Abstract
Interleukin-15 (IL-15) is a γ-common cytokine that plays an important role in the development, survival, and proliferation of natural killer (NK), NK T, and CD8+ T-cells. We administered IL-15 to recipients of an allogeneic bone marrow transplantation (allo BMT) to determine its effects on immune reconstitution. Posttransplantation IL-15 administration significantly increased donor-derived CD8+ T (mostly CD122+CD44+CD8+ T-cells), NK, and NK T-cells at day +28 in young and old recipients of allo BMT. This was associated with enhanced T-cell and NK-cell function. IL-15 stimulated homeostatic proliferation of donor CD8+ T-cells in recipients of carboxyfluorescein diacetate succinimidyl ester–labeled donor T-cell infusions. Posttransplantation IL-15 administration also resulted in a decrease in apoptotic CD8+ T-cells, an increase in Bcl-2–expressing CD8+ T-cells, and an increase in the fraction of Ki67+ proliferative NK and CD8+ T-cells in recipients of allo BMT. IL-15 did not exacerbate graft-versus-host disease (GVHD) in recipients of T-cell–depleted BMT but could aggravate GVHD in some cases in recipients of a T-cell–repleted BMT. Finally, we found that IL-15 administration could enhance graft-versus-leukemia activity. In conclusion, IL-15 can be administered safely to recipients of a T-cell–depleted allo BMT to enhance CD8+ T, NK, and NK T-cell reconstitution.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an important therapy for a variety of malignant and nonmalignant diseases. Immune deficiency after allogeneic HSCT is one of the major causes of early and late posttransplantation morbidity and mortality. Strategies to enhance posttransplantation immune reconstitution could decrease the incidence of fatal infectious complications, enhance graft-versus-tumor activity, and significantly increase the overall survival after allogeneic HSCT. Interleukin-7 (IL-7) and IL-15 are critical cytokines in lymphocyte development and homeostasis. Administration of IL-7, which plays an important role in the homeostasis of B and T lymphocytes, can improve immune reconstitution after syngeneic and allogeneic HSCT.1-5 The effect on immune reconstitution of administration of IL-15 to HSCT recipients has not been determined.
IL-15 is a pleiotropic cytokine that plays a role in natural killer (NK) development, innate and adaptive immune homeostasis, and the activation and homing of immune effector cells including NK cells, NK T-cells, and cytotoxic T lymphocytes.6 It binds to a receptor complex that consists of IL-15Rα, IL-2Rβ, and the γ-common chain.7-9 IL-15 is produced by antigen-presenting cells (APCs),10 bone marrow stroma,7-9 thymic epithelium,11 and epithelial cells in the kidney,8 skin,12 and intestines.13 IL-15 has nonredundant roles in the development and function of NK, NK T, and intestinal intraepithelial lymphocytes (IELs).6 It can stimulate cytolytic activity, cytokine secretion (tumor necrosis factor [TNF], interferon-γ [IFN-γ], granulocyte colony-stimulating factor [G-CSF]), and proliferation and survival of NK cells.8,14,15 IL-15 secretion by activated macrophages plays a role in the cross-talk between macrophages and NK cells during inflammation.6,16 IL-15 can act as a chemoattractant and activating agent for T-cells7-9,17 and has a proliferative and survival effect on CD8+ memory T-cells.18 IL-15 is required for optimal proliferation of CD8+ T-cells, and IL-15(-/-) mice cannot support homeostatic proliferation of CD8+ memory T-cells.19,20 Recently, IL-15 has been shown to have a role in the survival and antigen-independent expansion in vitro of naive CD8+ T-cells.21,22
Until now, few studies have evaluated the effects of IL-15 administration in vivo. Sprent and colleagues showed that the proliferation of CD8+ memory T-cells increased after a single dose of IL-15 in normal mice. However, IL-15 did not affect CD4+ memory T-cells, which could be due to their low expression of IL-2Rβ.18 Administration of IL-15 to mice also enhanced the antitumor activity after syngeneic bone marrow transplantation (BMT) and antigen-specific primary CD8+ T-cell responses following vaccination with peptide-pulsed dendritic cells.23,24
In this study, we used murine models to investigate the effects of IL-15 administration to recipients of an allogeneic BMT on immune reconstitution, graft-versus-host disease (GVHD), and graft-versus-leukemia (GVL) activity.
Materials and methods
Mice and BMT
Female C57BL/6J (B6, H-2b), C3FeB6F1 ([B6xC3H]F1, H-2b/k), B6D2F1/J (H-2b/d), B10.BR (H-2k), CBA/J (H-2k), and C57BL/6J (Ly5.1+) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). For some experiments, IL-15(-/-) mice were obtained from Taconic Laboratory (Germantown, NY). Mice used in BMT experiments were between 8 and 10 weeks or 10 months (CBA/J) of age. BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. Bone marrow (BM) cells were removed aseptically from femurs and tibias. Donor BM was T-cell depleted (TCD) by incubation with anti–Thy-1.2 for 30 minutes at 4°C followed by incubation with Low-TOX-M rabbit complement (Cedarlane Laboratories, Hornby, ON, Canada) for 40 minutes at 37°C. Splenic T-cells were obtained by purification over a nylon wool column, positive selection with anti-CD5 antibodies conjugated to magnetic beads (Miltenyi, Auburn, CA), or negative selection using fluorescein isothiocyanate (FITC) or phycoerythrin (PE)–conjugated lineage antibodies (NK1.1, Gr-1, Mac-1, B220), followed by anti-FITC or anti-PE antibodies coupled to magnetic beads (Miltenyi). Cells (5-10 × 106 BM cells with or without splenic T-cells) were resuspended in Dulbecco modified Eagle medium (DMEM, Life Technologies, Grand Island, NY) and transplanted by tail vein infusion (0.25 mL total volume) into lethally irradiated recipients on day 0. On day 0 prior to transplantation, recipients received 1300 cGy total body irradiation (137Cs source) as a split dose with a 3-hour interval between doses (to reduce gastrointestinal toxicity). Mice were housed in sterilized micro-isolator cages and received normal chow and autoclaved hyperchlorinated drinking water (pH 3.0).
Reagents and antibodies
Anti–murine CD16/CD32 FcR block (2.4G2) and all of the following fluorochrome-labeled antibodies against murine antigens were obtained from BD PharMingen (San Diego, CA): Ly-9.1 (30C7), CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD62L (MEL-14), CD122 (TM-B1), CD44 (IM7), CD45R/B220 (RA3-6B2), NK1.1 (PK136), CD11b (M1/70), CD25 (PC61), CD69 (H1.2F3), Bcl-2 (3F11), TCRαβ (H57-597), GR-1 (RB6-8C5), Annexin-V, 7-AAD, Ki67 (B56), isotype controls; rat IgG2a-κ (R35-95), rat IgG2a-λ (B39-4), rat IgG2b-κ (A95-1), rat IgG1-κ (R3-34), hamster IgG–group1-κ(A19-3), hamster IgG–group 1-λ(Ha4/8), and streptavidin-FITC, -PE, and -Per CP (peridinin chlorophyll protein). Recombinant human IL-15 was kindly provided by Amgen (Thousand Oaks, CA). Carboxyfluoroscein succinidyl ester (CFSE) was obtained from Molecular Probes (Eugene, OR).
Tissue culture medium consisted of RPMI-1640 or DMEM supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine.
Administration of IL-15
For immune reconstitution studies, IL-15 was given for 7 days at different time points after BMT (Table 1, 2.5 μg/d intraperitoneally). For GVHD studies, IL-15 was administered on days 0 to 10, 10 to 20, and 21 to 27 after BMT (2.5 μg/d intraperitoneally). In adoptive transfer experiments with CFSE-labeled T-cells, IL-15 was administered at a dose of 2.5 μg/d intraperitoneally from days 0 to 3.
Assessment of GVHD
The severity of GVHD was assessed with a clinical GVHD scoring system as previously described.25 Briefly, ear-tagged animals in coded cages were individually scored every week for 5 clinical parameters on a scale from 0 to 2: weight loss, posture, activity, fur, and skin. A clinical GVHD index was generated by summation of the 5 criteria scores (0-10). Survival was monitored daily. Animals with scores of at least 5 were considered moribund and were killed.
Cells
As described before, splenic T-cells were obtained by purification over a nylon wool column. Single cell suspensions were prepared from spleens according to standard protocols and used in fluorescence-activated cell-sorter (FACS) analysis. YAC-1, EL4, and A20 cell lines were obtained from American Type Culture Collection (Manassas, VA).
CFSE labeling
Cells were labeled with CFSE as previously described.26 Briefly, T-cells were incubated with CFSE at a final concentration of 2.5 μM in Hanks balanced salt solution (HBSS) at 37°C for 15 minutes. Cells were then washed 3 times with HBSS before intravenous injection.
Flow cytometric analysis
Cells were washed in FACS buffer (phosphate-buffered saline [PBS]/0.5% bovine serum albumin [BSA]/0.1% sodium azide), and 106 cells/mL were incubated for 20 minutes at 4° C with CD16/CD32 FcR block. Subsequently, cells were incubated for 30 minutes at 4° C with antibodies and washed twice with FACS buffer. The stained cells were resuspended in FACS buffer and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) with CellQuest software or with FlowJo software (Treestar, San Carlos, CA).
Intracellular stainings
Splenocytes were harvested, washed, and stained with primary (surface) fluorochrome-conjugated antibodies. Then they were fixed and permeabilized with the Cytofix/Cytoperm Kit (PharMingen, San Diego, CA) and subsequently stained with a secondary antibody. FACS analysis was conducted by gating for the designated populations.
Proliferation assay
Splenocytes (4 × 105 cells/well from recipients of allo BMT) were incubated for 5 days with irradiated (2000 cGy) host splenocytes as stimulators (2 × 105 cells/well) in 96-well plates. Cultures were pulsed during the final 18 hours with 1 μCi (0.037MBq)/well {3H} thymidine, and cells were harvested with a Topcount Harvester (Packard, Meriden, CT). The proliferation of the cells was determined as counts per minute (cpm).
Cytotoxicity assay
Target cells were labeled with 100 μCi (3.7 MBq)of 51Cr at 3 × 106 cells/mL for 1 hour at 37°C and 5% CO2. After 3 washes, labeled targets were plated at 2.5 × 103 cells/well in U-bottom plates (Costar, Cambridge, MA). Purified NK cells were added at various effector-to-target ratios in a final volume of 200 μL in triplicate and incubated for 4 hours at 37°C and 5% CO2. Subsequently, 35 μL of supernatant was removed from each well and counted in a gamma counter (Topcount-Packard, Meriden, CT) to determine experimental release. Spontaneous release was obtained from wells receiving target cells with medium, and total release was obtained from wells receiving 5% Triton X-100. Percent cytotoxicity was calculated by the following formula:
Delayed type hypersensitivity (DTH assay)
Mice were sensitized on day 0 by tail intravenous injection of 200 μL of 0.01% sheep red blood cells (SRBCs) (Colorado Serum, Denver, CO) in PBS solution. Sensitized animals were challenged at day 4 in the right hind footpad with 50 μL 20% SRBC suspension while the left hind footpad received the same volume of 50 μL PBS solution as a control. Footpad swelling was measured after 24 and 48 hours with a dial-thickness gauge (Mitutoyo, Kanagawa, Japan). The magnitude of the response was determined by subtracting measurements of PBS-injected left footpads from the experimental right one.
Statistics
All values are expressed as median ± a percentile range (25% and 75%). Statistical comparison of experimental data was performed with the nonparametric unpaired Mann-Whitney U test, whereas the Mantel-Cox log-rank test was used for survival data. A P value less than .05 was considered statistically significant.
Results
IL-15 administration to recipients of an allo TCD-BMT does not affect thymic cellularity except when given early after BMT
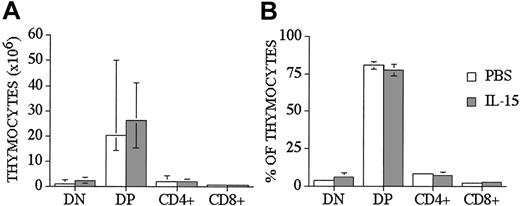
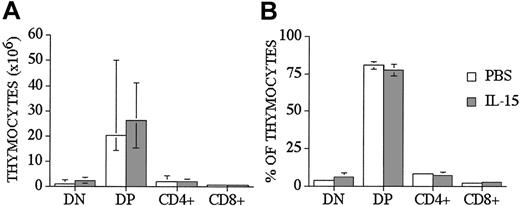
We used a well-described clinically relevant murine model for allogeneic BMT B10.BR → CBA/J27 (major histocompatibility complex [MHC]–matched [H-2k], but mismatched for minor histocompatibility antigens) to test the effects of IL-15 administration on immune reconstitution and GVHD. We administered human recombinant IL-15 intraperitoneally at a dose of 2.5 μg/d, which was previously shown to be an effective therapeutic dose. IL-15 was administered for no longer than 10 days because of the potential risk of neutralizing antibody formation with longer administration. Similar to our previous results with IL-7 administration to allo T-cell depleted (TCD) BMT recipients, we saw no effects of IL-15 on thymic and splenic T-cell reconstitution in allogeneic recipients when IL-15 was administered from days 0 to +7 after TCD-BMT and T-cell numbers analyzed on day +28 (Table 1 experiment 4 and data not shown). However, when we administered IL-15 either from days 0 to +7 (Table 1 experiment 4) or from day +14 to +21 (Table 1 experiment 1) after BMT to allogeneic recipients, we found a significant decrease in the total number of thymocytes when analyzed at earlier time points (days +14 and +21, respectively [Table 1]). However, we did not see this effect when we administered IL-15 to syngeneic recipients (Table 1 experiment 5) or one week later (days 21-27) to syngeneic or allogeneic recipients and analyzed at day 28 and day 42 after BMT (Table 1 experiments 1-3, 6). We concluded from these experiments that IL-15 administration early after allo BMT can decrease thymic cellularity in allogeneic recipients, but this inhibitory effect is only temporary and is not seen when IL-15 is administered at a later stage after allo BMT. We also analyzed thymocyte distribution, and we did not find any statistical differences in percentages and numbers of double-positive (DP), double-negative (DN), single CD4+, and single CD8+ thymocytes between control and IL-15–treated groups at day +28 after allo BMT (Figure 1). However, we did detect a small difference in the CD4/CD8 ratio between IL-15–treated and control recipients (3.6 ± 0.2 vs 2.8 ± 0.2, P < .05).
IL-15 administration does not affect thymocyte distribution in recipients of an allo TCD-BMT at day 28. Lethally irradiated (1300 cGy) CBA/J recipients received transplants of B10.BR TCD-BM (5 × 106) and 2.5 μg/d IL-15 or PBS (control) on days 21 through 27 by intraperitoneal injection. BMT recipients were killed at day 28, and percentage and absolute numbers of donor-derived cell populations in the thymi were calculated from total cell counts and multicolor flow cytometric analyses of thymocyte cells with anti-CD4 and anti-CD8. Donor or host origin was determined with anti-Ly 9.1, which is present on host leukocytes. Values represent the median cell numbers (n = 10) ± a percentile range (25% and 75%).
IL-15 administration does not affect thymocyte distribution in recipients of an allo TCD-BMT at day 28. Lethally irradiated (1300 cGy) CBA/J recipients received transplants of B10.BR TCD-BM (5 × 106) and 2.5 μg/d IL-15 or PBS (control) on days 21 through 27 by intraperitoneal injection. BMT recipients were killed at day 28, and percentage and absolute numbers of donor-derived cell populations in the thymi were calculated from total cell counts and multicolor flow cytometric analyses of thymocyte cells with anti-CD4 and anti-CD8. Donor or host origin was determined with anti-Ly 9.1, which is present on host leukocytes. Values represent the median cell numbers (n = 10) ± a percentile range (25% and 75%).
IL-15 administration to recipients of allo TCD-BMT increases donor derived CD8+ T, NK, and NK T-cells in the spleen
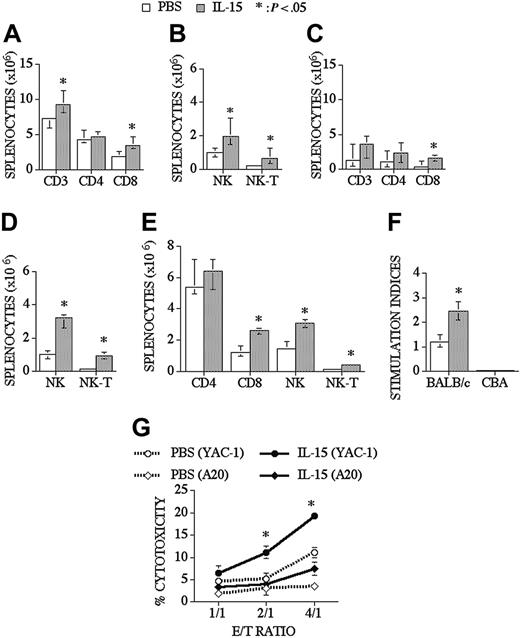
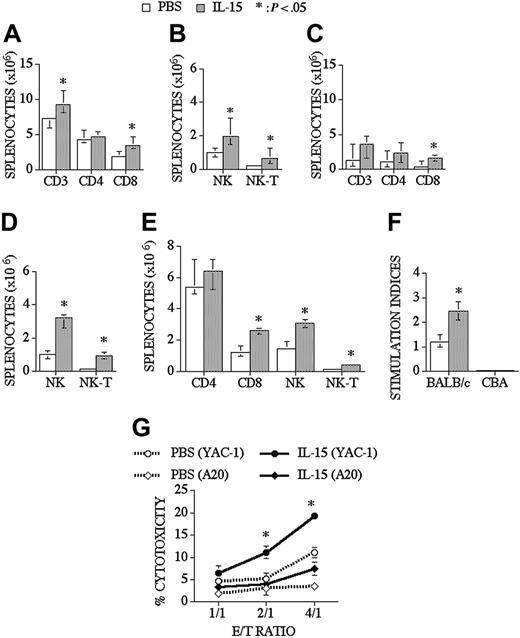
When we analyzed peripheral T-cell reconstitution in the spleen of recipients of an allo TCD-BMT, we found that IL-15 did not affect the chimerism of donor-derived T-cells, B cells, NK cells, and myeloid cells as assessed with a donor/host marker compared to control recipients (74% vs 78% for T-cells, 96% vs 96% for B cells, 96% vs 97% for NK cells, and 98% vs 99% for myeloid cells; control and IL-15–treated groups, respectively), and we observed that IL-15 could slightly enhance total splenic cellularity in some experiments. IL-15 administration significantly increased CD8+ T, NK, and NK T-cells in the spleen, but it did not change splenic CD4+ T-cell numbers (Figure 2A-B). Interestingly, IL-15 had even more profound effects on CD8+, NK, and NK T-cell reconstitution in older recipients of an allo TCD-BMT (Figure 2C-D). B-cell (B220+), monocyte (CD11b+, Gr-1-), and granulocyte (CD11b+Gr-1+) numbers were not changed after IL-15 administration when analyzed at day 28 and day 42 after allo TCD-BMT (data not shown). We also evaluated the effects of IL-15 on immune reconstitution in a parent-F1 model (B6 → [B6xC3H]F1) and again found significant increases in the numbers of NK, NK T, and CD8+ T-cells (Figure 2E).
IL-15 administration increases CD8+ T, NK, and NK T-cells in young and old recipients of an allo TCD-BMT. Lethally irradiated (1300 cGy) CBA/J recipients received transplants of B10.BR TCD-BM (5 × 106) and 2.5 μg/d IL-15 or PBS (control) on days 21 through 27 by intraperitoneal injection. BMT recipients were killed at day 28, and absolute numbers of donor-derived cell populations in the spleen were calculated from total cell counts and multicolor flow cytometric analyses of T-cells with anti-CD3, -CD4, -CD8, and -NK1.1 antibodies. NK cells were defined as NK1.1+CD3-, and NK T-cells were defined as NK1.1+CD3+. Data for young (3-month-old) recipients are shown in Figures 2A-B and for old (10-month-old) recipients in Figures 2C-D. (E) Lethally irradiated (1300 cGy) (B6xC3H)F1 recipients received transplants of B6 TCD-BM (5 × 106). Mice were treated and harvested as shown in Figure 2A-2B. (F) Splenocytes from IL-15– or PBS-treated allo TCD-BMT recipients were harvested day +28 and cultured for 5 days with irradiated BALB/c splenocytes (third party) or CBA splenocytes (host). 3H-thymidine was added during the final 18 hours of culture, and proliferation was determined. Stimulation index was calculated as Proliferation in MLR (cpm)–spontaneous proliferation (cpm)/spontaneous proliferation (cpm). (G) Mice received transplants and were treated as described Figure 2A-D and NK 1.1+, CD3- NK cells were sorted, and cytotoxicity against YAC-1 and A20 cell line was determined in a 51Cr release assay. Donor or host origin was determined with anti-Ly 9.1, which is present on host leukocytes. Values represent the median cell number ± 25% and 75% of the population (n = 4-20).
IL-15 administration increases CD8+ T, NK, and NK T-cells in young and old recipients of an allo TCD-BMT. Lethally irradiated (1300 cGy) CBA/J recipients received transplants of B10.BR TCD-BM (5 × 106) and 2.5 μg/d IL-15 or PBS (control) on days 21 through 27 by intraperitoneal injection. BMT recipients were killed at day 28, and absolute numbers of donor-derived cell populations in the spleen were calculated from total cell counts and multicolor flow cytometric analyses of T-cells with anti-CD3, -CD4, -CD8, and -NK1.1 antibodies. NK cells were defined as NK1.1+CD3-, and NK T-cells were defined as NK1.1+CD3+. Data for young (3-month-old) recipients are shown in Figures 2A-B and for old (10-month-old) recipients in Figures 2C-D. (E) Lethally irradiated (1300 cGy) (B6xC3H)F1 recipients received transplants of B6 TCD-BM (5 × 106). Mice were treated and harvested as shown in Figure 2A-2B. (F) Splenocytes from IL-15– or PBS-treated allo TCD-BMT recipients were harvested day +28 and cultured for 5 days with irradiated BALB/c splenocytes (third party) or CBA splenocytes (host). 3H-thymidine was added during the final 18 hours of culture, and proliferation was determined. Stimulation index was calculated as Proliferation in MLR (cpm)–spontaneous proliferation (cpm)/spontaneous proliferation (cpm). (G) Mice received transplants and were treated as described Figure 2A-D and NK 1.1+, CD3- NK cells were sorted, and cytotoxicity against YAC-1 and A20 cell line was determined in a 51Cr release assay. Donor or host origin was determined with anti-Ly 9.1, which is present on host leukocytes. Values represent the median cell number ± 25% and 75% of the population (n = 4-20).
IL-15 administration not only increased the number of T-cells in the spleen, and but it also resulted in a significant increase in posttransplantation T-cell function as measured in a third-party mixed lymphocyte reaction (MLR), without activity against host antigens (Figure 2F). We also evaluated T-cell function by measuring delayed type hypersensitivity (DTH) responses after foot pad injection of sheep red blood cells (SRBCs). First, we evaluated the effects of IL-15 administration (2.5 μg/d/intraperitoneally for 5 days) on DTH responses in normal mice that did not receive transplants and found that the IL-15–treated group had a significantly better DTH response than the control group (0.62 mm vs 0.43 mm, respectively, P < .05). We subsequently measured the DTH response in recipients of a minor Ag mismatched allogeneic BMT and observed a slight increase in DTH response in the IL-15 group at day 63 after BMT, but this was not significantly different from the control group (0.71 mm vs 0.6 mm). Finally, we evaluated the DTH response in an MHC-disparate model (B6 → BALB/c) and found an increased DTH response in the IL-15–treated group compared to the control group at day 70 after BMT (0.41 mm vs 0.19 mm, respectively). We conclude that posttransplantation IL-15 administration can enhance the DTH response in normal mice and in recipients of an allogeneic BMT.
We analyzed NK cell numbers and function in IL-15–treated allogeneic BMT recipients. Lethally irradiated recipients received TCD-BM and were treated with IL-15 from days +21 to +27. Mice were killed at day 28 after BMT, and NK1.1+CD3- splenic NK cells from each mouse were sorted to obtain purified NK cells (> 95% donor origin), which allowed a comparative analysis of NK activity on a per cell basis. A 51Cr release assay was performed using NK cell–sensitive (YAC-1) or control (A20) cell lines as targets. We found that the NK cell cytotoxicity significantly increased in the IL-15–treated group compared to the PBS-treated group (Figure 2G). These experiments demonstrate that posttransplantation IL-15 administration enhances NK cell activity as well as NK recovery.
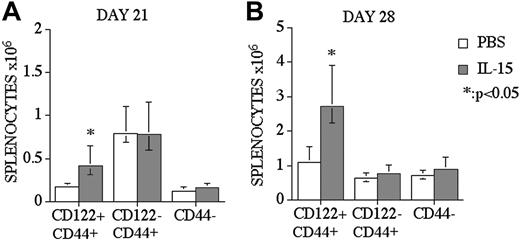
The effects of IL-15 administration on CD8+ T-cells in recipients of an allo TCD-BMT are limited to CD122+CD44+ memory-like T-cells
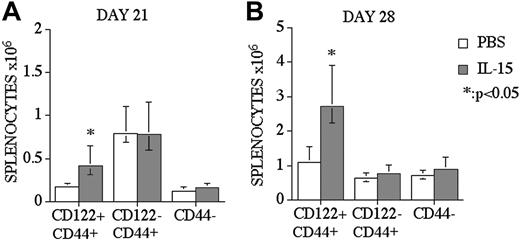
We analyzed CD8+ T-cell reconstitution and divided the CD8+ T-cell population into 2 subpopulations: CD44+ memory and CD44- naive CD8+ T-cells. CD44+ memory CD8+ T-cells were further subdivided by their CD122 expression, according to previous studies.28,29 We found that the increase of CD8+ T-cells after IL-15 administration in recipients of allo BMT was limited to CD122+CD44+ memory CD8+ T-cells, and we did not observe any effects on naive or CD122 memory CD8+ T-cells in IL-15–treated recipients at days 21 and 28 after BMT (Figure 3A-B). Interestingly, we found an increase of CD8+CD122+CD44+ T-cells at day 21 after BMT in IL-15–treated recipients, although we did not observe any difference in the total number of CD8+ T-cells between IL-15–treated and control groups at day 21 after BMT (data not shown).
IL-15 administration specifically enhances CD8+CD44+CD122+ memory T-cells in recipients of an allo TCD-BMT. Mice received transplants as described Figure 1 and received 2.5 μg/d IL-15 or PBS (control) on days 14 through 21 or 21 through 27 by intraperitoneal injection (panels A and B, respectively). Recipients were killed at day 21 or day 28, and absolute numbers of donor-derived cell populations in the spleen were calculated from total cell counts and multicolor flow cytometric analyses of T-cells with anti-CD8, -CD44, and -CD122 antibodies. Naive CD8 cells were defined as CD8+ CD44-(low) and memory/activated (MEM/ACT) CD8 cells were defined as CD8+ CD122+CD44+(high) and CD8+ CD122-CD44+(high). Donor or host origin was determined with anti-Ly 9.1, which is present on host leukocytes. Values represent the median cell number ± 25% and 75% of the population (n = 5).
IL-15 administration specifically enhances CD8+CD44+CD122+ memory T-cells in recipients of an allo TCD-BMT. Mice received transplants as described Figure 1 and received 2.5 μg/d IL-15 or PBS (control) on days 14 through 21 or 21 through 27 by intraperitoneal injection (panels A and B, respectively). Recipients were killed at day 21 or day 28, and absolute numbers of donor-derived cell populations in the spleen were calculated from total cell counts and multicolor flow cytometric analyses of T-cells with anti-CD8, -CD44, and -CD122 antibodies. Naive CD8 cells were defined as CD8+ CD44-(low) and memory/activated (MEM/ACT) CD8 cells were defined as CD8+ CD122+CD44+(high) and CD8+ CD122-CD44+(high). Donor or host origin was determined with anti-Ly 9.1, which is present on host leukocytes. Values represent the median cell number ± 25% and 75% of the population (n = 5).
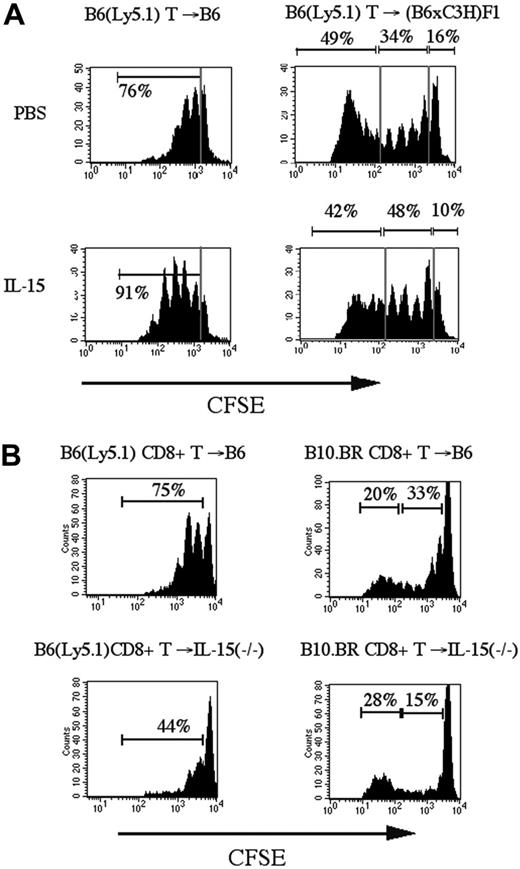
IL-15 administration increases homeostatic CD8+ T-cell proliferation in recipients of allogeneic T-cell infusion
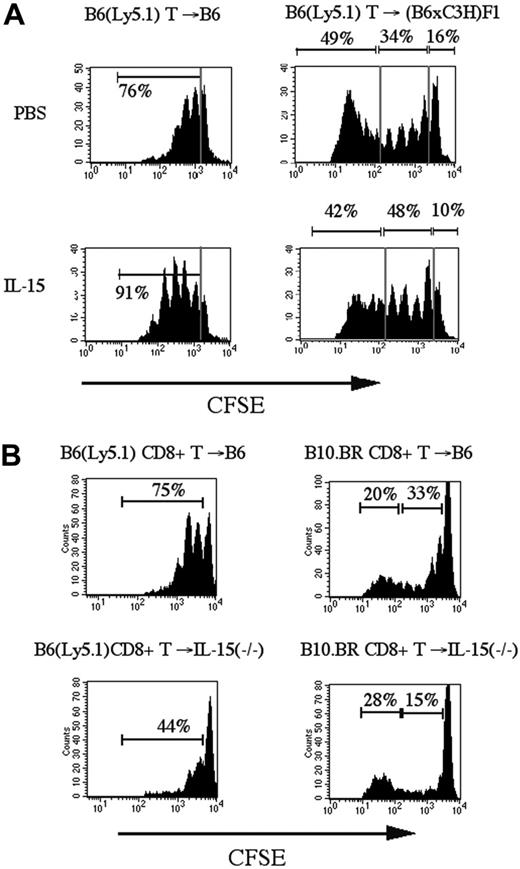
It has been shown previously that IL-7 and IL-15 are required for homeostatic proliferation of CD8+ memory T-cells.19 To assess the effects of IL-15 administration on the proliferative activity of alloreactive and nonalloreactive donor T-cells after transplantation, we transferred CFSE-labeled purified splenic T-cells from B6 (Ly5.1) donors into sublethally irradiated syngeneic (B6) and allogeneic ([B6xC3H]F1) recipients (an MHC-disparate model) and analyzed the number of cell divisions at day 3 according to CFSE intensity (Figure 4A). Of note, we have previously demonstrated that the irradiation dose (lethal or sublethal) of the recipient does not affect homeostatic or alloreactive expansion of donor T-cells over the relatively short period of 3 days in this strain combination as long as the dose is high enough to induce lymphopenia in the recipient.5 When transferred into a sublethally irradiated syngeneic recipient, B6 (Ly5.1) CD8+ T-cells undergo not more than 3 divisions in the first 3 days, which is consistent with homeostatic expansion with slow proliferative kinetics of CD8+ T-cells in a lymphopenic host. IL-15 administration to recipients of syngeneic CFSE-labeled T-cells resulted in increased homeostatic proliferation of CD8+ T-cells but not CD4+ T-cells (Figure 4A and data not shown).
IL-15 administration increases homeostatic proliferation of CD8+ T-cells in syngeneic and allogeneic recipients of CFSE labeled T-cell infusion. (A) Sublethally irradiated B6 (Ly5.1+) (SYN) or (B6xC3H)F1 (ALLO) recipients were infused with CFSE-labeled B6-purified T-cells (2 × 107). The recipients received 2.5 μg/d IL-15 or PBS (control) on days 0 to 3. On day 3, splenocytes were harvested and stained with anti-CD8 antibodies for flow cytometric analysis. A representative experiment of 3 is shown. (B) Sublethally irradiated B6 (Ly5.1+) or IL-15-/- recipients were infused with CFSE-labeled B6 (SYN) or B10.BR (ALLO) purified CD8+ T-cells (5 × 106). Harvesting, staining, and flow cytometric analysis of splenocytes were as in panel A. The corresponding percentages of the marked regions are shown in each plot.
IL-15 administration increases homeostatic proliferation of CD8+ T-cells in syngeneic and allogeneic recipients of CFSE labeled T-cell infusion. (A) Sublethally irradiated B6 (Ly5.1+) (SYN) or (B6xC3H)F1 (ALLO) recipients were infused with CFSE-labeled B6-purified T-cells (2 × 107). The recipients received 2.5 μg/d IL-15 or PBS (control) on days 0 to 3. On day 3, splenocytes were harvested and stained with anti-CD8 antibodies for flow cytometric analysis. A representative experiment of 3 is shown. (B) Sublethally irradiated B6 (Ly5.1+) or IL-15-/- recipients were infused with CFSE-labeled B6 (SYN) or B10.BR (ALLO) purified CD8+ T-cells (5 × 106). Harvesting, staining, and flow cytometric analysis of splenocytes were as in panel A. The corresponding percentages of the marked regions are shown in each plot.
In our previous studies, we found that CFSE-labeled T-cells transferred into sublethally irradiated allogeneic recipients could be separated into 3 populations of cells based upon their proliferation and cell surface antigen expression5 : (1) nonproliferating, (2) homeostatically proliferating, and (3) alloreactively proliferating cells. Interestingly, IL-15 administration increased homeostatically proliferating CD8+ T-cells in recipients of allogeneic CFSE-labeled T-cells, but we did not observe an effect on the rapidly proliferating alloreactive CD8+ T-cells (Figure 4A). The percentage of homeostatically proliferating CD8+ T-cells increased significantly in the IL-15–treated group compared to the control group (median: 49% vs 33%, respectively, P < .05 in 3 different experiments; n = 7). To further assess the differential effects of IL-15 on alloreactive and nonalloreactive donor T-cell populations, we analyzed CD8+ T-cell proliferation in sublethally irradiated IL-15–deficient recipients of CFSE-labeled CD8+ T-cell infusions from syngeneic and allogeneic donors. We found that homeostatic proliferation of CD8+ T-cells was decreased in IL-15–deficient syngeneic recipients (Figure 4B). In allogeneic IL-15–deficient recipients, we observed again a loss of homeostatic proliferation by nonalloreactive CD8+ T-cells, whereas the rapid proliferation of alloreactive T-cells was not affected. We conclude from these experiments that IL-15 is required for optimal homeostatic proliferation of nonalloreactive CD8+ T-cells in syngeneic and allogeneic recipients but not for the initial alloreactive proliferation of donor CD8+ T-cells in allogeneic recipients.
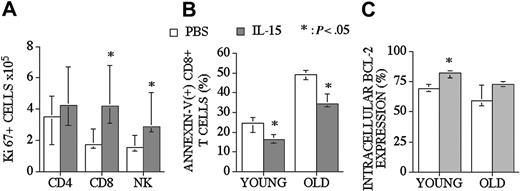
IL-15 administration increases CD8+ T and NK cell proliferation and decreases CD8+ T-cell apoptosis
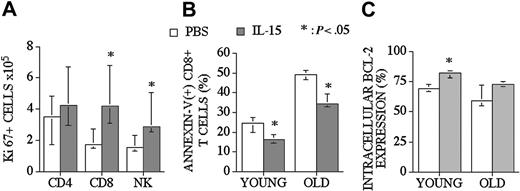
IL-15 can promote survival and enhance proliferation of CD8+ memory T-cells. In addition to our CFSE proliferation studies, we assessed proliferative effects of posttransplantation IL-15 administration by determining the intracellular levels of Ki67 (proliferative nuclear antigen) in recipients of an allo TCD-BMT at day 28. We found that IL-15 administration increased Ki67+ CD8+ T and NK cell numbers in the spleen but had no effect on Ki67+ CD4+ T-cells (Figure 5A).
IL-15 administration increases the proliferation of CD8+ T and NK cells and decreases the number of apoptotic CD8+ T-cells in recipients of an allo TCD-BMT. Mice received transplants as described Figure 1 and received 2.5 μg/d IL-15 or PBS (control) on days 21 through 27 by intraperitoneal injection. (A) Recipients were killed at day 28, and absolute numbers of donor-derived cell populations in the spleen were calculated from total cell counts and multicolor flow cytometric analyses of T-cells intracellularly stained with Ki67. (B) The percentage of apoptotic (Annexin-V(+) 7-AAD(-)) donor-derived CD8+ T-cells from young and old BMT recipients are shown. (C) The percentages of Bcl-2–expressing donor-derived CD8+ T-cells from young and old BMT recipients are shown. Values represent the median cell number ± 25% and 75% of the population (n = 5).
IL-15 administration increases the proliferation of CD8+ T and NK cells and decreases the number of apoptotic CD8+ T-cells in recipients of an allo TCD-BMT. Mice received transplants as described Figure 1 and received 2.5 μg/d IL-15 or PBS (control) on days 21 through 27 by intraperitoneal injection. (A) Recipients were killed at day 28, and absolute numbers of donor-derived cell populations in the spleen were calculated from total cell counts and multicolor flow cytometric analyses of T-cells intracellularly stained with Ki67. (B) The percentage of apoptotic (Annexin-V(+) 7-AAD(-)) donor-derived CD8+ T-cells from young and old BMT recipients are shown. (C) The percentages of Bcl-2–expressing donor-derived CD8+ T-cells from young and old BMT recipients are shown. Values represent the median cell number ± 25% and 75% of the population (n = 5).
To determine the antiapoptotic effect of IL-15 on CD8+ T-cells after an allogeneic TCD-BMT, we assessed the percentage of donor-derived apoptotic splenic CD8+ T-cells in young (3-month-old) and middle-aged (10-month-old) allogeneic recipients (Figure 5B). We observed a significant decrease in (Annexin-V + 7-AAD-) apoptotic T-cells in IL-15–treated old and young recipients of allo BMT when analyzed at day 28. We also evaluated intracellular Bcl-2 expression in T-cells in order to explain the mechanism of a decreased apoptotic fraction of CD8+ T-cells after allo BMT. We found increased numbers of Bcl-2–expressing CD8+ T-cells in young recipients (3-month-old) after IL-15 administration (Figure 5C).
The effects of posttransplantation IL-15 administration to recipients of an allo TCD-BMT on GVHD is dependent on the time of administration and the type of allo BMT
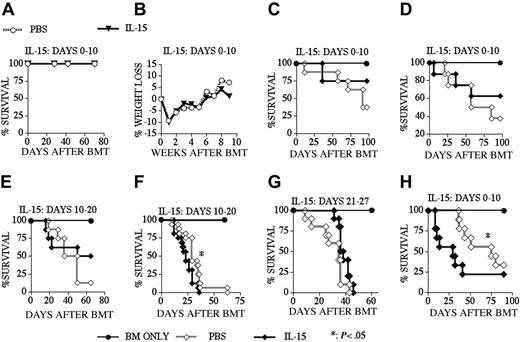
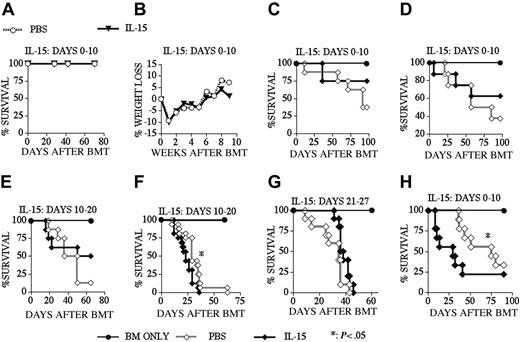
Because of its proliferative and antiapoptotic effects on CD8+ memory T-cells, IL-15 could potentially enhance GVHD or interfere with peripheral deletion, resulting in the survival of de novo–generated host-reactive T-cell clones in recipients of an allo BMT. To analyze these potentially detrimental effects of posttransplantation IL-15 administration, we first monitored IL-15–treated recipients of an allo TCD-BMT for mortality and morbidity (Figure 6A-B). We found no mortality during a 10-week follow-up period and detected no sign or symptoms of autoimmune disease (including weight loss) between IL-15– and PBS-treated recipients. We then induced GVHD in our recipients by adding splenic donor T-cells to the allogeneic TCD BM graft. The results from experiments in an MHC-matched GVHD model (B10.BR → CBA) demonstrated that IL-15 administration did not aggravate GVHD when we used a low donor T-cell dose (Figures 6C,E,G) independent of the dosing interval chosen: days 0 to 10 (Figure 6C), days 10 to 20 (Figure 6E), or days 21 to 27 (Figure 6G). In this model we only observed increased GVHD when we used a higher donor T-cell dose and administered IL-15 from days 10 to 20 (Figure 6F), but not earlier from days 0 to 10 (Figure 6D). We also found increased GVHD when we administered IL-15 to recipients of an MHC-disparate allograft in a different GVHD model: B6 → BALB/c (Figure 6H). Therefore, we conclude that IL-15 administration does not induce GVHD in recipients of an allo TCD-BMT, but can in some cases exacerbate GVHD in T-cell–replete allo BMT recipients.
IL-15 administration does not induce GVHD in recipients of an allo TCD-BMT. Lethally irradiated (1300 cGy) CBA mice (A-G) received transplants of B10.BR TCD-BM (5 × 106) or lethally irradiated (900 cGy) BALB/c mice (H) received transplants of B6 TCD-BM (5 × 106) with or without T-cells. PBS (control) or 2.5 μg/d IL-15 was administered by intraperitoneal injection from days 0 to 10 (A-D, H), 10 to 20 (E, F) and 21 to 27 (G) after allogeneic BMT. T-cell dose was 0.25 × 106 for panels C, E, and G and 0.5 × 106 for panels D, F, and H. Open diamonds represent the PBS (control) groups, and filled diamonds represent the IL-15–treated groups in all GVHD experiments (C-H). Kaplan-Meier survival curves are shown (n = 8-16 mice each group).
IL-15 administration does not induce GVHD in recipients of an allo TCD-BMT. Lethally irradiated (1300 cGy) CBA mice (A-G) received transplants of B10.BR TCD-BM (5 × 106) or lethally irradiated (900 cGy) BALB/c mice (H) received transplants of B6 TCD-BM (5 × 106) with or without T-cells. PBS (control) or 2.5 μg/d IL-15 was administered by intraperitoneal injection from days 0 to 10 (A-D, H), 10 to 20 (E, F) and 21 to 27 (G) after allogeneic BMT. T-cell dose was 0.25 × 106 for panels C, E, and G and 0.5 × 106 for panels D, F, and H. Open diamonds represent the PBS (control) groups, and filled diamonds represent the IL-15–treated groups in all GVHD experiments (C-H). Kaplan-Meier survival curves are shown (n = 8-16 mice each group).
IL-15 administration can increase graft-versus-tumor activity in recipients of an allo BMT
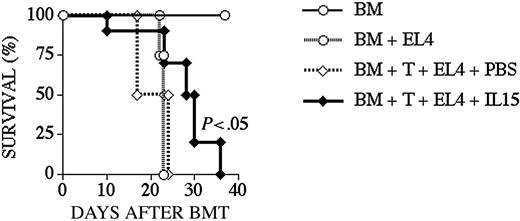
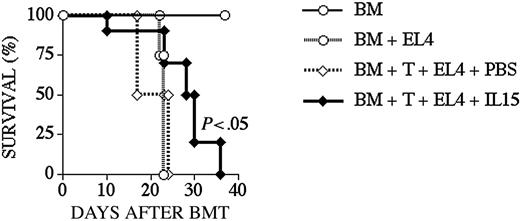
We used the B10.BR → B6 strain combination for our graft-versus-tumor (GVT) experiments as previously described.30 To test the effects of IL-15 administration on GVT activity, we administered IL-15 from day 0 to +10 to B6 recipients of B10.BR TCD BM + T-cells, which also received EL4 lymphoma cells at the time of BMT. All animals that died during the course of the experiment underwent an autopsy to determine the cause of death. We found that most recipients died from lymphoma, but the IL-15–treated group had a modest but significant prolonged survival (Figure 7; Table 2).
IL-15 administration increases GVT activity in recipients after an allo BMT. Lethally irradiated (1300 cGy) B6 recipients received transplants of B10.BR TCD-BM cells (5 × 106) with the addition of B10.BR splenic T-cells (0.5 × 106) on day 0. Recipients also received EL4 cells (5 × 104) on day 0. The cause of death was determined by necropsy and is shown in Table 2. Survival is depicted as a Kaplan-Meier curve, and each group contained 10 animals.
IL-15 administration increases GVT activity in recipients after an allo BMT. Lethally irradiated (1300 cGy) B6 recipients received transplants of B10.BR TCD-BM cells (5 × 106) with the addition of B10.BR splenic T-cells (0.5 × 106) on day 0. Recipients also received EL4 cells (5 × 104) on day 0. The cause of death was determined by necropsy and is shown in Table 2. Survival is depicted as a Kaplan-Meier curve, and each group contained 10 animals.
Discussion
Posttransplantation immune deficiency is associated with a risk of opportunistic infections in recipients of an allo HSCT, especially in the first months after transplantation. Several studies have shown that recipients of an unrelated HLA-matched HSCT have greater mortality from infections than from leukemic relapse.31-35 For example, a recent study demonstrated that opportunistic infections were the leading cause of posttransplantation mortality in patients with chronic myeloid leukemia (CML), who received an unrelated HLA-matched allo-HSCT.36 Moreover, this study showed that fungal and viral infections comprise 70% of the infection mortality after BMT. Opportunistic infections are also a major problem after T-cell–depleted BMT, especially in the adult population.37 Therefore, any improvement in posttransplantation immune reconstitution would result in decreased morbidity and mortality from infection and could enhance quality of the life after an HSCT. In this study, we have shown that IL-15 administration to young and old recipients of an allo BMT enhances CD8+ T, NK, and NK T-cell reconstitution. To our knowledge, this is the first study demonstrating the effects of IL-15 administration on immune reconstitution after allogeneic BMT.
Previously, studies in IL-15(-/-) mice have shown that IL-15 is not required for thymic T-cell development. Thymic cellularity, weight, and cell distribution in IL-15(-/-) mice were comparable with their littermates except for a loss of thymic NK T-cells.38 IL-15Rα and IL-2/15Rβ are expressed in the thymus,9,39 but the function of these receptors in thymic development are not clearly defined. IL-2/15Rβ has a role in negative selection, and IL-2/15Rβ(-/-) mice develop autoimmune disorders that are lethal at approximately 12 weeks of age.40 Reya et al found that a significant fraction of Sca-1+ Lin- precursor cells in day-12 fetal livers express IL-2/15Rβ (CD122) on their surface.41 They hypothesized that these cells represent early lymphoid precursors in fetal life, which can enter the thymus and give rise to T-cells. Finally, in vitro studies on IL-15 could exert a proliferative effect on human thymocytes (preferentially on CD8+ T-cells). IL-15 was found to be a growth factor for human thymocytes with preferential effects on CD8+ cells39 or on CD8+ thymocytes from mice deficient for suppressor of cytokine signaling-1 (SOCS 1), which was accompanied by an up-regulation of Bcl-XL and CD44 expression.42 However, the in vivo effects of IL-15 administration on T-cell lymphopoiesis in the thymus of normal mice or mice that received transplants had not been studied before. We used an allogeneic TCD murine BMT model comparable to an HLA-matched unrelated TCD-HSCT and found that IL-15 administration did not affect chimerism and thymic reconstitution except when we administered IL-15 early after transplantation and detected a temporary decrease in thymic cellularity.
In contrast to its effects on the thymus, we found that posttransplantation IL-15 administration resulted in a significant increase in splenic CD8+ T, NK, and NK T-cell reconstitution in young and old recipients of an allo TCD-BMT. An IL-15–dependent increase in the proliferation of CD8+ T-cells (especially in CD44+ memory T-cells) has been shown in previous studies,18,29,43 whereas IL-15 has no effect on CD4+ T-cell proliferation and survival.18,19 Although CD8+ T-cell reconstitution is more rapid than CD4+ T-cell reconstitution, CD8+ T-cell function is initially impaired. For example, the reactivation of cytomegalovirus (CMV) in allo BMT recipients was associated with a lack of functional antigen-specific CD8+ T-cells (as determined by cytokine secretion) rather than a deficiency in the recovery of sufficient numbers of CMV-specific T-cells.44 Increased antimicrobial activity has been observed in IL-15 transgenic mice and after IL-15 administration.45,46 In addition, it has been shown that IL-15 is required for proliferative renewal of virus-specific memory CD8+ T-cells.47 Our MLR and DTH data indicate that the administration of IL-15 after allo BMT can increase the response of antigen-specific T-cells against foreign antigens (Figure 1F). Therefore, posttransplantation administration of IL-15 could enhance the reconstitution of antimicrobial immunity.
NK cell numbers and function recover rapidly and reach normal values within 1 month of transplantation.48,49 In mice, increased NK cell cytotoxicity (compared to mice that did not receive transplants) was found at day 17 after TCD-BMT.50 In the early posttransplantation period NK cells may provide an important defense against infections while specific immunity is still recovering; therefore, increased NK cell numbers and function due to IL-15 administration after BMT might increase host defense. In addition, alloreactive NK cells have been implicated in GVT activity and the decrease of GVHD by eliminating the host APC after allo BMT.51
IL-15 can increase CD8+ T-cell numbers after allo BMT by increasing T-cell proliferation as shown in our Ki67 and CFSE experiments (Figures 4, 5); on the other hand, IL-15 also can decrease apoptosis in donor-derived CD8+ T-cells, possibly through increasing intracellular Bcl-2 levels (Figure 5). Zhang et al reported that only IL-15 caused a prominent increase in Bcl-2 expression in comparison to other γc cytokines after 18 hours in vitro culture, and they also suggested that increased Bcl-2 expression (especially in old mice) was associated with a decrease in spontaneous apoptosis.43 Despite decreased numbers of apoptotic CD8+ T-cells, we have not found a significant increase in Bcl-2 expression in old mice. It could be that different antiapoptotic molecules such as Bcl-XL might play an additional role in apoptosis in old recipients.52
The use of any lymphoid growth factor in recipients of an allo BMT can increase the risk of GVHD and autoimmune-like disorders. We did not detect any sign of GVHD or other antihost immunity in IL-15–treated recipients of TCD-BMT. Interestingly, we found that IL-15 specifically increased the homeostatic proliferation of nonalloreactive CD8+ T-cells in recipients of CFSE-labeled donor T-cells. IL-15 along with IL-7 can regulate CD8+ T-cell homeostatic proliferation, and in the absence of both cytokines homeostatic proliferation fails to occur.19,20 Our findings in IL-15(-/-) recipients showed that IL-15 is required not only for homeostatic CD8+ T-cell proliferation in recipients of a syngeneic graft, but also in recipients of an allograft. In contrast to recipients of a TCD-BMT, recipients of a T-cell–repleted allograft can develop more GVHD morbidity and mortality when IL-15 is administered under certain conditions. These data suggest that initial clinical studies regarding posttransplantation IL-15 administration should be limited to recipients of TCD HSCT without GVHD.
Unlike IL-2Rα and IL-7Rα expression, IL-15Rα is expressed on naive, activated, and memory CD8+ T-cells, and IL-15 is involved in all stages of T-cell activation: initiation, clonal expansion, contraction, and maintenance of memory T-cells.53,54 IL-15 administration increases antitumor and antimicrobial activity in mice55-59 and was less toxic than IL-2.55 Katsanis et al23 administered IL-15 in the second week after BMT to recipients of a syngeneic BMT with lymphoma and found a significantly better survival in the IL-15–treated group. We found that IL-15 administration can increase GVT activity without increasing GVHD, and we are currently studying the effects of IL-15 administration on posttransplantation adoptive T-cell therapies and tumor vaccination.
In conclusion, posttransplantation IL-15 administration can safely and effectively enhance the reconstitution of CD8+ T, NK, and NK T-cells in recipients of an allogeneic TCD-BMT and is capable of enhancing GVT activity. This could result in the development of IL-15 as a posttransplantation therapy to prevent or treat opportunistic infections, facilitate immunization against microbial or tumor antigens, increase NK and T-cell–mediated GVT activity, or enhance the efficacy of posttransplantation adoptive therapy with donor T or NK cells.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2003-09-3344.
Supported by grants HL69929 and HL72412 from the National Institutes of Health (M.R.M.v.d.B.) and by a translational research grant from the Leukemia & Lymphoma Society (M.R.M.v.d.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the staff of the Research Animal Resource Center for excellent animal care and Drs Richard O'Reilly and Eric Pamer for helpful discussions and support throughout the project. The authors also would like to thank Marsinay Smith, Thomas van Huystee, and Suzanne McGoldrick for their assistance. M.R.M.v.d.B. is the recipient of a Damon Runyan Scholar Award of the Cancer Research Fund and a research award from the V scholar program of the V Foundation. T.H.T. is the recipient of a research award from the Boehringer Ingelheim Fonds.