palis for critical review of the manuscript.