Comment on Brown et al, page 812
FMS-like tyrosine kinase 3 (FLT3) is proving to be an attractive therapeutic target for a variety of hematologic malignancies including the most common childhood cancer, acute lymphoblastic leukemia (ALL).
The cure rate for childhood acute lymphoblastic leukemia (ALL) has increased steadily over the past 3 to 4 decades with event-free survival (EFS) now approaching 75% to 80%. Yet despite this success, many challenges remain: (1) 25% of children fail treatment and their prognosis is poor, (2) many children are overtreated exposing them to potentially serious side effects, and (3) less progress has been made in certain subgroups such as infants whose blasts harbor an 11q23 rearrangement involving the MLL gene. The article by Brown and colleagues in this issue of the journal is extremely relevant to each one of these challenges.FIG1
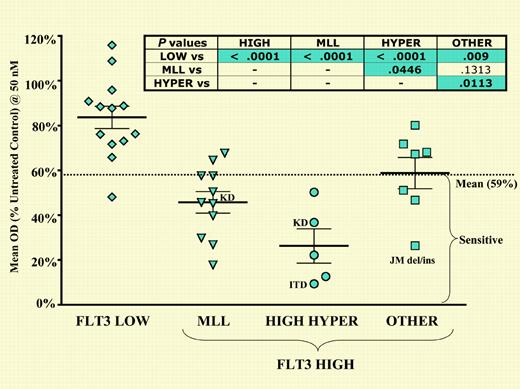
Primary ALL blasts with high-level FLT3 expression, particularly those with MLL gene rearrangements, high hyperdiploidy, and FLT3 juxtamembrane domain mutations, are differentially sensitive to FLT3 inhibition. See the complete figure in the article beginning on page 812.
Primary ALL blasts with high-level FLT3 expression, particularly those with MLL gene rearrangements, high hyperdiploidy, and FLT3 juxtamembrane domain mutations, are differentially sensitive to FLT3 inhibition. See the complete figure in the article beginning on page 812.
Molecularly targeted therapy was a dream rather than a reality until recently. The target explored by Brown and colleagues is the FMS-like tyrosine kinase-3 (FLT3), a member of the well-known class III receptor tyrosine kinases that also include c-kit and platelet-derived growth factor receptor (PDGFR). FLT3 is aberrantly expressed in many leukemias including 90% of AML cases and almost all cases of B-precursor ALL (reviewed in Stirewalt and Radich1 ). Activating mutations of FLT3 including internal tandem duplications (ITDs) and kinase domain mutations are seen in 22% of childhood AML cases, and the presence of ITD mutations correlates with a poor outcome. While ITD mutations are uncommon in childhood ALL, 18% of MLL rearranged leukemias and almost one third of hyperdiploid blasts harbor mutations in either the kinase or juxtamembrane domains.1
FLT3 is an attractive therapeutic target and a number of inhibitors have been developed that disrupt activated (phosphorylated) FLT3. The authors have tested the activity of one inhibitor, CEP-701, against ALL cell lines and clinical samples. They show that 50% of the cell lines and samples tested were sensitive to CEP-701. MLL rearranged (82%) and high hyperdiploid (100%) cases showed unusual sensitivity to this agent. Inhibition correlated with high expression and constitutive phosphorylation of FLT3. Biochemical analysis confirmed loss of phosphorylation and the initiation of apoptosis upon exposure to CEP-701 in vitro.
These results indicate that FLT3 inhibition may benefit 2 groups of ALL patients with distinctly different outcomes. Given such differences in survival, integration of FLT3 inhibitors will likely proceed along different paths. The poor survival of infants mandates rapid integration of new agents such as FLT3 inhibitors. In this circumstance, the development of clinical trial designs that seek to evaluate the efficacy of new agents in the context of conventional chemotherapy seems warranted. It must be recognized that these agents have undergone testing in adults, and preclinical work provides some insight into appropriate schedules.2-5
For good-risk patients, such as hyperdiploid cases, integration awaits further experience with FLT3 inhibitors in adults and children. Close to one third of B-precursor ALL samples contain a hyperdiploid karyotype so that a randomized trial could be considered. What would be the end point of such a trial? Clearly an improvement in EFS should always be a goal since even a 90% cure rate is not acceptable for these children. However, replacing more toxic agents such as anthracyclines with less harmful molecularly targeted drugs is another very important aim. Similar to the success of combination chemotherapy, treatment with a combination of molecularly targeted agents will likely replace more conventional treatments in the years to come. A major focus in clinical research is to accelerate this evolution in therapy. ▪