Comment on Heller et al, page 4664
Heller and colleagues show that platelet Mpl receptor expression is decreased in patients with FPD/AML (MIM 601399) and put forward a potential mechanism for thrombocytopenia in such patients.
Patients with familial platelet disorder with predisposition to acute myelogenous leukemia (FPD/AML) are characterized by an autosomal dominant thrombocytopenia, abnormal platelet function, and a propensity to develop acute myelogenous leukemia (AML). A key finding in patients with FPD/AML has been the demonstration by Song et al1 of mutations in one allele of the hematopoietic transcription factor consisting of a DNA-binding protein AML1, also known as CBFA2 and RUNX1, and CBFβ. These mutations have been in the AML1 runt-homology domain responsible for DNA binding and dimerization with CBFβ. AML1-deficient mice have embryonic lethality with absence of fetal liver hematopoiesis and die of central nervous system hemorrhage. AML+/- mice have a 15% reduction in platelet count2 and to date have not been shown to develop AML, in striking contrast to the human AML1 haplodeficiency where about 30% of the subjects progress to AML.
A striking feature in FPD/AML is moderate thrombocytopenia, the mechanisms of which are not well delineated. Patients with thrombocytopenia have impaired megakaryopoiesis evidenced by decreased megakaryocyte (MK) colony formation in bone marrow or peripheral blood.1 In mice, AML1 haplodeficiency induces a 50% decrease in long-term repopulating stem cells with a paradoxical increase in multilineage progenitors.2 In human bone marrow, MKs but not erythroblasts show strong immunostaining for AML1, which is upregulated during megakaryocytic differentiation in primary human progenitor cultures.3 Multiple lines of evidence link MK differentiation, protein kinase C (PKC), and AML1. PKC signaling has a critical role in MK differentiation; PKC activators have long been used to induce MK differentiation in cell lines. PCK-ϵ and PKC-θ are implicated in MK lineage commitment.4 PKC-ϵ cooperates with the transcription factor GATA-1 in the activation of the glycoprotein IIb (GPIIb) promoter.3 Of particular relevance, platelet PKC-θ expression (protein and mRNA) has been reported to be decreased in AML1 haplodeficiency,5 suggesting a potential mechanism for the impaired megakaryopoiesis and platelet production. This PKC deficiency has also been associated with impaired platelet responses to activation, including in protein phosphorylation and activation of GPIIb-IIIa.6 FIG1
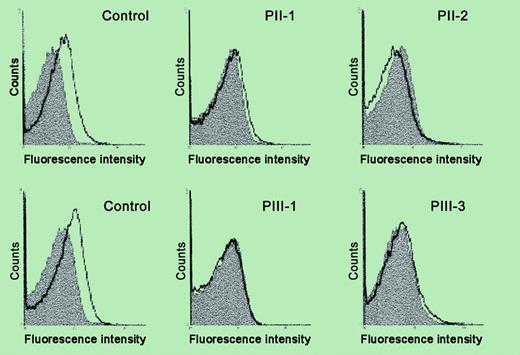
Analysis of platelet surface Mpl expression by flow cytometry. See the complete figure in the article beginning on page 4664.
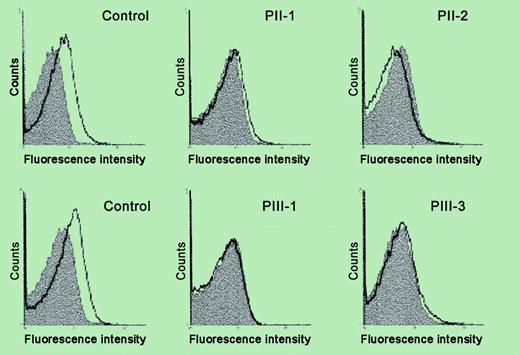
Analysis of platelet surface Mpl expression by flow cytometry. See the complete figure in the article beginning on page 4664.
In the current issue, Heller et al show decreased platelet surface expression and mRNA of the Mpl receptor in a family with AML1 haplodeficiency, along with decreased thrombopoietin-stimulated protein tyrosine phosphorylation as a functional concomitant. This provides a potential mechanism for the thrombocytopenia and suggests that the Mpl gene is regulated by AML1, although the latter needs to be established by demonstration of direct regulation of Mpl by AML1. Interestingly, the mutation in this family is in the C-terminal region of AML1 whereas previously reported patients have had mutations in the conserved Runt domain. These studies constitute an important piece in unraveling the pathogenetic mechanisms leading to thrombocytopenia and impaired platelet function in patients with FPD/AML. ▪