Abstract
Embryonic stem (ES) cells have the potential to serve as an alternative source of hematopoietic precursors for transplantation and for the study of hematopoietic cell development. Using coculture of human ES (hES) cells with OP9 bone marrow stromal cells, we were able to obtain up to 20% of CD34+ cells and isolate up to 107 CD34+ cells with more than 95% purity from a similar number of initially plated hES cells after 8 to 9 days of culture. The hES cell–derived CD34+ cells were highly enriched in colony-forming cells, cells expressing hematopoiesis-associated genes GATA-1, GATA-2, SCL/TAL1, and Flk-1, and retained clonogenic potential after in vitro expansion. CD34+ cells displayed the phenotype of primitive hematopoietic progenitors as defined by co-expression of CD90, CD117, and CD164, along with a lack of CD38 expression and contained aldehyde dehydrogenase–positive cells as well as cells with verapamil-sensitive ability to efflux rhodamine 123. When cultured on MS-5 stromal cells in the presence of stem cell factor, Flt3-L, interleukin 7 (IL-7), and IL-3, isolated CD34+ cells differentiated into lymphoid (B and natural killer cells) as well as myeloid (macrophages and granulocytes) lineages. These data indicate that CD34+ cells generated through hES/OP9 coculture display several features of definitive hematopoietic stem cells.
Introduction
Human embryonic stem (hES) cells represent a unique population of cells capable of self-renewal and differentiation. hES cells give rise to tissues from all 3 germ layers upon injection into immunodeficient mice or when induced to form embryoid bodies in vitro.1,2 Recently, the potential of hES cells to differentiate into hematopoietic lineage has been demonstrated. During embryoid body differentiation or upon coculture with S17 bone marrow stromal cell line, hES cells give rise to endothelial cells with hemangioblastic properties3 and colony-forming cells (CFCs).4-6 The addition of a combination of cytokines and bone-morphogenic protein-4 (BMP-4) strongly promotes hES cell hematopoietic differentiation during embryoid body development.5 These results clearly demonstrate the usefulness of hES cells as an alternative source of hematopoietic precursors that potentially can be used for studies of hematopoietic ontogeny and hematopoietic cell transplantation in humans. However, this requires reproducible methods for large-scale production of hematopoietic stem cells from hES cells.
The macrophage colony-stimulating factor (M-CSF)–deficient stromal cell line OP9 has been used successfully to induce mouse ES cell differentiation into myeloid, lymphoid, erythroid, and megakaryocytic lineage cells.7-9 Here we describe OP9 coculture for hematopoietic differentiation of hES cells. OP9 coculture allowed observation of the process of hematopoietic differentiation of hES cells and was superior to S17 and MS-5 coculture in the production of CD34+ cells and CFCs. We demonstrated that CD34+ cells generated in the OP9 system gave rise to B, natural killer (NK), and myeloid cell lineages, indicating that cells with definitive hematopoietic potential could be obtained from hES cells. Another important advantage of OP9 coculture was the ability, within a short period of time and without added cytokines, to generate a large number of CD34+ cells that can be isolated using magnetic sorting. In addition, hES cells/OP9 coculture could represent a powerful in vitro model for analyzing the earliest stages of hematopoietic development that are not accessible in human embryos.
Materials and methods
Cell culture
The hES cell lines (H1 passages 23-45 and H9 passages 27-38) were maintained in an undifferentiated state by weekly passage on mouse embryonic fibroblast (MEF) layers as previously described.10 At least 5 different batches of H1 and 3 different batches of H9 karyotypically normal cell lines were used. The OP9 mouse bone marrow stromal cell line was obtained from Dr Toru Nakano (Research Institute for Microbial Diseases, Osaka University, Japan). This cell line was maintained on gelatinized 10-cm dishes (BD Bioscience, Bedford, MA) in OP9 growth medium consisting of α-modified minimum essential media (α-MEM) (Invitrogen, Carlsbad, CA) supplemented with 20% non–heat-inactivated defined fetal bovine serum (FBS; HyClone Laboratories, Logan, UT). Mouse bone marrow stromal cell lines S17 and MS-5 were obtained from Dr Kenneth Dorshkind (University of California, Los Angeles) and the German Tissue Culture Collection, respectively. These cells were maintained in α-MEM supplemented with 10% FBS (Invitrogen).
Hematopoietic differentiation of hES cells in coculture with OP9, S17, and MS-5 cells
For hES cell differentiation, OP9 cells were plated onto gelatinized 6-well plates or 10-cm dishes in OP9 growth medium. After formation of confluent cultures on days 4 and 5, half of the medium was changed, and cells were cultured for an additional 3 to 4 days. Undifferentiated hES cells were harvested by treatment with 1 mg/mL collagenase IV (Invitrogen) and dispersed by scraping to maintain the cells in small clumps. Concurrently, hES cultures growing under the same conditions were used to obtain single cell suspension for counting. The hES cells were added to OP9 cultures at a density of 1.5 × 106/20 mL per 10-cm dish or 0.3 × 106/4 mL per well of a 6-well plate in α-MEM supplemented with 10% FBS (HyClone) and 100 μM monothioglycerol (MTG; Sigma, St Louis, MO). The hES cell/OP9 cocultures were incubated for up to 10 days at 37°C in normoxic conditions and 5% CO2 with a half-medium change on days 4, 6, and 8. Cells were harvested every day, and single-cell suspension was prepared by treatment of the hES cell/OP9 cocultures with collagenase IV (Invitrogen; 1 mg/mL in α-MEM) for 20 minutes at 37°C, followed by treatment with 0.05% trypsin-0.5 mM EDTA (ethylenediaminetetraacetic acid) (Invitrogen) for 15 minutes at 37°C. Cells were washed twice with phosphate-buffered saline (PBS)–5% FBS, filtered through a 100-μM cell strainer (BD Biosciences, Palo Alto, CA), counted, and used for clonogenic and flow-cytometric assays and gene expression analysis. Culture and analysis of hES cells growing on S17 and MS-5 cell lines were performed in a similar manner.
Because comparable results were obtained for H1 and H9 hES cell/OP9 cocultures, we reported pooled data for both cell lines in these studies.
Positive selection of CD34+ cells by magnetic sorting
A single-cell suspension from days 8 and 9 of hES cell/OP9 cocultures was labeled with CD34 paramagnetic monoclonal antibodies (mAbs) using Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech, Auburn, CA) as recommended by the manufacturer and processed through an LS+ separation column attached to a Midi-MACS separation unit (Miltenyi Biotech) to obtain the magnet-retained fraction of purified CD34+ cells. Purity of isolated CD34+ cells, as determined by flow cytometry, was generally more than 95% at a single column run, and cell viability, as evaluated by trypan blue exclusion, was always higher than 95%.
Clonogenic progenitor cell assay
Hematopoietic clonogenic assays were performed in 35-mm low adherent plastic dishes (Stem Cell Technologies, Vancouver, BC, Canada) using a 1 mL/dish of MethoCult GF + H4435 semisolid medium (Stem Cell Technologies) consisting of 1% methylcellulose, 30% FBS, 1% bovine serum albumin (BSA), 50 ng/mL stem cell factor (SCF), 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 20 ng/mL interleukin-3 (IL-3), 20 ng/mL IL-6, 20 ng/mL granulocyte colony-stimulating factor (G-CSF), and 3 units/mL erythropoietin. Cells from hES cell/OP9 coculture were plated at various densities depending on the day of differentiation: 1 to 5 days, 2 × 105/mL; 6 days, 1 × 105/mL; 7 to 8 days, 5 × 104/mL; and 9 to 10 days, 2 × 104/mL. Sorted CD34+ cells were plated at 2 × 103/mL. Undifferentiated hES cells were tested at densities up to 5 × 105/mL, and no CFCs were found. All clonogenic progenitor assays were performed in duplicate. CFCs were scored after 14 to 21 days of incubation according to their colony morphology as erythroid (E-CFC), granulocyte, erythroid, macrophage, megakaryocyte (GEMM-CFC), granulocyte-macrophage (GM-CFC), and macrophage (M-CFC). Cytospin preparations from single colonies were made using a cytospin centrifuge (Shandon, Pittsburgh, PA). The cytospins were fixed with methanol and stained with Wright stain (Sigma) to confirm the cell content of appropriate colonies. The frequency of CFC was calculated per 106 total cells.
Simultaneous lymphomyeloid differentiation of CD34+ cells in vitro
CD34+ cells were seeded on 6-well plates (5 × 104 cells/well) with preestablished irradiated (50 Gy) monolayer of MS-5 stromal cells in the complete medium (4 mL/well) consisting of α-MEM supplemented with 10% FBS (HyClone), 100 μM MTG and the following human cytokines: SCF, 50 ng/mL; the class III receptor tyrosine kinase ligand (Flt3-L), 50 ng/mL; IL-3, 10 ng/mL; and IL-7, 20 ng/mL (Peprotech, Rocky Hill, NJ). Separate cultures were additionally supplemented with 20 ng/mL IL-15 to induce NK cell maturation. Half of the medium was changed every fifth day with complete medium without IL-3. After 21 days of incubation, single cell suspension was harvested by treatment of CD34+/MS-5 cocultures with collagenase IV/hyalouronidase IV solution (1 mg/mL and 0.05 mg/mL in α-MEM, respectively) for 20 minutes at 37°C, followed by treatment with nonenzymatic cell dissociation solution (Invitrogen) for 30 minutes at 37°C. Cells were used for flow cytometric and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis.
Short-term culture of isolated CD34+ cells
CD34+ cells were cultured for 5 days in serum-free expansion medium (SFEM) (Stem Cell Technologies) or α-MEM medium containing 10% FBS (HyClone) and 400 μM MTG at a density of 2.5 × 104/mL in 35-mm low adherent plastic dishes (Stem Cell Technologies) in the presence of SCF, 100 ng/mL; Flt3-L, 100 ng/mL; thrombopoietin (TPO), 50 ng/mL; vascular endothelial growth factor (VEGF), 5 ng/mL (Peprotech); and BMP4, 10 ng/mL (R&D Systems; Minneapolis, MN). Cells were analyzed for surface phenotype and clonogenic potential.
Phenotype analysis by flow cytometry
Cells were prepared in PBS containing 0.05% sodium azide, 1 mM EDTA, 2% FBS, and 2% normal mouse serum (Sigma) and were labeled with a combination of monoclonal antibodies (mAbs). For analysis of perforin expression, cells were permeabilized using Fix&Perm reagents (Caltag, Burlingame, CA). Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) with CellQuest acquisition software (BDIS). List mode files were analyzed by FlowJo software (Tree Star, Ashland, OR). The mAbs were preliminarily tested for cross-reactivity with OP9 and mouse bone marrow mononuclear cells. Only the following mAbs without detectable cross-reactivity with murine cells were selected: KDR-PE (R&D Systems); CD19-APC, CD34-PerCP-Cy5.5, CD38-PE, CD117-PerCP-Cy5.5, HLA-DR-PE (BDIS); CD14-FITC, CD31-FITC, CD41a-PE, CD43-FITC, CD45-PE/APC, CD90-APC, CD164-FITC, CD184-APC, perforin-FITC (BD Pharmingen); CD56-PE, CD133-PE (Miltenyi Biotech); and CD10-APC, CD66b-FITC (Caltag). Control staining with appropriate isotype-matched control mAbs (BD Pharmingen) was included to establish thresholds for positive staining and background for linear-scaled mean fluorescence intensity (MFI). The percentage of positive cells was calculated as percent of positive cells stained with specific mAb – percent of background staining with corresponding isotype control. The ΔMFI was calculated as MFI of cells stained with specific mAb – MFI of cells stained with corresponding isotype control. Linear-scaled MFI was used as an indicator of relative antigen density on given cells. Results were presented as a percent of positive cells and/or ΔMFI ± standard deviation (SD).
Rhodamine 123 exclusion assay
106 isolated CD34+ cells were incubated with 0.1 μg/mL rhodamine (Rho) (Molecular Probes, Eugene, OR) in 1 mL RPMI-1640 medium containing 15 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 2% FBS (assay medium) for 30 minutes at 37°C. Cells were washed with and resuspended in assay medium and incubated for 40 minutes at 37°C without or with 50 μM verapamil (Sigma) to reveal Rho exclusion activity. After washing with PBS-FBS, cells were labeled with CD45-APC mAb, resuspended in PBS containing 2 μg/mL propidium iodide (PI) (Sigma), and analyzed by flow cytometry. A minimum of 2 × 105 events from live-gated, PI-negative cells were acquired. Rholow cells were defined as those showing less fluorescence in the FL-1 channel than exhibited by verapamil-treated samples.
Aldehyde dehydrogenase staining
Aldehyde dehydrogenase (ALDH) staining of CD34+ cells was performed using ALDEFLUOR kit (Stem Cell Technologies) according to instructions provided by the manufacturer. Control samples were established using diethylaminobenzaldehyde (DEAB), an ALDH inhibitor. Cells also were stained for CD45, and dead cells were excluded using PI staining. Samples were analyzed by flow cytometry.
Immunocytochemistry
Cytospins of hES cells or isolated CD34+ cells were stained using anti–Oct-4 mAb (Santa Cruz Biotechnology, Santa Cruz, CA) and ABC peroxidase kit (Vector Laboratories, Burlingame, CA).
Gene expression analysis by conventional and real-time quantitative PCR
Total RNA was isolated from cells using RNAwiz (Ambion, Austin, TX). Human bone marrow, thymus, and fetal liver RNA were purchased from Clontech (Palo Alto, CA). All RNA samples were treated with DNAfree reagent (Ambion) to remove potentially contaminating DNA. The cDNA was prepared from 1 μg total RNA using oligo(dT) primer (Ambion) and Omniscript RT kit (QIAGEN, Valencia, CA). Quantitative PCR (QPCR) was performed using Brilliant SybrGreen QPCR kit (Stratagene, La Jolla, CA). The specified genes were amplified for 40 cycles, and PCR reactions were analyzed with ABI Prism 7700 (Applied Biosystems, Foster City, CA). Regular PCR was performed using Taq PCR kit (QIAGEN) for each transcript to check the size of the amplified product and its sequencing to ensure authenticity. QPCR reactions were done using 2 μL RT products per reaction according to the instructions from the manufacturer with the annealing temperature optimized for each primer. Transcripts of target genes were amplified along with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using human-specific primers. GAPDH was selected as the housekeeping gene since its expression remains constant during ES cell differentiation in culture.11 Each reaction was performed in duplicate. All QPCR products were analyzed on 2.0% agarose gels to exclude false-positive readings due to primer dimers. Comparative quantification of the target gene expression in the samples was performed based on cycle threshold (Ct) normalized to GAPDH using the ΔΔCt method.12 The relative expression of target genes in hES cell/OP9 cocultures as well as hES cell–derived CD34+ and CD34– cell populations was compared with the level of the same gene expression in bone marrow samples using the following equation:
The primers used (Table 1), except β-actin primer, were human gene–specific and did not amplify cDNA from mouse cells (OP9, MS-5, and MEFs).
Results
Distinctive efficiency of OP9 stromal cells in the induction of hES cell hematopoietic differentiation
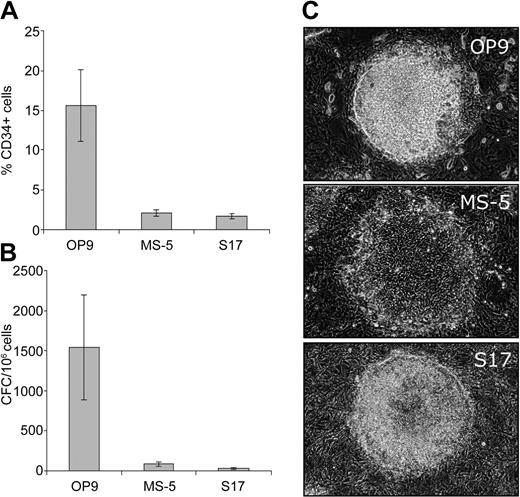
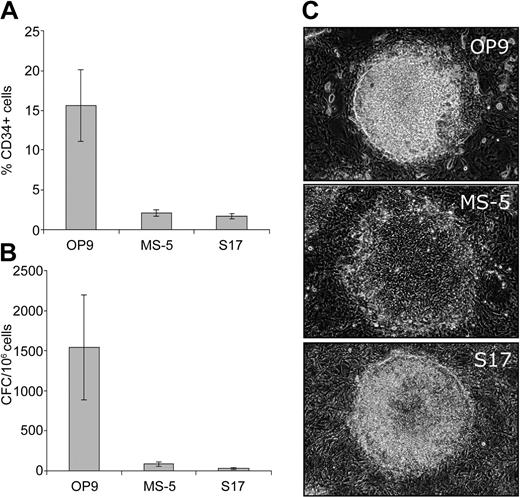
Several bone marrow stromal cell lines have been shown to support hematopoietic differentiation of ES cells in mice and humans.4,7,13 To find the optimal bone marrow stromal cell line for induction of hematopoietic differentiation, we cocultured hES cells on OP9, S17, and MS-5 cell lines. We found that the OP9 cell line was superior to both the MS-5 and S17 cell lines. After 7 days of culture, a substantial number of CD34+ cells and CFCs were generated in the OP9 coculture, while significantly fewer CD34+ cells and CFCs were observed in the MS-5 and S17 cocultures (Figure 1A-B). On day 4 of the OP9 coculture, colonies morphologically similar to mesodermal colonies described in the mouse ES cell/OP9 coculture appeared.14 These colonies displayed dense, elevated central portions composed of stacked, large, round cells (Figure 1C). The hES cell colonies in MS-5 and S17 cultures were different and appeared as flat, doughnut-shaped groups of cells with empty centers that tended to spread peripherally (Figure 1C).
Distinct hemogenic properties of OP9 cell line. Induction of CD34+ expression (A) and CFCs (B) after 7 days of coculture of H1 hES cells on OP9, MS-5, and S17 bone marrow stromal cell lines. Results are mean ± SD of 3 experiments ± SD. (C) Morphology of differentiated hES cell colonies after 4 days of coculture with OP9, MS-5, and S17 cells. Images were captured with an inverted Leica DM1RB microscope (Leica Microsystems, Bannockburn, IL) using 5 × objective with numerical aperture 0.12 and acquired through Spot RT camera and Spot software (Diagnostic Instruments, Sterling Heights, MI).
Distinct hemogenic properties of OP9 cell line. Induction of CD34+ expression (A) and CFCs (B) after 7 days of coculture of H1 hES cells on OP9, MS-5, and S17 bone marrow stromal cell lines. Results are mean ± SD of 3 experiments ± SD. (C) Morphology of differentiated hES cell colonies after 4 days of coculture with OP9, MS-5, and S17 cells. Images were captured with an inverted Leica DM1RB microscope (Leica Microsystems, Bannockburn, IL) using 5 × objective with numerical aperture 0.12 and acquired through Spot RT camera and Spot software (Diagnostic Instruments, Sterling Heights, MI).
Emergence of CD34+ cells in hES cell/OP9 coculture is associated with the acquisition of clonogenic hematopoietic potential and the up-regulation of hematopoiesis-associated genes
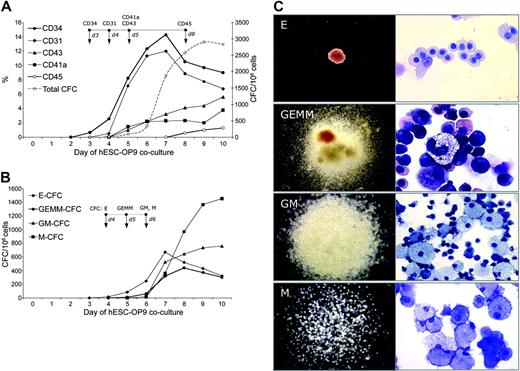
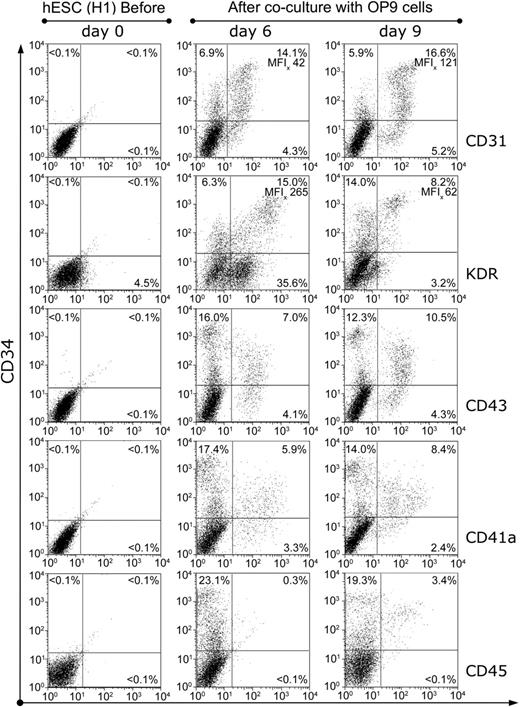
To examine the timeframe of hematopoiesis onset and to define the phenotype of early hematopoietic progenitors in the hES cell/OP9 coculture, we evaluated CFCs and the expression of hematopoiesis-associated molecules on undifferentiated hES cells and on hES cells after 1 to 10 days of differentiation. hES cells maintained strictly in an undifferentiated state did not express CD34, CD31, CD43, CD41a, or CD45 (Figure 2A and 3). In the OP9 coculture, CD34+ cells first appeared on day 3 of culture and gradually peaked at day 7 (Figure 2A). CD43+ and CD41a+ cells appeared 2 days later within the CD34+ population and gradually increased by day 10 of culture (Figure 2A). The temporal kinetics of the CD31+ cells closely followed that of CD34+ cells; however, CD31+ cells were first seen one day after the appearance of CD34+ cells. CD45+ cells appeared much later, on day 8 of culture (Figure 2A). Figure 3 shows representative, multicolor flow cytometric analysis of the expression of hematopoiesis-associated molecules by undifferentiated and differentiated hES cells on days 6 and 9 of OP9 coculture.
Induction of hematopoietic molecules and CFCs during hES cells/OP9 coculture. (A) Percentages of CD34+, CD31+, CD43+, CD41a+, and CD45+ cells (left Y-scale) as determined by flow cytometry, and total numbers of CFCs (right Y-scale) in hES cell/OP9 coculture at days 1 through 10. Day 0 indicates undifferentiated hES cells. (B) Kinetic of different CFC types in hES cell/OP9 coculture. Panels A and B represent mean results from 6 experiments (H1 = 3; H9 = 3); arrows on the top of each figure point to the earliest day when expression of indicated markers or CFCs was detectable. (C) Morphology (left column) and Wright staining of cytospins (right column) of different CFC types. Images captured with an inverted Leica DM1RB microscope (Leica Microsystems) using 5 × objective with numerical aperture 0.12 (left column) and Olympus BX51 microscope (Olympus America Inc., Melville, NY) using 40 × with numerical aperture 0.75 (GM-CFC, M-CFC) and 100 × with numerical aperture 1.30 (E-CFC, GEMM-CFC) objectives (right column) and acquired through Spot RT camera and Spot software (Diagnostic Instruments).
Induction of hematopoietic molecules and CFCs during hES cells/OP9 coculture. (A) Percentages of CD34+, CD31+, CD43+, CD41a+, and CD45+ cells (left Y-scale) as determined by flow cytometry, and total numbers of CFCs (right Y-scale) in hES cell/OP9 coculture at days 1 through 10. Day 0 indicates undifferentiated hES cells. (B) Kinetic of different CFC types in hES cell/OP9 coculture. Panels A and B represent mean results from 6 experiments (H1 = 3; H9 = 3); arrows on the top of each figure point to the earliest day when expression of indicated markers or CFCs was detectable. (C) Morphology (left column) and Wright staining of cytospins (right column) of different CFC types. Images captured with an inverted Leica DM1RB microscope (Leica Microsystems) using 5 × objective with numerical aperture 0.12 (left column) and Olympus BX51 microscope (Olympus America Inc., Melville, NY) using 40 × with numerical aperture 0.75 (GM-CFC, M-CFC) and 100 × with numerical aperture 1.30 (E-CFC, GEMM-CFC) objectives (right column) and acquired through Spot RT camera and Spot software (Diagnostic Instruments).
Sequential phenotypic analysis of differentiated hES cells in OP9 coculture. Representative phenotype of undifferentiated (day 0) and differentiated H1 hES cells on day 6 and day 9 of OP9 coculture. Single cell suspension from hES cell/OP9 coculture obtained at the indicated time was labeled with CD34 (y-axis) and with CD31, KDR, CD43, CD41a, or CD45 mAbs as indicated in right side of each row (x-axis). Numbers indicate percentages of positive cells and MFI (x-scale) in corresponding quadrants.
Sequential phenotypic analysis of differentiated hES cells in OP9 coculture. Representative phenotype of undifferentiated (day 0) and differentiated H1 hES cells on day 6 and day 9 of OP9 coculture. Single cell suspension from hES cell/OP9 coculture obtained at the indicated time was labeled with CD34 (y-axis) and with CD31, KDR, CD43, CD41a, or CD45 mAbs as indicated in right side of each row (x-axis). Numbers indicate percentages of positive cells and MFI (x-scale) in corresponding quadrants.
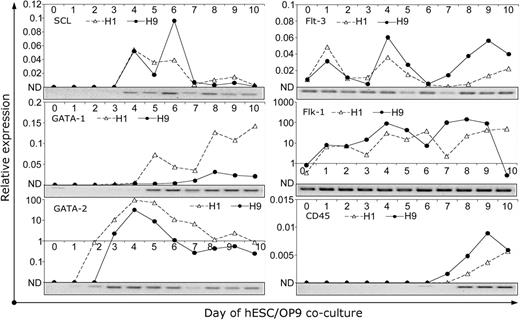
Kinetic analysis of CFC emergence demonstrated that E-CFCs were induced on day 4 of culture, the day following the appearance of CD34+ cells (Figure 2B). Myeloid (GM, M) and mixed (GEMM) CFCs appeared later, along with the induction of CD43 and CD41a expression on CD34+ cells. After day 7 of culture, we observed predominant expansion of myeloid CFCs and gradual decrease of E-CFCs and GEMM-CFCs (Figure 2B). As shown in Figure 4, transcription factors associated with hematopoiesis such as SCL, GATA-1, and GATA-2 were not expressed in undifferentiated hES cells. GATA-1 and GATA-2 were detected on days 2 and 3 of hES cell differentiation, coincident with the appearance of CD34+ cells, while SCL expression was detected a day later. GATA-2 and SCL expression peaked on days 4 to 6 of differentiation and then gradually decreased, while GATA-1 expression gradually increased up to day 10 of culture (Figure 4). PCR analysis demonstrated that undifferentiated hES cells already expressed several genes essential for hematopoietic development, such as Flk-1 and Flt-3. However, at least 2 major waves of Flk-1 and Flt-3 up-regulation that were coincident with the appearance of CD34+ (days 3 and 4) and CD45+ cells (days 7 to 9) were observed in hES cells/OP9 cocultures (Figure 4).
Kinetic analysis of hematopoiesis-associated gene expression by QRT-PCR. Human-specific primers listed in Table 1 were used to amplify indicated genes during H1 and H9 differentiation in OP9 coculture. Pictures under each graph show corresponding agarose gel electrophoresis of QPCR from H1 cell samples. Gene expression relative to bone marrow RNA was calculated using ΔΔCt method as described in “Materials and methods.”
Kinetic analysis of hematopoiesis-associated gene expression by QRT-PCR. Human-specific primers listed in Table 1 were used to amplify indicated genes during H1 and H9 differentiation in OP9 coculture. Pictures under each graph show corresponding agarose gel electrophoresis of QPCR from H1 cell samples. Gene expression relative to bone marrow RNA was calculated using ΔΔCt method as described in “Materials and methods.”
These data clearly demonstrated that the OP9 bone marrow stromal cell line efficiently induced hematopoietic differentiation of hES cells and reproduced early stages of hematopoietic development.
Isolated hES cell–derived CD34+ cells are highly enriched in cells with phenotypic and functional hematopoietic properties
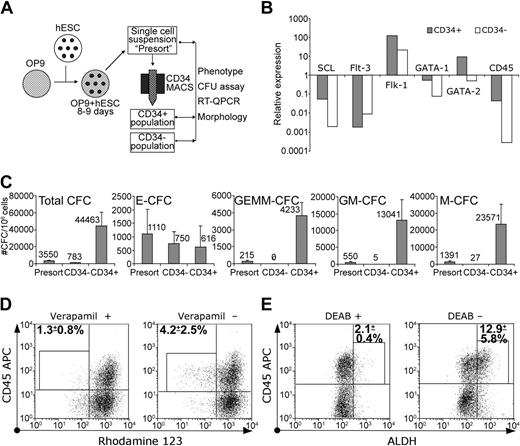
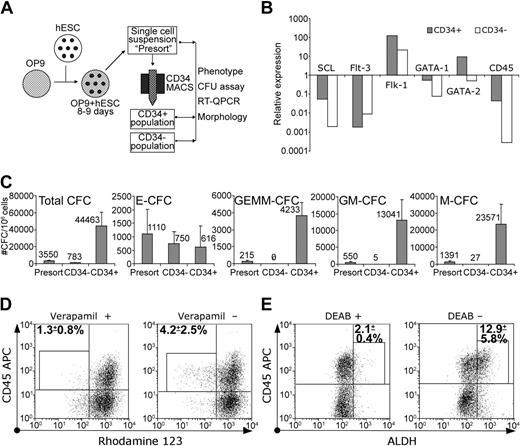
Since CD34 is considered the most reliable marker for embryonic and adult hematopoietic stem cells,15-17 we examined whether hematopoietic progenitors would arise in CD34+ populations in the OP9 coculture. Using the magnetic separation technique, we were able to obtain up to 107 CD34+ cells with more than 95% purity from a similar number of the initially plated hES cells after 8 to 9 days of culture (Table 2; Figure 5). CD34+ selection markedly enriched all CFCs, except E-CFCs. The most significant enrichment was seen for the multilineage progenitors, GEMM-CFCs. All GEMM-CFCs were found within the CD34+ population, and none were found within the CD34– population. The CD34– population contained minimal numbers of M-CFC and GM-CFCs (Figure 5C). The levels of SCL, Flk-1, GATA-1, and GATA-2 expression by QPCR were substantially higher within the CD34+ population as compared to the CD34– population (Figure 5B). These results demonstrated that hES cell–derived hematopoietic progenitors were restricted mostly to the CD34+ population.
Isolated CD34+ cells revealed hematopoietic progenitor potential. (A) Schematic diagram of the protocol used for CD34+ cells' isolation and analysis. (B) Relative expression of hematopoiesis-associated genes in CD34+ and CD34– populations by QPCR. (C) Clonogenic potential of H1 CD34+ and CD34– cells. Results are mean ± SD of 4 experiments. (D) Rho efflux and CD45 expression by isolated CD34+ cells were analyzed by flow cytometry. The gates used to distinguish Rholow population of CD34+CD45+ cells and percentages of Rholow cells within this gate (mean ± SD of 3 experiments; H1 = 2, H9 = 1) are indicated. (E) ALDH and CD45 expression by isolated CD34+ cells were analyzed by flow cytometry. The gates used to distinguish the ALDHhigh population of CD34+CD45+ cells and percentages of ALDHhigh cells within the gates (mean ± SD of 3 experiments; H1 = 2, H9 = 1) are indicated.
Isolated CD34+ cells revealed hematopoietic progenitor potential. (A) Schematic diagram of the protocol used for CD34+ cells' isolation and analysis. (B) Relative expression of hematopoiesis-associated genes in CD34+ and CD34– populations by QPCR. (C) Clonogenic potential of H1 CD34+ and CD34– cells. Results are mean ± SD of 4 experiments. (D) Rho efflux and CD45 expression by isolated CD34+ cells were analyzed by flow cytometry. The gates used to distinguish Rholow population of CD34+CD45+ cells and percentages of Rholow cells within this gate (mean ± SD of 3 experiments; H1 = 2, H9 = 1) are indicated. (E) ALDH and CD45 expression by isolated CD34+ cells were analyzed by flow cytometry. The gates used to distinguish the ALDHhigh population of CD34+CD45+ cells and percentages of ALDHhigh cells within the gates (mean ± SD of 3 experiments; H1 = 2, H9 = 1) are indicated.
Bone marrow multipotential hematopoietic cells possess the ability to efflux dyes such as Hoechst 33342 and Rhodamine 123 (Rho)18-20 and express a high level of ALDH.21,22 We found that cells with the verapamil-sensitive ability to efflux Rho constituted a minor but consistent fraction of CD34+CD45+ cells (Figure 5D). Rho-effluxing cells were not found within either the CD34+CD45– subset (Figure 5D) or undifferentiated hES cells (not shown). In addition, cells rich in ALDH activity were identified within isolated CD34+ cells, predominantly within the CD34+CD45+ subset (Figure 5E).
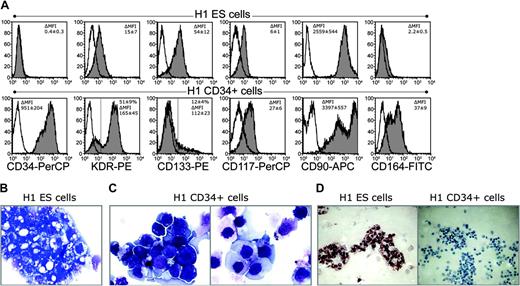
Several molecules, such as CD90, CD117, and CD133, which are known to be expressed on primitive hematopoietic progenitors,17,18,23,24 were found to be expressed on undifferentiated hES cells (Figure 6A). The hES cell–derived CD34+ cells up-regulated expression of CD90, CD117, CD164, but down-regulated the expression of CD133 (Figure 6A). Most of the CD34+ cells were CD31+ and CD38– (Table 2; Figures 3 and 7C). A significant proportion of CD34+ cells coexpressed vascular endothelial growth factor receptor 2 (VEGFR2 or KDR; Figure 6A). CD45+, CD41a+, CD43+, and CXCR4+ subsets were identified within CD34+ cells (Table 2; Figure 3). Like the CD34+ primitive hematopoietic progenitors that arise in the human embryo,16 hES cell–derived CD34+ cells did not express lineage-specific markers CD3, CD19, and CD14 (data not shown).
Phenotype and morphology of undifferentiated hES cells and hES cell–derived CD34+ cells. (A) Comparative expression of hematopoiesis-associated surface molecules on undifferentiated hES cells (top row) and isolated CD34+ cells (bottom row). Depicted histograms represent H1 cells, MFI values in the right histogram corner are mean ± SD of 9 experiments (H1 = 6, H9 = 3). (B) Morphology of Wright-stained cytospins of undifferentiated H1 hES cells. (C) Wright-stained cytospins of isolated H1 CD34+ cells demonstrate 2 different populations. (D) Oct-4 immunostaining of undifferentiated H1 ES cells and H1-derived CD34+ cells. CD34+ cells are Oct-4 negative. Images were captured with an Olympus BX51 microscope (Olympus America Inc.) using 20 × with numerical aperture 0.5 (D) and 100 × with numerical aperture 1.30 (B,C) objectives; and acquired through Spot RT camera and Spot software (Diagnostic Instruments).
Phenotype and morphology of undifferentiated hES cells and hES cell–derived CD34+ cells. (A) Comparative expression of hematopoiesis-associated surface molecules on undifferentiated hES cells (top row) and isolated CD34+ cells (bottom row). Depicted histograms represent H1 cells, MFI values in the right histogram corner are mean ± SD of 9 experiments (H1 = 6, H9 = 3). (B) Morphology of Wright-stained cytospins of undifferentiated H1 hES cells. (C) Wright-stained cytospins of isolated H1 CD34+ cells demonstrate 2 different populations. (D) Oct-4 immunostaining of undifferentiated H1 ES cells and H1-derived CD34+ cells. CD34+ cells are Oct-4 negative. Images were captured with an Olympus BX51 microscope (Olympus America Inc.) using 20 × with numerical aperture 0.5 (D) and 100 × with numerical aperture 1.30 (B,C) objectives; and acquired through Spot RT camera and Spot software (Diagnostic Instruments).
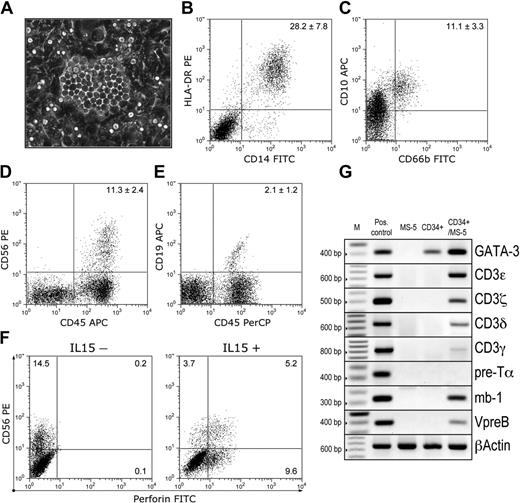
Development of myeloid, NK, and B cells from hES cell–derived CD34+ cells. CD34+ cells were cultured on MS-5 stromal cells as described in “Materials and methods.” (A) CD34+ cells gave rise to phase-dark cobblestone-shaped colonies underneath the stroma starting from day 7 of coculture. Image captured with an inverted Leica DM1RB microscope (Leica Microsystems), using 20 × objective with numerical aperture 0.3 and acquired through Spot RT camera and Spot software (Diagnostic Instruments). (B-F) Phenotype of the cells generated after 21 days of CD34+/MS-5 coculture. Flow cytometric analysis demonstrated the presence of CD14+HLA-DR+ macrophages (B) and CD66b+CD10+ mature granulocytes (C) within the CD45+ gated population. In addition, CD56+CD45+ NK cells (D) and CD19+CD45+ B-cell precursors (E) were evident. Numbers in the upper right corner indicate percentages of positive cells in the corresponding quadrant (mean ± SD of 4 experiments; H1 = 2, H9 = 2). (F) IL-15–induced expression of perforin in CD56+ cells. CD34+ cells were cultured on MS-5 cells without (left panel) or with IL-15 (right panel). Cells were stained with CD56-PE and CD45-APC mAbs followed by permeabilization and staining with perforin-FITC mAbs. Dot plots represent CD45+ gated cells. (G) Analysis of gene expression in isolated CD34+ cells and cells after 21 days of CD34+/MS-5 coculture by RT-PCR. Positive controls are as follows: human bone marrow (GATA-3); peripheral blood lymphocytes (CD3ϵ, CD3δ, CD3γ, CD3ξ); thymus (pre-Tα); fetal liver (mb-1, VpreB). Transcripts of the studied genes were not amplified from MS-5 cells alone. M indicates DNA markers (100-bp ladder).
Development of myeloid, NK, and B cells from hES cell–derived CD34+ cells. CD34+ cells were cultured on MS-5 stromal cells as described in “Materials and methods.” (A) CD34+ cells gave rise to phase-dark cobblestone-shaped colonies underneath the stroma starting from day 7 of coculture. Image captured with an inverted Leica DM1RB microscope (Leica Microsystems), using 20 × objective with numerical aperture 0.3 and acquired through Spot RT camera and Spot software (Diagnostic Instruments). (B-F) Phenotype of the cells generated after 21 days of CD34+/MS-5 coculture. Flow cytometric analysis demonstrated the presence of CD14+HLA-DR+ macrophages (B) and CD66b+CD10+ mature granulocytes (C) within the CD45+ gated population. In addition, CD56+CD45+ NK cells (D) and CD19+CD45+ B-cell precursors (E) were evident. Numbers in the upper right corner indicate percentages of positive cells in the corresponding quadrant (mean ± SD of 4 experiments; H1 = 2, H9 = 2). (F) IL-15–induced expression of perforin in CD56+ cells. CD34+ cells were cultured on MS-5 cells without (left panel) or with IL-15 (right panel). Cells were stained with CD56-PE and CD45-APC mAbs followed by permeabilization and staining with perforin-FITC mAbs. Dot plots represent CD45+ gated cells. (G) Analysis of gene expression in isolated CD34+ cells and cells after 21 days of CD34+/MS-5 coculture by RT-PCR. Positive controls are as follows: human bone marrow (GATA-3); peripheral blood lymphocytes (CD3ϵ, CD3δ, CD3γ, CD3ξ); thymus (pre-Tα); fetal liver (mb-1, VpreB). Transcripts of the studied genes were not amplified from MS-5 cells alone. M indicates DNA markers (100-bp ladder).
Undifferentiated hES cells and MACS-selected hES cell–derived CD34+ cells were stained with Wright solution and examined microscopically (Figure 6B-C). Morphologically, at least 2 major subpopulations of cells can be identified within hES cell–derived CD34+ cells. Most of the CD34+ cells had a high nuclear cytoplasmic ratio, dark blue cytoplasm, and a nucleus with 2 or 3 large nucleoli, and they resembled bone marrow hematopoietic blast cells (Figure 6C, left panel). The second, smaller population consisted of cells with an abundant light-blue cytoplasm and a relatively small nucleus with inconspicuous nucleoli (Figure 6C, right panel). This population may have represented endothelial precursors. Both subpopulations of isolated CD34+ cells were considerably different from undifferentiated hES cells, which on cytospins tended to form clumps composed of large cells with vacuolated cytoplasms and an irregular hyperchromatic nuclei (Figure 6B). In contrast to undifferentiated hES cells, CD34+ cells expressed neither Oct-4 transcription factor specific for totipotent ES cells25 (Figure 6D) nor TRA-1-60 and TRA-1-81 hES cell markers2 (data not shown). These findings indicate advanced differentiation of CD34+ cells and rule out contamination of isolated CD34+ cells by undifferentiated hES cells.
hES cell–derived CD34+ cells produce both lymphoid and myeloid cells upon coculture with MS-5 stromal cells
To demonstrate the lymphomyeloid potential of hES cell–derived hematopoietic progenitors, we cultured isolated CD34+ cells on the MS-5 stromal cell line, which supports myeloid and B-lymphoid human hematopoiesis.26-28 During MS-5 coculture, CD34+ cells developed adherent hematopoietic colonies as well as cobblestone-like colonies under the stroma first detected on day 7 of coculture (Figure 7A). After 21 days, a large population of CD45+ cells (58.5% ± 3.4% of total cells in coculture) was detected by flow cytometry, and granulocytes, macrophages, and lymphoid cells were identified morphologically on cytospins (data not shown). The CD45+ population included CD14+HLA-DR+ macrophages (Figure 7B), CD10+CD66b+ mature granulocytes (Figure 7C), CD56+ NK cells (Figure 7D), and CD19+ B cells (Figure 7E). To further prove that the CD56+ population represents NK cells, we added to some cultures IL-15, which induces maturation of NK precursors into perforin-positive cytolytic NK cells.29-31 As shown in Figure 7F, perforin-expressing CD56+ NK cells were generated in cultures with IL-15. CD56–perforin+ cells also were detected when cells were cultured with IL-15. These cells may represent immature macrophage precursors that have been shown to acquire perforin expression upon IL-2 stimulation.32 In addition, in CD34+/MS-5 cocultures, we detected CD3ϵ, CD3δ, and CD3ξ transcripts, which are known to be expressed in embryonic and fetal NK cells,33 as well as mRNAs for VpreB and Igα (CD79a or mb-1) components of pre–B-cell receptor complex (Figure 7G). We found very low but consistent expression of CD3γ in CD34+/MS-5 coculture; however, pre-Tα, a component of the pre–T-cell receptor complex, was not detected by PCR. Thus, the lymphopoiesis in CD34+/MS-5 coculture is mostly restricted to NK and B-cell lineages, and a different culture system is required to direct the differentiation of CD34+ cells into T lymphocytes. Altogether, these results provide strong evidence that CD34+ cells generated in hES/OP9 coculture possess the capacity to generate both lymphoid and myeloid cells.
hES cell–derived CD34+ cells retain clonogenic potential after in vitro expansion
To evaluate the possibility of in vitro expansion, we cultured CD34+ cells in a medium without serum or with 10% FBS in the presence of SCF, Flt3L, TPO, BMP4, and VEGF. We were able to achieve approximately 5-fold expansion of total cells and 2- to 4-fold expansion of CFCs after 5 days of culture (Table 3). However, the phenotype and CFC potential of cells expanded in serum-free and serum-containing media were different. Most of the cells expanded in serum-free medium maintained more primitive phenotype as defined by co-expression of CD34 and CD45 and the lack of CD38 expression. These cells retained multilineage GEMM-CFC potential. In contrast, cells expanded in serum-containing media were mostly CD45+CD34–, acquired a high level of CD38 expression, and were enriched in M-CFCs with a loss of GEMM-CFCs (Table 3). These data demonstrated that hES cell–derived CD34+ cells could potentially be expanded in vitro in serum-free media with the retention of hematopoietic potential.
Discussion
Our study represents the first report that describes hematopoietic differentiation of hES cells using the OP9 bone stromal cell line. The OP9 coculture has been used successfully for hematopoietic differentiation of mouse and nonhuman primate ES cells and to obtain multilineage hematopoietic progenitors as well as mature hematopoietic cells such as lymphocytes and megakaryocytes, which cannot be obtained using the embryoid body method.7-9,34 Differentiation of hES cells through OP9 coculture was similar to that observed previously in murine ES cell/OP9 coculture:8,14 Mesodermal colonies appeared at day 4 of culture, and the first CFCs were detected on days 4 and 5 of culture with decrease of E-CFCs on days 8 and 9 of culture. Formation of mesodermal-type colonies in the OP9 coculture can be observed under the microscope. Single-cell suspension of hES cells growing on OP9 cells can be obtained easily by mild enzymatic digestion and analyzed by flow cytometry and CFC assay. In the OP9 coculture, the peak of hematopoietic differentiation occurred at least 1 week earlier (days 7 to 9 of culture) when compared to the embryoid body method and S17 coculture (days 15 to 22 of culture).4,5 In contrast to the S17 or MS-5 coculture, hES cells differentiated on OP9 cells gave rise to CD34+CD45+ cells and produced a much higher number of CFCs.4,35 However, OP9 cells are very sensitive to variations in maintenance conditions, including medium source and serum lot, which can affect the ability of OP9 cells to support hematopoiesis.
The strong hematopoiesis-promoting activity of OP9 is at least partially attributed to the lack of M-CSF production,14 since M-CSF inhibits early hematopoiesis.36 However, murine M-CSF is not active on human cells37 and therefore should not be a factor, which explains differences in the efficiency of hES cell hematopoietic differentiation through coculture with various mouse bone marrow stromal cell lines. We think that the recently identified mKirre protein,38 differences in expression of Notch ligands, or other unidentified factors, rather than lack of M-CSF production, are accountable for the distinct hemogenic properties of OP9 cells.
Our results indicate that the first hematopoietic progenitors with clonogenic potential can be detected in the OP9 coculture before the emergence of CD45+ cells. Clonogenic progenitors arising in yolk sac and aorta-gonad-mesonephros at 32 days of gestation in the human embryo were abundant within CD34+CD45+ populations, while CD34+CD45– cells were devoid of CFCs.16 Likewise, CFCs were found only in CD45+ cells in the embryoid body system.5 In the murine system, ES cell/OP9 coculture reproduces a pattern of the hematopoiesis observed in developing embryos.14,39,40 Therefore, the OP9 coculture appears to be a suitable model for evaluation of the earliest stages of hematopoietic differentiation within CD45– populations, which could not be easily accessible in the human embryo or the embryoid body method. Recently, CD41 was identified as the earliest marker of hematopoiesis in murine embryos.41-43 We have shown that the appearance of CD41a+ and CD43+ cells in the OP9 coculture coincided with the appearance of CFCs (Figure 2). Whether CD41 can be used to define early hematopoietic progenitors in humans is currently under investigation in our laboratory.
Phenotypically, CD34+ cells obtained through the OP9 coculture were similar to primitive bone marrow hematopoietic progenitors as well as to intraembryonic hematopoietic precursors as defined by co-expression of CD90, CD117, and CD164, and by the lack of CD38 and lineage-specific marker expression.17,18,23,24,44 However, only 12% of hES cell–derived CD34+ cells expressed CD133, while CD133 was detected on 20% to 60% of bone marrow, peripheral blood, and fetal liver CD34+ cells.24,45 The presence of CXCR4 on hES cell–derived CD34+ cells may indicate their homing potential to bone marrow.46 CD34+ cells derived through the OP9 coculture were highly enriched in clonogenic progenitors. The CFC frequency in hES cell–derived CD34+ cells (approximately 4%) was comparable with the CFC frequency in human bone marrow CD34+ cells,17,45,47 and the lack of E-CFC enrichment by CD34+ selection is consistent with the progressive loss of CD34 expression by erythroid progenitors with advanced maturation.47 As expected, CD34+ populations expressed much higher levels of SCL, GATA-1, GATA-2, Flk-1, and CD45, as compared to CD34– cells. A lower level of Flt-3 expression in CD34+ cells was consistent with their the most primitive hematopoietic progenitor features.17
Hematopoietic stem cells can be identified based on the efflux of fluorochrome dyes such as Rho and Hoechst 3334218-20 and by a high level of ALDH expression.21,22 Dye-excluding and ALDHbright bone marrow cells are highly enriched for repopulating cells and are present in multiple species.19,48-50 Similar to bone marrow CD34+ cells, the Rho efflux in hES cell–derived CD34+ cells was inhibited by verapamil, pointing to P-glycoprotein–mediated transport. The finding of Rho-extruding cells and ALDHbright cells within hES cell–derived CD34+ cells suggested that these cells may contain a subpopulation with hematopoietic stem cell activity.
Here we demonstrated lymphomyeloid differentiation of hES cells which, so far, has not been reported. The ability to generate, simultaneously, lymphoid and myeloid progenitors is considered a distinctive feature of stem cells having definitive hematopoietic potential. In humans, lymphomyeloid progenitors have been found in para-aortic splanchnopleura, while yolk sac hematopoietic cells have been restricted to myelopoiesis.26-28 These data indicate that hES cell/OP9 coculture recapitulates major events observed during embryonal hematopoietic development, including formation of lymphomyeloid progenitors that can be found within para-aortic splanchnopleura.
The hallmark of hematopoietic stem cells is their capacity to establish long-term multilineage engraftment. Recently, hematopoietic cells able to be engrafted in conditioned adult mice have been derived from mouse ES cells transduced with Bcr/Abl or HoxB4.51,52 So far, no engraftment of hES cell–derived hematopoietic cells has been reported. The lack of a system for producing large numbers of hES cell–derived hematopoietic precursors has, at least in part, delayed engraftment studies of hES cells. The OP9 coculture allowed us to obtain CD34+ cells highly enriched in hematopoietic progenitors in sufficient quantities for genetic manipulation as well as for transplantation in immunodeficient mice.
In summary, our data clearly indicate that CD34+ populations obtained by differentiation of hES cells in coculture with OP9 cells are enriched in cells with features of hematopoietic progenitors and stem cells. Preliminary in vivo engraftment experiments are under way in our laboratory to prove that cells with hematopoietic stem cell potential can be generated from hES cells.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-04-1649.
Supported by Defense Advanced Research Projects Agency grant DRP5-UWM and National Institutes of Health grant number P51 RR000167 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rachel Lewis for expert technical advice; Dr Dong Chen and Dr Aimen Shaaban for review of the manuscript; Dr T. Nakano and Dr K. Dorshkind for providing bone marrow stromal cell lines; and Lynn Schmidt for editorial assistance.