Abstract
Hematopoietic progenitor/stem cell homing to the bone marrow requires the concerted action of several adhesion molecules. Endothelial P- and E-selectins play an important role in this process, but their ligands on a large subset of neonate-derived human CD34+ cells are absent, leading to a reduced ability to interact with the bone marrow (BM) microvasculature. We report here that this deficiency results from reduced α1,3-fucosyltransferase (FucT) expression and activity in these CD34+ cells. Incubation of CD34+ cells with recombinant human FucTVI rapidly corrected the deficiency in nonbinding CD34+ cells and further increased the density of ligands for both P- and E-selectins on all cord blood–derived CD34+ cells. Intravital microscopy studies revealed that these FucTVI-treated CD34+ cells displayed a marked enhancement in their initial interactions with the BM microvasculature, but unexpectedly, homing into the BM was not improved by FucTVI treatment. These data indicate that, although exogenous FucT enzyme activity can rapidly modulate selectin binding avidity of cord blood CD34+ cells, further studies are needed to understand how to translate a positive effect on progenitor cell adhesion in bone marrow microvessels into one that significantly influences migration and lodgement into the parenchyma.
Introduction
The selectin family of adhesion molecules comprises 3 members (E-, L-, P-selectin) that mediate interactions among endothelial cells, leukocytes, and platelets.1-3 Selectins are particularly efficient in capturing free-flowing leukocytes from the circulation, decelerating their movement through a slow rolling motion on the vessel wall. This feat is accomplished by the rapid on and off binding rates of selectins with their ligands. Cell rolling provides the leukocyte with an ideal mechanism to survey the endothelium for chemokines and chemoattractants which can signal and activate β2 integrins that, in turn, mediate cell arrest and extravasation. Mice lacking either endothelial selectins or β2 integrins display severe defects in leukocyte recruitment into inflammatory sites.4-7
Accumulating data indicate that the adhesion pathways mediating the homing of hematopoietic progenitor and stem cells (HPCs/HSCs) following transplantation involve the cooperative functions of multiple adhesion molecules, including α4 integrins,8,9 β2 integrins,10 endothelial selectins,11-13 CD44,9,14,15 and their various counter-receptors. Thus, rather than using a specific homing receptor, hematopoietic stem cells appear to use adhesion molecules that are also expressed on mature leukocytes, but in distinct combinational sets.13 Specificity in the homing process is likely provided, at least in part, by the repertoire of functional adhesion molecules on HPCs/HSCs, the constitutive expression of specific adhesion receptors (E-selectin and vascular adhesion molecule-1 [VCAM-1]16 ) in the bone marrow (BM), and the presentation of the chemokine stromal-derived factor-1 (SDF-1 or CXCL12) by bone marrow endothelial cells which promote integrin activation, cell arrest, and subsequent migration toward the BM parenchyma.17
Human hematopoietic stem cells can be derived from 3 different sources for clinical BM transplantation: BM, peripheral blood after induced mobilization (mPB), and neonatal blood (cord blood, CB). CB-derived progenitors represent a promising source of human hematopoietic stem cells since they are widely available and their use is associated with reduced graft-versus-host disease.18 However, transplantation using CB cells has been mostly limited to children due to the limited number of cells available in a placenta.19 While investigating the differential adhesion pathways mediating the initial interactions of human CD34+ cells in the bone marrow of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, we found that neonate-derived CD34+ cell interactions were significantly reduced compared with those obtained from adult sources. Further studies revealed that a large subset (∼ 30%) of CD34+ cells, enriched in CD34+ CD38low/neg stem cells, expressed a nonfunctional form of P-selectin glycoprotein ligand-1 (PSGL-1), the main selectin ligand on hematopoietic cells.20
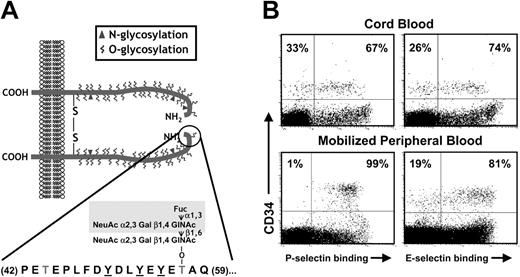
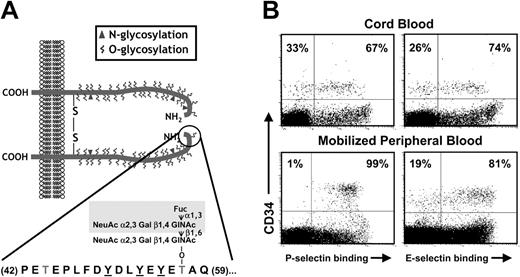
The key posttranslational modifications required for selectin binding have been particularly well characterized for PSGL-1.21 PSGL-1 is a homodimeric mucin that can bind all 3 selectins and contributes to the recruitment of leukocytes22,23 and human and mouse HPCs to the bone marrow.13,20 A short acidic 20–amino acid N-terminal segment of PSGL-1 (Figure 1A) contains an O-glycosylated threonine residue which, when presented in the context of sulfated tyrosines, can mediate high-affinity binding to P-selectin.24 Tyrosine sulfation, required for P-selectin but not E-selectin binding, can be accomplished by 1 or more tyrosylprotein-sulfotransferases (TPSTs).25 The α2,3sialylated and α1,3fucosylated tetrasaccharide sialyl Lewis X (sLex, NeuAcα2,3Galβ1,4[Fucα1,3]GlcNAc) appears to represent the major carbohydrate moiety that can interact with all 3 selectins.26 sLex is presented on branched O-linked polylactosamine structures synthesized by the core 2 β1,6 N-acetylglucosaminyltransferase (C2GnT) enzyme.27 Mice deficient in C2GnT present defects in adhesion to both P- and E-selectins.28 While it is likely that multiple α2,3sialyltransferases contribute to the sialylation of selectin ligands,29 2 α1,3fucosyltransferases (FucT VII and IV) are expressed at significant levels in leukocytes.30 The critical role for fucosylation in the synthesis of selectin ligand was revealed in experiments using mice null for FucTVII or both FucTVII and FucTIV, which show a phenotype similar to endothelial selectin knockouts.31,32 The role for fucose in selectin binding is further illustrated by patients presenting a mutation in the guanosine diphosphate (GDP)–fucose transporter gene33,34 who present a syndrome of leukocyte adhesion deficiency (LAD type II).
Selectin ligands on CB-CD34+ cells. (A) Structure of PSGL-1. PSGL-1 is homodimeric membrane mucin. A short N-terminal segment (residues 42-59; encircled) is critical for P-selectin binding. It includes 3 tyrosine residues that can be sulfated (underlined) and an important O-glycan (Thr57) that carries the tetrasaccharide sLex (shaded area). (B) Binding of P- and E-selectin on CB-CD34+ cells is heterogeneous. Dot plots depict CD34 staining and P- or E-selectin binding in CB- and mobilized peripheral blood (mPB) mononuclear cells (MNCs). Numbers indicate the percentage of CD34+ cells binding selectins (right quadrants) or not (left quadrants). Note that while all CD34+ cells from mPB bind P-selectin, a subset of CB-CD34+ cells does not bind P-selectin. Binding of E-selectin is heterogeneous in both CB and mPB CD34+ cells.
Selectin ligands on CB-CD34+ cells. (A) Structure of PSGL-1. PSGL-1 is homodimeric membrane mucin. A short N-terminal segment (residues 42-59; encircled) is critical for P-selectin binding. It includes 3 tyrosine residues that can be sulfated (underlined) and an important O-glycan (Thr57) that carries the tetrasaccharide sLex (shaded area). (B) Binding of P- and E-selectin on CB-CD34+ cells is heterogeneous. Dot plots depict CD34 staining and P- or E-selectin binding in CB- and mobilized peripheral blood (mPB) mononuclear cells (MNCs). Numbers indicate the percentage of CD34+ cells binding selectins (right quadrants) or not (left quadrants). Note that while all CD34+ cells from mPB bind P-selectin, a subset of CB-CD34+ cells does not bind P-selectin. Binding of E-selectin is heterogeneous in both CB and mPB CD34+ cells.
In the present report, we demonstrate that impaired selectin binding in the subset of CD34+ cells is due to reduced fucosylation of PSGL-1. We show that enforced fucosylation by a brief incubation with a recombinant FucT enzyme can correct the abnormality in nonbinding cells and can further increase selectin-mediated adhesion of all CD34+ cells.
Material and methods
Antibodies and selectin chimeras
Anti-human PSGL-1 (KPL1), anti-sLex (HECA 452), and fluorescein isothiocyanate (FITC)–conjugated anti-human CD38 were purchased from PharMingen (San Diego, CA), phycoerythrin (PE)–conjugated anti-human CD34 (clone AC136) was from Miltenyi Biotech (Bergisch Gladbach, Germany), and FITC-conjugated anti-human CD45 was from Immunotech (Marseille, France). Human P-selectin–immunoglobulin G (IgG) chimeras were a gift from Dr Robert Schaub (Wyeth, Cambridge, MA). Murine E- and P-selectin–IgM chimeras were produced by transfection of 293T cells with selectin-IgM DNA vector using the diethylaminoethyl (DEAE)–Dextran method31 or Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
Human samples and CD34+ cell isolation and culture
CB samples were obtained from normal full-term deliveries (38 weeks) in accordance with protocols approved by the Internal Review Board of Mount Sinai School of Medicine. CD34+ cells were purified using the CD34-isolation mini-MACS (magnetic cell sorting) kit (Miltenyi) or the EasySep kit (StemCell Technologies, Vancouver, BC, Canada) as previously described.20
For intravital microscopy experiments, purified human CD34+ cells were fluorescently labeled by incubation with 20 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 30 minutes at 6°C and washed thrice in Roswell Park Memorial Institute medium (RPMI) before intracarotid injection into mice.
For cell culture, purified CD34+ cells were washed once in Iscoves modified Dulbecco medium (IMDM) and resuspended in culture medium consisting of IMDM, 10% fetal bovine serum (FBS), 1% deionized bovine serum albumin (dBSA), 100 ng/mL recombinant human (rh) stem cell factor (SCF), 100 ng/mL rh–Flt-3 ligand, and 10 ng/mL rhThrombopoietin (TPO) (all from R&D Systems, Minneapolis, MN). In some experiments, 2% dBSA was used, and FBS was substituted for 160 μg/mL lecithin/cholesterol (Sigma, St Louis, MO), 10 μg/mL rh-insulin (Novo-Nordisk, Princeton, NJ), and 200 μg/mL transferrin (Sigma). Fresh medium was added every other day. These culture conditions were chosen because they can maintain and expand the primitive stem cell fraction35 and both the P-selectin binding and nonbinding populations in cord blood CD34+ cells (unpublished data, A.H. and P.S.F., August, 2000).
Mice
NOD/SCID mice were originally from the Jackson Laboratory (Bar Harbor, ME) and bred in isolators within the barrier facility of Mount Sinai. Mice were fed with sterilely irradiated chow and autoclaved water. Experimental procedures on animals were approved by the Animal Care and Use Committee at the Mount Sinai School of Medicine.
Intravital microscopy of the bone marrow
Six- to 9-week-old splenectomized NOD/SCID mice were prepared for bone marrow intravital microscopy as described previously.20 After anesthesia, mice were injected via the left common carotid artery catheter with 7 × 106 non–labeled human MNCs to block nonspecific retention sites. Then, untreated CFSE-labeled CD34+ cells were injected. When cells were no longer detected in the circulation (approximately 15 minutes), the same mouse was injected with FucTVI-treated CD34+ cells. Hemodynamic parameters were calculated as previously reported20 and were as follows in the experiments shown in this study: mean vessel diameter = 39 ± 2 μm; Vmax = 1652 ± 222 μm/s; wall shear rate = 204 ± 25 s–1. Any cell traveling below the critical velocity (Vcrit) was considered to be rolling on the vessel wall. Cells that remained stationary for 5 seconds were considered “arrested” cells.
α1,3-Fucosyltransferase and sialidase treatment
Cord blood–derived CD34+ cells (0.5 to 5 × 106) or MNCs (20 to 80 × 106) were washed once in Hanks balanced salt solution (HBSS) buffered with 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, and then incubated in 250 μL HEPES-buffered HBSS/0.1% human serum albumin (HSA; HHH buffer), containing 10 mM MnCl2, 1 mM guanosine diphosphate (GDP)–fucose (Calbiochem, San Diego, CA) and 20 mU/mL recombinant human FucTVI (Calbiochem), for 40 minutes at 37°C. In some experiments, CD34+ cells either untreated or previously treated with FucTVI were incubated with 90 mU/mL neuraminidase (Roche Diagnostics GmbH, Mannheim, Germany) in 50 μL HHH buffer, for 1 hour at 37°C. The reaction was stopped by washing the cells with phosphate-buffered saline (PBS) containing 0.5% BSA and 2 mM ethylenediaminetetraacetic acid (EDTA; PEB buffer) at 4°C, and cells were analyzed by flow cytometry for selectin chimera binding.
Flow cytometry, selectin chimera binding assay, and cell sorting
For 3-color staining, purified CD34 cells were incubated with PE-conjugated anti-CD34 and biotin-conjugated rat anti-sLex (HECA 452) antibodies. After washing once in binding buffer (RPMI containing 5% FBS), cells were incubated in supernatants from transfected 293T cells expressing P- or E-selectin chimeras and then with FITC-conjugated anti-human IgM antibody (1:50 dilution) and cyanine 5 (Cy5)–-conjugated streptavidin (both from Jackson ImmunoResearch, West Grove, PA) were added. Control samples were performed in the presence of 5 mM EDTA. Double-staining of CD34 and P- or E-selectin ligands was accomplished as previously described.20 In some experiments, triple staining of CB-MNCs was performed with PE-conjugated anti-CD34, FITC-conjugated anti-CD38, and the P- or E-selectin chimeras detected with a Cy5-conjugated anti-human IgM antibody. All incubations were performed for 15 minutes at 6°C, and analysis was performed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA).
For cell sorting, freshly purified or cultured CB-derived CD34+ cells were triple-labeled for CD34 and sLex antigen expression and for P-selectin binding. Cells were first labeled with PE-conjugated anti-CD34 and biotinylated–HECA-452 antibodies as indicated in the previous paragraph, then washed once and incubated with 3 μg (per million cells) human P-selectin–IgG chimera that had been preconjugated with 0.15 μg biotinylated Protein A. Previous studies showed that this concentration saturates P-selectin ligands on CD34+ cells.20 Cells were subsequently incubated with an excess of streptavidin-FITC (1.5 μg; Jackson ImmunoResearch) and washed once and sorted using a FACSVantage (Becton Dickinson). All incubations were performed for 15 minutes at 6°C in a medium containing RPMI and 5% FBS.
Chemotactic and static adhesion assays
Transwell migration of CD34+ cells toward CXCL12 (R&D Systems) and static adhesion assays of CD34+ cells on rhVCAM-1 (R&D Systems) were performed essentially as described.36 For the adhesion assay, the bound fraction of CFSE-labeled CD34+ cells was quantified using a fluorescence analyzer (HTS 7000 Plus; Perkin Elmer, Norwalk, CT).
ELISA-based α1,3-fucosyltransferase assay
For detection of FucT activity in sorted CD34+ cells, we modified the method described by Räbinä et al,37 which allows one to quantify FucT activity lysates derived from relatively few cells. Briefly, 6 × 105 cells were resuspended in 100 μLof50 μM morpholinepropanesulfonic acid (MOPS)–NaOH pH 7.4, 150 mM NaCl buffer, and lysed by sonication on ice by two 6-second bursts on a microprobe sonicator. Cell extracts were adjusted to 1% final concentration of Triton X-100 from a 10% stock, and protein concentration of the extracts was determined by the BCA (bicinchoninic acid) Protein Assay kit (Pierce, Rockford, IL). Then 10 μg/mL of the acceptor 3′sialyl-N-acetyllactosamine-BSA (14 atom spacer; V-labs, Covington, LA) was immobilized on enzyme-linked immunosorbent assay (ELISA) plates (Corning, Corning, NY) in 50 μL sodium bicarbonate pH 8.8 buffer, overnight at 4°C. Wells were blocked with 150 μL of a 0.5% BSA solution in the bicarbonate buffer for 1 hour at room temperature. After washing the wells twice with H2O, 50 μL cell extracts diluted 1:2 in assay buffer (50 μM MOPS-NaOH pH 7.4, 50 μM GDP-fucose, 6 mM MnCl2, and 0.5% Triton X-100) were added in triplicates to the wells (50 μg protein per triplicate). After 2 hours at 37°C, the wells were washed thrice in a buffer containing PBS with 0.5% BSA and 0.05% Tween-20 and incubated with 50 μL solution of 0.5 μg/mL HECA 452 in PBS/BSA 0.5% buffer for 1 hour at room temperature. After washing thrice, the wells were incubated with peroxidase-labeled anti-rat IgM antibody for another hour at room temperature. After washing, 50 μL per well of tetramethyl-benzidine (TMB; Sigma) peroxidase substrate was added to the wells and incubated for 30 minutes at room temperature. The reaction was stopped by addition of 50 μL 1M HCl, and the plate was read at 450 nm in a MicroQuant ELISA plate reader (Bio-Tek Instruments, Winooski, VT).
Homing assays
Purified CD34+ cells or total MNCs from CB samples were treated or not with the FucTVI as indicated in “α1,3-fucosyltransferase and sialidase treatment” and intravenously injected into sublethally irradiated (400 cGy) NOD/SCID mice. Mice were killed 12 to 16 hours after injection, femoral BM cells were harvested, and MNCs (containing all human CD34+ cells) were isolated using a 65% Percoll discontinuous gradient. Cells were labeled with PE-labeled anti-human CD34 and FITC-labeled antibodies to either anti-human CD45 or CD38, or a FITC-labeled control antibody. Cells were washed in PEB buffer and analyzed by flow cytometry using the TO-PRO3 dye (Molecular Probes) to exclude dead cells. A minimum of 4 × 105 viable cells were analyzed per sample.
Semiquantitative RT-PCR
RNA was isolated from cultured CD34+ cells sorted on the basis of P-selectin binding using the Trizol reagent (Life Technologies), and cDNA was prepared using the ThermoScrip RT-PCR (reverse transcriptase–polymerase chain reaction) system (Invitrogen) as indicated by the manufacturer. PCR amplification of the cDNA was carried out following the conditions previously reported to be below the plateau of amplification using the Taq polymerase (Invitrogen). For β-actin the primers used were forward, 5′-TGGGTCAGAAGGACTCCTATG-3′, and reverse, 5′-CAGGCAGCTCATAGCTCTTCT-3′, and PCR was performed as follows: 95°C for 5 minutes followed by 25 cycles of 94°C for 50 seconds, 56°C for 50 seconds and 72°C for 50 seconds, and a final extension at 72°C for 7 minutes.38 The same conditions were used for amplification of C2GnT with the following primers: forward, 5′-AAAATGCTTCCTCCACTC-3′, and reverse, 5′-TCAGTGTTTTAATGTCTC-3′.38 For the FucTVII gene the primers used were forward, 5′-CTCGGACATCTTTGTGCCCTATG-3′, and reverse, 5′-CGCCAGAATTTCTCCGTAATGTAG-3′, and the PCR was performed as follows: 36 cycles of 94°C for 1 minute, 66°C for 1 minute, and 72°C for 1 minute.39 For the TPST1 gene the primers used were forward, 5′-AAGATGGTTGGAAAGCTGAAGC-3′, and reverse, 5′-TTCTCATCCACCGTTCAGGATG-3′, and the PCR was performed as follows: 94°C for 5 minutes followed by 30 cycles of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 50 seconds.40 The same PCR conditions as for TPST1 were used for the TPST2 gene, using the following primers: forward, 5′-AGCATGCGCCTGTCGGTGCG-3′, and reverse, 5′-CACTTGGAGAGCGCTTCCAG-3′.40
Statistical analysis
All values are reported as mean ± SEM. Statistical significance for 2 paired or unpaired groups was assessed by the Student t test.
Results
Cord blood CD34+ cells that do not bind P-selectin lack the sialyl Lewis antigen and do not interact with E-selectin
We have previously found a significant difference in the expression of P-selectin ligands in cord blood CD34+ cells compared with adult-derived CD34+ cells in that approximately 30% of CB-CD34+ cells did not bind P-selectin despite the expression of the PSGL-1 glycoprotein, suggesting that PSGL-1 lacks key posttranslational modification(s) in these cells (Figure 1A-B; Hidalgo et al20 ). In contrast, the E-selectin binding profile of CD34+ cells was similar between neonatal and adult-derived cells in that approximately 20% to 30% of CD34+ cells did not bind E-selectin (Figure 1B; Hidalgo et al20 ).
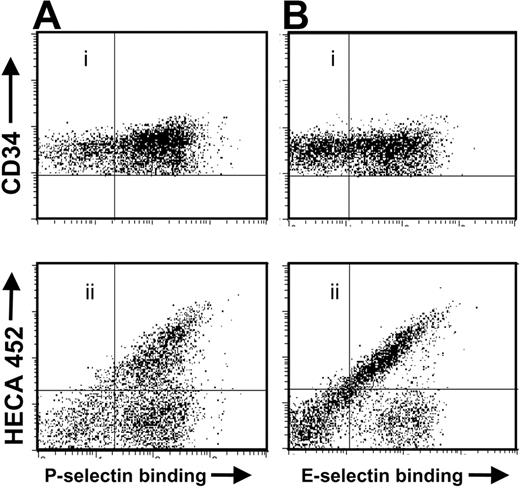
To gain more insight into the defect, cord blood cells were triple-stained for sLex antigen, CD34, and P- or E-selectin ligand expression and analyzed by fluorescence activated cell sorting (FACS). As shown in Figure 2A-B, approximately 30% of CB-CD34+ cells did not bind to either P- or E-selectin. Cells negative for selectin ligands (SLneg) did not express sLex as determined by HECA-452 staining (Figure 2Aii, Bii). In some samples, a subset of CD34+ cells bound to P- or E-selectins but did not express the HECA-452 antigen (Figure 2Aii, Bii, right lower quadrants).
CB-CD34+ cells that do not bind selectins are negative for sLex expression. Cord blood cells were stained for CD34, sLex (clone HECA-452), and assayed for (A) P-selectin or (B) E-selectin binding. Shown is a representative experiment of 6.
CB-CD34+ cells that do not bind selectins are negative for sLex expression. Cord blood cells were stained for CD34, sLex (clone HECA-452), and assayed for (A) P-selectin or (B) E-selectin binding. Shown is a representative experiment of 6.
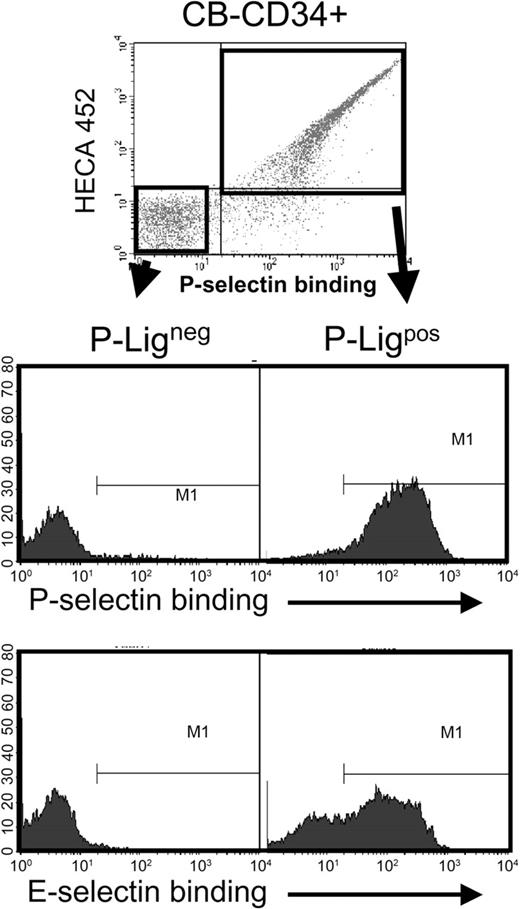
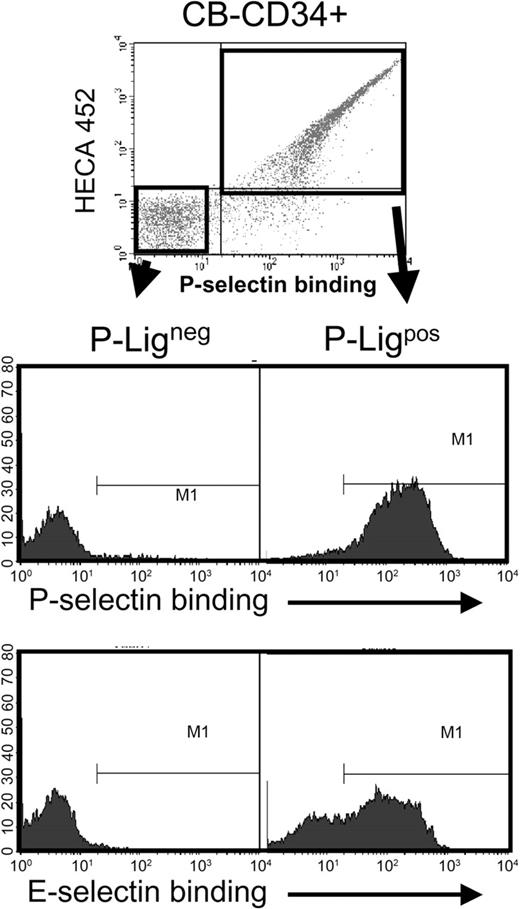
The similar profile in sLex expression and P- or E-selectin binding suggested that the same cell population might in fact be negative for the ligands of both selectins. To assess this issue, CD34+ cells were sorted on the basis of P-selectin binding using a P-selectin–IgG chimera preconjugated with biotinylated protein A and sLex expression, incubated with EDTA to remove soluble P-selectin and then re-assayed for P- and E-selectin binding (using the IgM chimeras). As shown in Figure 3, cells that did not bind P-selectin were also unable to interact with E-selectin, while most, but not all, P-selectin binding CD34+ cells interacted with E-selectin. Thus, these results suggest that the defect in selectin ligands is in a pathway common to the synthesis of ligands for both endothelial selectins.
CB-CD34+ cells that do not bind P-selectin are also negative for E-selectin ligands. CB-CD34+ cells were sorted on the basis of P-selectin binding (P-Ligneg and P-Ligpos) using the gates shown in the upper panel. Sorted cells were then analyzed for P- or E-selectin binding. Shown is a representative experiment of 3. Note that many cells that bound to P-selectin did not interact with E-selectin.
CB-CD34+ cells that do not bind P-selectin are also negative for E-selectin ligands. CB-CD34+ cells were sorted on the basis of P-selectin binding (P-Ligneg and P-Ligpos) using the gates shown in the upper panel. Sorted cells were then analyzed for P- or E-selectin binding. Shown is a representative experiment of 3. Note that many cells that bound to P-selectin did not interact with E-selectin.
Reduced expression and activity of α1,3 fucosyltransferase in SLneg cells
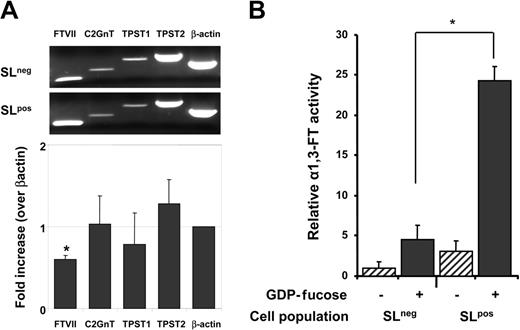
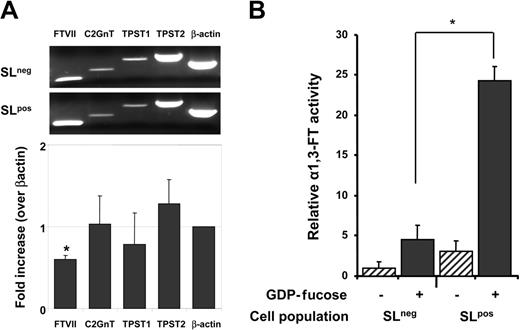
We extracted mRNA from sorted SLneg and SLpos cells to assess the expression of transferases involved in the posttranslational maturation of PSGL-1 by semiquantitative RT-PCR. The relative expression of tyrosilprotein-sulfotransferases (TPST1 and TPST2) was similar between the 2 populations. Since the same population of cells does not bind P- and E-selectins, this result was expected because tyrosine sulfation is not required for E-selectin binding.41 While there was no differential expression of C2GnT, the expression of FucTVII was significantly lower (by 40%) in SLneg cells compared with SLpos cells (Figure 4A).
Reduced expression of the FucTVII gene and reduced fucosyltransferase activity in SLneg cells. Cultured CB-CD34+ cells were sorted on the basis of P-selectin binding and sLex expression. (A) RNA was extracted for RT-PCR analyses. The bar graph shows the ratio ± SEM of mRNA levels in SLneg/SLpos for each of the genes indicated. Data were normalized for β-actin expression. *P = .003 compared with the β-actin control; n = 4 independent experiments. (B) Cell lysates were assayed for FucT activity. Controls were performed in the absence of a fucose donor (GDP-fucose; hatched bars) for each cell population. Bars represent mean ± SEM. *P = .001, n = 4 independent experiments.
Reduced expression of the FucTVII gene and reduced fucosyltransferase activity in SLneg cells. Cultured CB-CD34+ cells were sorted on the basis of P-selectin binding and sLex expression. (A) RNA was extracted for RT-PCR analyses. The bar graph shows the ratio ± SEM of mRNA levels in SLneg/SLpos for each of the genes indicated. Data were normalized for β-actin expression. *P = .003 compared with the β-actin control; n = 4 independent experiments. (B) Cell lysates were assayed for FucT activity. Controls were performed in the absence of a fucose donor (GDP-fucose; hatched bars) for each cell population. Bars represent mean ± SEM. *P = .001, n = 4 independent experiments.
Since mRNA levels may not correlate well with actual enzymatic activity, we evaluated FucT activity using a sensitive ELISA-like assay.37 This assay measures the ability of a given cell extract to add the high-energy fucose donors (GDP-fucose) to 3′sialyl-N-acetyllactosamine acceptors. The reaction generates the sLex antigen which can then be recognized by the HECA-452 antibody and detected by ELISA. Preliminary experiments revealed that the assay was sensitive and specific since a strong signal was detected with lysates from as little as 5 × 105 HL60 cells, and only background signal was detected when HL60 cell lysates or recombinant FucTVI were tested in the absence of the GDP-fucose donor (data not shown). Lysates from sorted SLneg and SLpos cells were thus prepared and subjected to the FucT assay. Strikingly, SLneg cells displayed about 20% of the FucT activity detected in SLpos cells (Figure 4B). Little signal was noted in both populations when GDP-fucose was omitted. These experiments strongly suggest that reduced fucosylation might be responsible for the absence of selectin ligands in these CD34+ cells.
Incubation with FucTVI restores selectin ligands on cord blood CD34+ cells
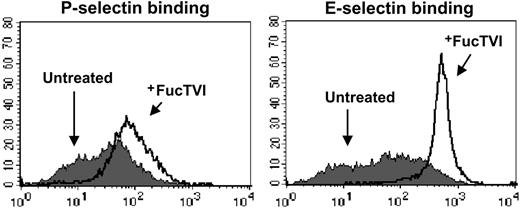
We reasoned that if impaired fucosylation was the sole cause for the deficient selectin binding of SLneg cells, treatment of CD34+ cells with recombinant FucT might restore selectin binding. Cord blood–derived CD34+ cells were thus incubated in the presence or absence of recombinant human FucTVI and GDP-fucose for 40 minutes at 37°C, after which the cells were tested for selectin binding by FACS. We chose to use FucTVI because it was commercially available, and it has previously been demonstrated to increase sLex expression on murine neutrophils.42 We found that FucTVI treatment dramatically increased the percentage of CB-CD34+ cells binding P-selectin (Figure 5; Table 1). Similarly, virtually all cells were able to bind to E-selectin following FucTVI treatment (Figure 5). A similar induction of the sLex antigen was achieved by this treatment (Table 1). Interestingly, not only the percentage of CD34+ cells able to bind both selectins was increased, but also the overall density of selectin ligands on all cells was greatly increased (by 38% and 345% for P- and E-selectin, respectively; Figure 5; Table 1). Incubation of sorted SLneg cells with FucTVI also resulted in the induction of selectin ligands, indicating that these cells are not lost during FucTVI treatment (data not shown).
Treatment with recombinant human fucosyltransferase VI enhances P- and E-selectin binding activity in CB-CD34+ cells. Purified CB-CD34+ cells were treated with rhFucTVI for 40 minutes at 37°C in the presence of GDP-fucose (+FucTVI, open histograms) or left untreated (filled histograms) and then analyzed for both P- and E-selectin binding. See Table 1 for complete data and statistics.
Treatment with recombinant human fucosyltransferase VI enhances P- and E-selectin binding activity in CB-CD34+ cells. Purified CB-CD34+ cells were treated with rhFucTVI for 40 minutes at 37°C in the presence of GDP-fucose (+FucTVI, open histograms) or left untreated (filled histograms) and then analyzed for both P- and E-selectin binding. See Table 1 for complete data and statistics.
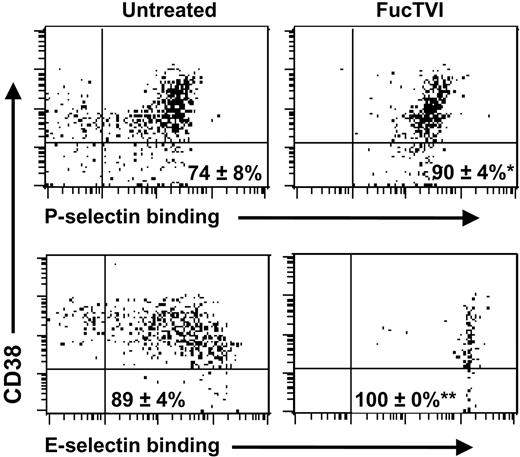
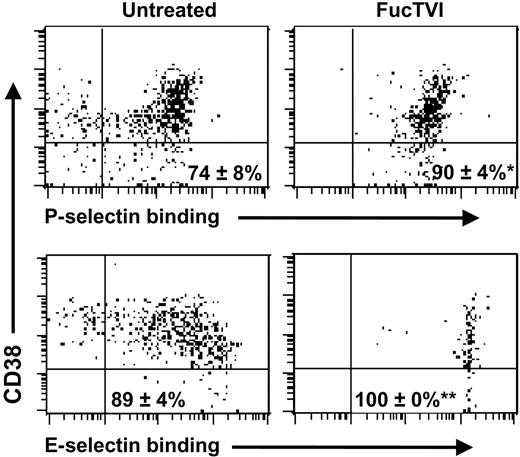
Because CD34+ cells comprise a heterogeneous population of which immature progenitors represent only a minor proportion characterized by low or absent expression of CD38, we next analyzed whether treatment with FucTVI also induced selectin ligands on CD34+CD38lo/neg cells. As shown in Figure 6, treatment with FucTVI also induced both P- and E-selectin ligands on CD34+CD38lo/neg cells, to a level similar to that of total CD34+ cells.
Treatment with recombinant human fucosyltransferase VI enhances P- and E-selectin binding activity on CB-CD34+CD38lo/neg cells. CB-MNCs were treated with rhFucTVI for 40 minutes at 37°C in the presence of GDP-fucose or left untreated, stained for CD34 and CD38 expression and binding of P- or E-selectin. Shown is CD38 expression and selectin binding of gated CD34+ cells. Numbers on each panel indicate mean ± SEM percentage of CD34+CD38lo/neg cells that bind the selectins. *P = .02, **P = .07, compared with untreated samples; n = 5 donors.
Treatment with recombinant human fucosyltransferase VI enhances P- and E-selectin binding activity on CB-CD34+CD38lo/neg cells. CB-MNCs were treated with rhFucTVI for 40 minutes at 37°C in the presence of GDP-fucose or left untreated, stained for CD34 and CD38 expression and binding of P- or E-selectin. Shown is CD38 expression and selectin binding of gated CD34+ cells. Numbers on each panel indicate mean ± SEM percentage of CD34+CD38lo/neg cells that bind the selectins. *P = .02, **P = .07, compared with untreated samples; n = 5 donors.
To determine whether PSGL-1 was a target of fucosylation, FucTVI-treated CD34+ cells were incubated with the KPL1 antibody, which inhibits the P-selectin binding region of PSGL-1 and abrogates all P-selectin–mediated binding of CD34+ cells,20,43 and then assayed for P-selectin binding. Preincubation with KPL1, but not a control antibody, resulted in complete blockage of P-selectin binding (data not shown), indicating that PSGL-1 is a major target of fucosylation and accounts for all P-selectin–mediated binding of FucTVI-treated cells.
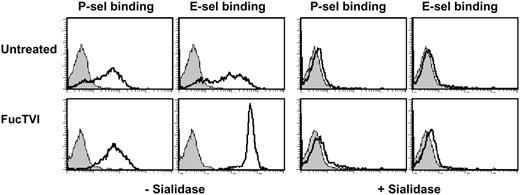
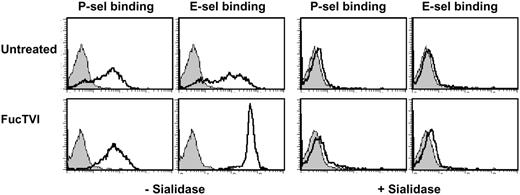
To exclude the possibility that deficient sialylation contributed to the lack of selectin ligand expression in SLneg cells, FucTVI-treated and control CD34+ cells were incubated with sialidase and selectin chimera binding assessed by FACS. We found that P- and E-selectin binding to FucTVI-treated or control CD34+ cells was completely abolished after the removal of sialic acid (Figure 7), indicating that the levels of sialylation in SLneg cells are sufficient for the synthesis of selectin ligands. Altogether, these experiments indicate that defective α1,3-fucosylation is the sole abnormality responsible for impaired selectin binding in SLneg cells. The dramatic increase in the density of functional selectin ligands by a short incubation with exogenous FucTVI provides evidence for a simple and efficient method to induce ligands for P- and E-selectins on human progenitor cells.
Selectin ligands on CB-CD34+ cells are sialylated. Purified FucTVI-treated or control CB-CD34+ cells were incubated with sialidase or vehicle and assayed for P- and E-selectin binding. Sialidase treatment completely abrogated selectin binding in both FucTVI-treated and control CB-CD34+ cells. Shown is 1 of 3 experiments with the same result. Filled histograms represent control chimera binding performed in the presence of 5 mM EDTA.
Selectin ligands on CB-CD34+ cells are sialylated. Purified FucTVI-treated or control CB-CD34+ cells were incubated with sialidase or vehicle and assayed for P- and E-selectin binding. Sialidase treatment completely abrogated selectin binding in both FucTVI-treated and control CB-CD34+ cells. Shown is 1 of 3 experiments with the same result. Filled histograms represent control chimera binding performed in the presence of 5 mM EDTA.
Enhanced selectin ligand function on cord blood CD34+ cells increases their capacity to interact with the BM microvasculature
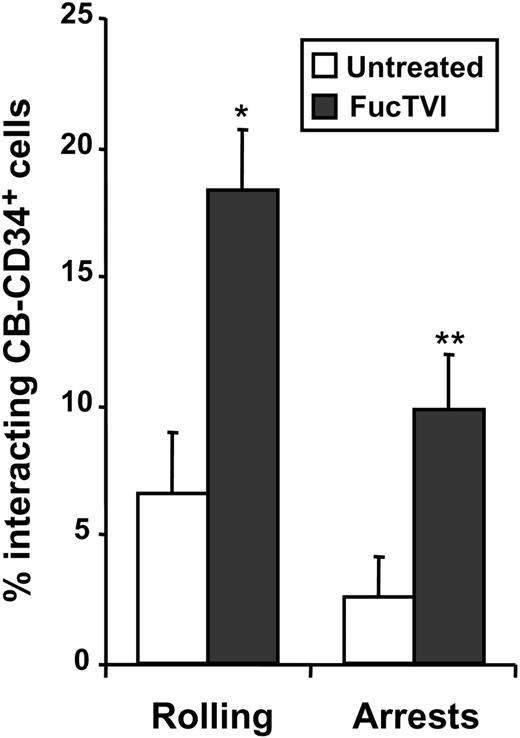
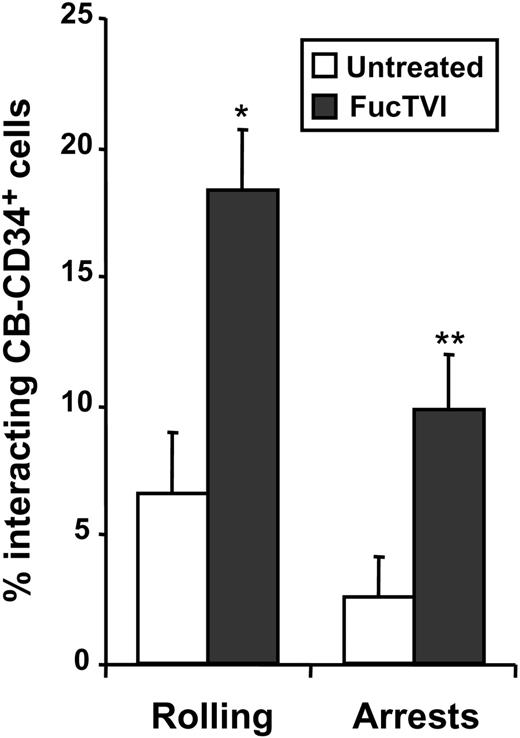
We have previously shown using intravital microscopy of the BM in NOD/SCID mice that the ability of CB-D34+ cells to interact with BM microvessels was significantly reduced compared with CD34+ cells derived from adult donors.20 To evaluate whether the acquisition of P- and E-selectin ligands can improve the capacity of CB-CD34+ cells to interact with the BM microvasculature in vivo, we evaluated the initial interactions of FucTVI-treated or untreated CB-CD34+ cells with BM microvessels of NOD/SCID mice. As shown in Figure 8, FucTVI treatment resulted in a sharp increase in the proportion of cells able to interact with the BM microvasculature of NOD/SCID mice compared with untreated cells (2.8-fold increase; Figure 8). Importantly, the proportion of cells capable of arresting on the BM microvasculature was also dramatically increased upon treatment with FucTVI (3.9-fold increase; Figure 8). These experiments indicate that improved selectin ligand function in CB-CD34+ cells can lead to enhanced interactions with the BM microvessels in vivo, and they confirm the notion that lower selectin ligand activity in these cells was responsible for their reduced capacity to interact with BM vessels compared with CD34+ cells from other sources.20
FucTVI treatment enhances the capacity of CB-CD34+ cells to interact with BM microvessels. Purified CB-CD34+ cells were treated with FucTVI or left untreated, fluorescently labeled, and injected into NOD/SCID mice prepared for BM intravital microscopy. The numbers of cells interacting with the BM microvasculature were determined by analysis of video recordings. Shown is mean ± SEM percentage of interacting CD34+ cells from 7 independent fluorescence intravital microscopy experiments. *P = .0001; **P = .01 comparing FucTVI-treated versus untreated.
FucTVI treatment enhances the capacity of CB-CD34+ cells to interact with BM microvessels. Purified CB-CD34+ cells were treated with FucTVI or left untreated, fluorescently labeled, and injected into NOD/SCID mice prepared for BM intravital microscopy. The numbers of cells interacting with the BM microvasculature were determined by analysis of video recordings. Shown is mean ± SEM percentage of interacting CD34+ cells from 7 independent fluorescence intravital microscopy experiments. *P = .0001; **P = .01 comparing FucTVI-treated versus untreated.
Enforced fucosylation of cord blood CD34+ cells does not enhance homing to the BM
To determine whether the increased ability of FucTVI-treated CB-CD34+ cells to interact with the BM microvasculature in vivo could result in increased homing ability, sublethally irradiated NOD/SCID mice were injected with untreated or FucTVI-treated total mononuclear cells or purified CB-CD34+ cells, and the number of human CD34+ cells homed in the femoral BM was assessed 12 to 16 hours after injection. Unexpectedly, no significant differences in the total number of homed CB-CD34+ cells were found with or without FucTVI treatment (Table 2). Similarly, the number of homed primitive CD34+/CD38low/neg cells was not affected by FucTVI treatment (Table 2). Interestingly, we observed that in both groups the majority of homed CB-CD34+ cells had the CD38lo/neg phenotype (82% ± 2%; n = 12 mice), as has been previously reported.44
Fucosylation of cord blood CD34+ cells does not alter the function of other homing receptors
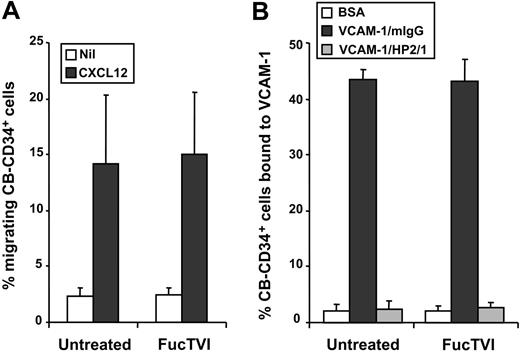
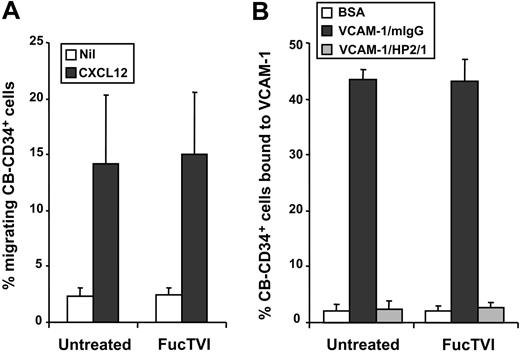
Since FucTVI can add fucose moieties to sialylated and nonsialylated lactosamine acceptors on multiple glycoproteins and glycolipids,42,45 enforced fucosylation may have affected the function of other homing receptors on the surface of CD34+ cells. To investigate this possibility, we analyzed the in vitro function of the α4 integrin and the chemokine receptor CXCR4, 2 receptors that play important roles in progenitor homing to the BM.8,46,47 Freshly purified CB-CD34+ cells, treated with FucTVI or untreated, were analyzed for chemotaxis toward CXCL12, the ligand of CXCR4. We found no significant difference in the capacity of treated or untreated cells to migrate toward CXCL12 (Figure 9A). Similarly, there was no difference in the ability of FucTVI-treated and untreated CD34+ cells to bind to immobilized VCAM-1 in a static adhesion assay (Figure 9B). Thus, these results suggest that enforced fucosylation with FucTVI does not alter the function of other important homing receptors on CB-CD34+ cells.
FucTVI treatment does not affect the function of other homing receptors on cord blood CD34+ cells. Purified CB-CD34+ cells, FucTVI-treated or untreated, were assayed for (A) chemotaxis toward the chemokine CXCL12 and (B) adhesion to rhVCAM-1. Adhesion to VCAM-1 was specific since it was completely inhibited by an anti-α4 integrin antibody (HP2/1). No difference was observed in the activities of CXCR4 and α4 integrins between FucTVI-treated or untreated cord blood CD34+ cells. Shown are mean ± SEM values; n = 6 for panel A and n = 4 for panel B.
FucTVI treatment does not affect the function of other homing receptors on cord blood CD34+ cells. Purified CB-CD34+ cells, FucTVI-treated or untreated, were assayed for (A) chemotaxis toward the chemokine CXCL12 and (B) adhesion to rhVCAM-1. Adhesion to VCAM-1 was specific since it was completely inhibited by an anti-α4 integrin antibody (HP2/1). No difference was observed in the activities of CXCR4 and α4 integrins between FucTVI-treated or untreated cord blood CD34+ cells. Shown are mean ± SEM values; n = 6 for panel A and n = 4 for panel B.
Discussion
We have identified in this report the molecular mechanism responsible for the deficiency in selectin binding in a large subset of cord blood CD34+ cells derived from full-term deliveries. We show that the nonbinding cells display a sharp reduction in α1,3 fucosyltranferase activity, which leads to deficits in both P- and E-selectin–mediated interactions in the bone marrow microvasculature. This adhesion deficit was fully correctable by a brief incubation of CD34+ cells or total MNCs with recombinant FucTVI. In fact, FucTVI treatment produced supraphysiologic levels of selectin binding activity on all cord blood CD34+ cells, leading to improved initial interactions with bone marrow microvessels but, surprisingly, not to improved homing into the BM parenchyma.
Regulation of α 1,3 fucosyltranferase expression
The contrast in the expression and activity of FucT between neonatal and adult CD34+ cells suggest regulated expression of FucT during ontogeny. Previous studies in human embryos have shown that the myeloid FucT was expressed very early (8 weeks) in all tissues and that FucTs of different specificities were then induced.48 There are also examples of postnatal induction of FucT activities in other organs. For instance, up-regulation of FTVII expression 2 days after birth was described in high endothelial venules (HEVs) of murine lymph nodes.49 In the intestinal mucosa, a shift from sialylation to fucosylation, associated with induction of fucosyltransferase activities, has been reported following the introduction of dietary fibers in weaning rats.50 This suggests that the net fucosyltranferase activities in certain tissues can be modulated after birth. The significant reduction in FucTVII mRNA in SLneg cells suggests that their reduced FucT activity is likely regulated at the transcriptional level and not from endogenous protein inhibitors that could target the FucT enzyme(s) or GDP-fucose.51
Cytokine-mediated transcriptional regulation of the FucTVII gene expression has been reported in adult T lymphocytes. Most circulating human T cells express PSGL-1 glycoprotein but are unable to bind P-selectin. When cultured in vitro for several days, stimulated T cells could bind P-selectin, and binding was associated with up-regulation of FucT but not C2GnT activity.52 Subsequent studies have shown that interleukin 12 (IL-12)53 and transforming growth factor β1 (TGF-β1)54 can induce FucTVII gene transcription in CD4+ T cells, while the T helper type 2 (Th2) cytokine IL-4 was inhibitory.53 Mindful of these studies, we started the present work by growing CB-CD34+ cells in the presence of hematopoietic cytokines (SCF, Flt-3 ligand, and TPO) known to transiently expand HSCs35 with or without the presence of matrix proteins (fibronectin, fibrinogen, hyaluronic acid, or collagen type I), anti-adhesion receptor antibodies or chimeric proteins (P-selectin–Ig, E-selectin–Ig, VCAM-Ig, PSGL-1–Ig, anti–VLA-4, or anti-PSGL-1,) or another cytokine (TGF-β1), with the rationale that ligation of surface receptor(s) might trigger signals that induce the maturation of PSGL-1. None of the conditions tested, however, affected the proportion of CD34+ cells that bound to selectins after up to 3 days of cultivation (A.H. and P.S.F., unpublished data, September 2001). Since we show here that selectin ligands are hypofucosylated or a-fucosylated in a subset of CD34+ cells, the inability of TGF-β1 to induce selectin ligands in these experiments suggests that the FucTVII gene in HSCs may not respond to the same regulatory pathways as those of T cells.
Selectin ligands on cord blood CD34+ cells
The effect of FucTVI on selectin ligand densities on CD34+ cells was dramatic. The response of cord blood CD34+ cells appears to differ from that of mature mouse neutrophils in which sLex epitopes were induced by FucTVI without a significant augmentation of their ability to bind P- or E-selectin.42 We found that even after enforced fucosylation, PSGL-1 remains the sole P-selectin ligand on CD34+ cells, indicating that PSGL-1 is a target for the FucTVI. The dramatic up-regulation of E-selectin ligands by FucTVI treatment suggests most E-selectin ligands are only partially fucosylated under physiologic conditions. The apparent preferential fucosylation of P-selectin ligands over E-selectin ligands in CD34+ cells is consistent with the results obtained from patients with LAD type II treated with oral fucose in which neutrophil P-selectin ligands were rapidly restored but E-selectin ligands required higher doses administered over longer periods.55,56 This may result from a preferential fucosylation of O-glycans over N-glycans in conditions in which FucT activity is limiting.57 It is interesting that enforced fucosylation of CD34+ cells produced a highly homogenous E-selectin binding population that formed a single narrow peak by flow cytometry (Figure 5). This does not mean, however, that E-selectin ligands are carried by a single glycoprotein. In fact, this possibility is unlikely. The exact nature of E-selectin ligands is still unclear even in mature neutrophils, but PSGL-1, CD44,14 and E-selectin ligand-1 (ESL-1)58 represent prime candidates. We have recently shown, for example, that PSGL-1 can mediate approximately 60% of the E-selectin–dependent progenitor homing activity in the mouse.13 Enforced fucosylation may thus represent a useful tool to identify novel E-selectin ligands on CD34+ cells.
Can increased adhesion improve HSC engraftment?
Since the relatively small number of harvested cord blood cells limits the availability of donors available for transplantation in adults, and because we have previously shown that many cord blood CD34+ CD38– stem cells do not bind selectins,20 we hypothesized that a correction of this “defect” would provide a way to enhance progenitor homing and engraftment in the bone marrow and, therefore, may increase the cord blood stem cell donor pool. While we found a dramatic effect on cell rolling and adhesion in the bone marrow microvasculature, homing studies unexpectedly did not reveal a significant improvement in the migration to the bone marrow. Several possibilities should be considered to explain our results.
One possibility is that FucTVI-mediated fucosylation may have altered the function of other surface glycoproteins or glycolipids that contribute to homing into BM. FucTVI is relatively nonspecific since it can add fucose to both sialylated and nonsialylated acceptors, whereas the natural leukocyte FucTVII exhibits restricted acceptor specificity to α2,3sialylated oligosaccharides.59 Although we show here that α4 integrin and CXCR4 functions were unchanged in vitro, the possible interference of neofucosylation with other cell surface molecules cannot be excluded. FucTVI is mostly found in the plasma, but, interestingly, it was reported to be stored in Weibel-Palade bodies of endothelial cells (also the site of P-selectin storage).60 Although the present results clearly indicate that it can generate selectin ligands, FucTVI does not appear to be a physiologic contributor on leukocytes since it cannot rescue selectin ligand deficiency in mice lacking the FucTVII enzyme.31 It will be important to evaluate in the future whether the more selective fucosylation achieved by FucTVII can improve cord blood CD34+ cell homing and engraftment.
One should also consider the possibility that enforced fucosylation of selectin ligands may have a deleterious effect on CD34+ cells. There is evidence that selectin ligands may negatively affect hematopoietic progenitor growth. For example, mice deficient in both endothelial selectins display enhanced myelopoiesis in the BM with displacement of erythroid activity in the spleen, although it is difficult to separate the possibility of a direct effect on HPCs from a secondary stimulation by increased levels of hematopoietic cytokines.5 Studies in mice lacking FucTVII indicated that FucTVII–/– progenitors do not home efficiently to the BM, but the number of HSCs in steady-state FucTVII–/– BM was increased.61 This suggests that FucTVII may play dual roles in facilitating homing and engraftment and in maintaining stem cell numbers. Consistent with a role of selectin ligands in HSC growth and survival, the engagement of CD34+ cells with P-selectin or E-selectin inhibited growth in vitro and induced apoptosis, but this effect spared the most primitive progenitors.43,62 Although we have not noticed significant apoptosis in cultured FucTVI-treated CD34+ cells (A.H. and P.S.F., unpublished data, May 2003), whether FucTVI-treated CD34+ cells are prone to apoptosis in vivo is currently unknown.
It is also possible that the generation of “super sticky” CD34+ cells may exhibit increased lodgement in nonhematopoietic organs. While we clearly show using intravital microscopy that FucTVI-treated CD34+ cells that reach the BM display increased rolling and adhesion on the endothelium, it is conceivable that the absolute numbers of CD34+ cells reaching the BM microcirculation is altered by a steal phenomenon. Low levels of constitutive expression of endothelial selectins have been detected by various organs using sensitive I125 antibody labeling.63 In particular, our recent data suggest that P-selectin expression in the spleen recruits HPCs in that organ.13 However, splenic lodgement cannot explain the lack of improved BM homing in the present experiments since our recipient mice had been splenectomized.
It is interesting that studies have suggested that enhanced chemotaxis through CXCR4-induced expression47 or function64 can improve HPC homing. Since the chemotactic response could potentially limit the benefit of enhanced selectin-mediated adhesion, it will also be important in the future to design studies that will investigate the combined effects of improved stem cell adhesion by forced fucosylation and treatments that enhance chemotaxis. These approaches may lead to better ways to transplant cord blood stem cells with greater efficiency.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-03-1026.
Supported by the National Institutes of Health (grants DK-56638 and HL-69438) (P.S.F.). A.H. was supported in part by a grant from the Cooley's Anemia Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Note added in proof. While this paper was in press, another study by Xia et al. (Blood 2004, 104:3091-3096) has shown that exogenous fucosylation can enhance selectin ligand function on CB-CD34+ cells. A recent study (Christopherson et al.; Science 2004; 305:1000-1003) also suggests that inhibition of bone marrow SDF-1/CXCL12 degradation may enhance HPC/HSC homing.
We thank the Labor and Delivery Team at Mount Sinai Medical Center for assistance in obtaining neonatal blood. We also thank Drs. Robert S. Schaub and John Lowe for providing reagents.