Abstract
The optimal approach to stem cell transplantation in children with immunodeficiency who lack a matched family donor is controversial. Unrelated donor stem cell transplantation gives equivalent outcome to mismatched family donor stem cell transplantation in severe combined immunodeficiency, whereas unrelated donors may be preferable in non–severe combined immunodeficiency children. However, unrelated donor stem cell transplantation with conventional conditioning regimens has been associated with significant treatment-related toxicity, particularly in non–severe combined immunodeficiency patients with preexisting organ dysfunction. We report the outcome of a series of 33 consecutive unrelated donor transplantations performed at our center in children with primary immunodeficiency using a reduced-intensity conditioning regimen between 1998 and 2001. We have compared these outcomes with a retrospective control cohort of 19 patients who underwent transplantation with myeloablative conditioning between 1994 and 1998. All children in both groups had primary engraftment. There was no statistical difference in the speed of immune reconstitution or incidence of graft-versus-host disease between the 2 groups. Overall survival was significantly better in the reduced-intensity conditioning group: 31 (94%) of 33 patients survived, compared with 10 (53%) of 19 in the myeloablative conditioning group (P = .014). We conclude that the reduced-intensity conditioning regimen results in improved survival and reduced transplantation-related mortality compared with myeloablative conditioning in high-risk patients undergoing unrelated donor transplantation.
Introduction
Allogenic stem cell transplantation (SCT) is curative for congenital immunodeficiencies, but unfortunately only about 10% of these children will have a matched family donor (MFD).1 The optimal approach for children who do not have a MFD is unclear. Related HLA-nonidentical transplantations have been performed since the early 1980s, when better T-cell depletion strategies became available,2-4 and the obvious advantage of a haploidentical transplantation is that the donor is readily available. However, the intense T-cell depletion required for these procedures has posed many problems, including graft failure,5 poor immune reconstitution necessitating “boost” transplantations,6 and susceptibility to viral infections.7 Another approach is to use an unrelated donor (UD), and there have been successful long-term engraftments and immune reconstitution after the use of T-replete UD stem cell transplants,8 but this has to be counterbalanced against the risk of graft-versus-host disease (GvHD). For patients with severe combined immunodeficiency (SCID), this problem has been largely overcome, as over the years, the results of matched unrelated donor (MUD) and mismatched family donors have improved significantly and are comparable with the results after HLA-identical transplantations.6,9 By contrast, this has not been the case with the non-SCID transplantations where there has been no improvement in survival since 1985, whatever the donor origin or HLA compatibility.9 Antoine et al9 report a 59% 3-year survival after MUD SCT for non-SCID patients using myeloablative conditioning (MAT), and Fischer et al7 report a 45.5% survival in 22 patients with non-SCID primary immunodeficiency (PID) from closely matched donors (phenotypically identical related, matched unrelated donors, and 1-antigen [Ag]–mismatched related). In both series, the major causes of death were infections and toxicity.
Because of the concerns over toxicity associated with the MAT regimens, some groups have tried and reported successes using reduced-intensity conditioning (RIC) regimens for hematologic malignancies.10-12 With many of these regimens, reliable engraftment can be achieved without myeloablation and with reduced regimen-related toxicity. The intense pretransplantation immunosuppression and T-cell alloreactivity are important for cure of hematologic malignancies using RIC. In PIDs, with RIC, the aim is to create an immunologic platform of host and donor tolerance using pre- and posttransplantation immunosuppression. T-cell alloreactivity is less important for cure in these diseases. We have previously reported 8 patients with PID and significant organ dysfunction who received transplants of a fludarabine/melphalan-based RIC regimen. All children survived the procedure with minimal toxicity and GvHD, and with good engraftment and immune reconstitution.13 Following this, we have now used the RIC regimen in more than 50 patients. Here we report our results on 33 consecutive UD transplantations and have compared them with a retrospective control cohort of 19 patients who received a MAT transplant.
Patients, materials, and methods
Between April 1994 and January 2002, 52 children with PID underwent SCT from an UD at Great Ormond Street Hospital, London. This study cohort is divided into 2 groups based on the conditioning regimen received. Between April 1994 and December 1998, 19 children received a MAT transplant, and between October 1998 and January 2002, 33 children received a RIC transplant. Patient characteristics before SCT are outlined in Table 1. The 2 groups were matched for the major prognostic factors defined by Antoine et al,9 in particular, the number of children with B-negative SCID and the presence of pulmonary complications before transplantation. The only variable that differed significantly between the groups was age, with the RIC group considerably older at transplantation compared with the MAT group. Informed consent was gained in writing from patients or parents prior to the transplantation procedure in all cases, and the reduced-intensity continuing protocol was registered with the local Research and Development Office protocol no: 99MH11.
In the RIC group, 6 children (18%) had SCID and 27 children (82%) had non-SCID immunodeficiencies. In the MAT group, 7 children (37%) had SCID and 12 children (63%) had non-SCID immunodeficiencies. The molecular diagnoses of the patients with SCID are outlined in Table 2, and characterization of the immunodeficiency phenotype in the non-SCID T-cell deficiencies is provided in Table 3. The median age at transplantation in the RIC group was 5.9 years (range, 0.19-18 years), and in the MAT group it was 1.9 years (range, 0.39-13.08 years). Of the children, 73% in the MAT group and 74% in the RIC group were older than one year at the time of transplantation. In the RIC group, 21 (64%) of 33 donors were fully matched serologically for class I and by molecular techniques for class II antigens; 11 (33%) of 33 were 1-antigen mismatches; and there was 1 child who underwent a 2-antigen mismatched transplantation. Of donors in the MAT group, 11 (58%) of 19 were fully HLA compatible. Of the mismatches (8 [42%] of 19 patients), 6 were 1-antigen mismatches and 2 were 2-antigen mismatches.
Preparative regimen, transplantation, and supportive care
Marrow was used as the source of hematopoietic stem cells in all transplantations in both groups. The RIC group was conditioned with fludarabine (150 mg/m2), melphalan (140 mg/m2), and alemtuzumab (Campath 1H; 0.2 mg/kg day -8 to -4, n = 14) or anti–thymocyte globulin (ATG, 2.5 mg/kg day -2 to +2, n = 19). All children received T-replete grafts. The MAT group was conditioned with busulphan (16 mg/kg) and cyclophosphamide (200 mg/kg). T-cell depletion (TCD) was used in all but 2 of the MAT transplantations. TCD was performed with YTH 66.9 (Campath 1M) with donor serum as a source of complement in vitro, together with in vivo Campath 1G (0.2 mg/kg day -12 to -8); an add-back of 5 × 104 CD3 cells/kg was given with the graft. Cyclosporin was used as GvHD prophylaxis for the RIC group, and in the MAT group cyclosporin and methotrexate (10 mg/m2 on days 3, 6, 11, and 18) were used for GvHD prophylaxis.
Supportive care was identical in both groups. All children received granulocyte colony-stimulating factor (G-CSF) 5 μg/kg from day 8 until neutrophil recovery to more than 1 × 109/L. Antimicrobial prophylaxis during the transplantation period consisted of aciclovir 750 to 1500 mg/m2 from day -1 to -3 and then 1200 to 2400 mg/m2 per day orally to 6 months; itraconazole 5 mg/kg per day to count recovery; cotrimoxazole adjusted to body surface area from neutrophil recovery to 6 months or normalization of phytohemagglutinin (PHA) stimulation index; and penicillin V 250 to 500 mg/d from discharge to 2 years after transplantation. Patients with sclerosing cholangitis received anticryptosporidial prophylaxis with paramomycin 30 mg/kg per day orally or azithromycin 10 mg/kg per day orally, or both. All patients received intravenous immunoglobulin (IVIg) 0.5 g/kg every 3 weeks during the transplantation period, and this was continued until CD4 count was more than 300 per μL and serum immunoglobulin A and M levels had normalized. Blood and urine samples of all patients were screened weekly by DNA polymerase chain reaction (PCR) and detection of early antigen fluorescent (DEAFF) test, respectively, to check for cytomegalovirus (CMV). In the MAT group, adenovirus and Epstein-Barr virus (EBV) screening were performed when symptoms such as unresolving pyrexia, lymphadenopathy, or unexplained hepatitis developed. In the RIC group, additional prospective monitoring by weekly DNA PCR for EBV and adenovirus on the peripheral blood was performed. About 40% of children in both groups developed adenovirus in the stools. This was not routinely treated, and the majority of children with adenovirus in stools did not develop viremia. Only reactivations in the blood will be further detailed. CMV reactivation was treated with ganciclovir. Adenoviremia was treated with withdrawal of immunosuppression where possible and intravenous ribavirin or cidofovir. Early EBV reactivation was treated with withdrawal of immunosuppression where possible and rituximab. Semiquantitative analysis of EBV viral loads was performed every week. Based on these, children received between 1 and 4 doses of rituximab at weekly intervals. Late EBV reactivations were either treated with rituximab or a “wait-and-watch” policy with careful monitoring of viral loads was adopted.
Engraftment and immune reconstitution studies
Engraftment studies were performed in patients alive one month after transplantation who had neutrophil recovery (defined as neutrophils > 0.5 × 109/L on 2 consecutive days). Engraftment was assayed on the peripheral blood using XY fluorescence in situ hybridization (FISH; sex-mismatched patient-donor) or variable number of tandem repeat (VNTR) analysis (sex matched). Lineage-specific chimerism analysis was possible after 1998. Mixed chimerism (MC) is defined as the presence of more than 5% host-derived cells on more than one occasion in the whole blood. This is further categorized into high-level MC (95%-50% donor chimerism), low-level MC (49%-10% donor chimerism), or very low-level MC (< 10% donor chimerism). Acute GvHD was assessed using the Seattle criteria. Chronic GvHD is defined as GvHD occurring 100 days or more after bone marrow transplantation (BMT) and is graded as none, extensive, or limited. Immune reconstitution was studied at 1, 2, 3, 6, 9, 12, and 15 months by fluorescence-activated cell sorter (FACS) analysis of peripheral blood mononuclear cells using fluorescein isothiocyanate–phycoerythrin–labeled antibodies against CD3, CD4, and CD19; immunoglobulin A and M levels; and assay of PHA stimulation index (defined as the ratio of baseline–maximal stimulated levels of 3H-thymidine uptake in a 3-day culture of peripheral blood mononuclear cells stimulated with a range of concentrations of PHA). Age-specific normal ranges for CD3, CD4, and CD19 were used to ascertain times to normalization.14 Children alive and with evidence of engraftment 6 months after transplantation were included in the analysis of immune reconstitution. Children were immunized at 18 months after transplantation if they were off immunoglobulin replacement therapy and did not have GvHD. Vaccine responses were checked 4 weeks after vaccination.
The median duration of follow-up for the RIC group is 40 months and for the MAT group it is 104 months.
Transplantation-related mortality (TRM) is defined as death occurring within 100 days of transplantation, due to transplantation-related causes.
TREC analysis
CD4+ and CD8+ cells were isolated from Ficolled blood using anti-CD4 or anti-CD8 antibody-coated magnetic beads (Miltenyi, Harrow, United Kingdom). T-cell receptor excision circle (TREC)15 levels were analyzed by real-time quantitative polymerase chain reaction (PCR) assay. Cells were lysed using a solution of proteinase K (Roche, Milan, Italy), nonidet P-40 (NP40), and Tween 20 and the lysate used in duplicate as the template for real-time PCR. These were run on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany) under standard reaction conditions and cycling parameters. The primers and probe are previously published.16 For each run, a standard curve was generated from duplicate samples of 5-fold serially diluted known copies of plasmid DNA, obtained by inserting a human signal joint TREC fragment in the pCR-Blunt Vector (Invitrogen; Life Technologies, Paisley, United Kingdom). The threshold cycle (Ct) value for each duplicate was determined at the point where the fluorescence exceeded the manually set threshold limit. This value was then compared with the standard curve to determine the starting copy number. To normalize for cell equivalents, the β actin gene was quantified under identical real-time PCR conditions used for TREC quantification; primers and probe were previously published.16 As per the TREC assay, a standard curve was generated per plate using serial dilutions of a fragment of the β actin gene inserted into the pCR-Blunt Vector.
Statistical analysis
The MAT and RIC groups were compared with respect to age, pretransplantation diagnosis, presence of pretransplantation comorbidities, and type of donors. These variables were analyzed using an exact method to account for the sample sizes and the different-sized groups, and chi-square was used where appropriate. The end-points of survival were determined using the Kaplan-Meier product limits methods, and comparisons of survival distribution were performed using the log-rank test.
Results
Engraftment and chimerism
All children in both groups had primary engraftment.
RIC group
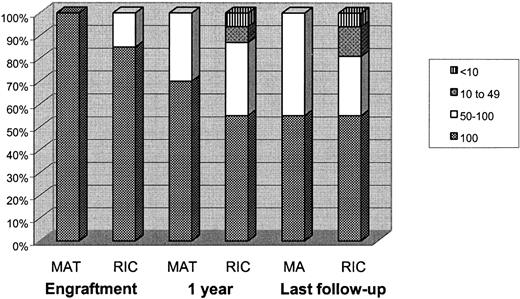
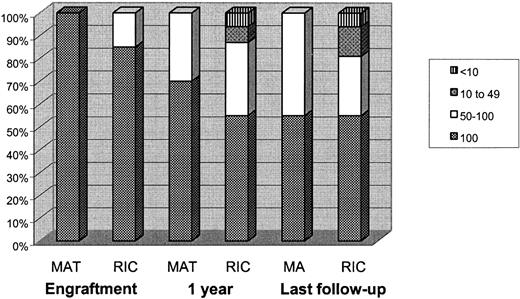
The median time to neutrophil recovery was 13 days (range, 8-34 days) and to an unsupported platelet count of more than 20 × 109/L was 16 days (range, 9-100 days). One child continued to have an unsupported platelet count of more than 20 × 109/L throughout, and one child was platelet-dependent at discharge after a protracted BK virus–induced hemorrhagic cystitis. As shown in Figure 1, at one month after transplantation, 32 of 33 children were alive and engrafted. Of these, 82% (n = 27) of children had 100% donor chimerism, and 15% (n = 6) had high-level MC. At one year after transplantation, 31 of 33 children were alive. Of these, 55% (n = 17), 32% (n = 10), 6.5% (n = 2), and 6.5% (n = 2) had 100% donor chimerism, high-level MC, low-level MC, and very low-level MC, respectively. These figures were not significantly different at last follow-up where 55% (n = 17), 26% (n = 8), 13% (n = 4), and 6% (n = 2) of children had 100% donor chimerism, high-level MC, low-level MC, and very low-level MC, respectively. Both children who had very low-level MC were following a one-antigen mismatched transplantation. They have been restarted on prophylactic medications.
Chimerism in the 2 groups at engraftment, at one year after transplantation, and at last follow-up. At one year after transplantation, 45% of children in the RIC group and 36% of children in the MAT group developed MC. The low-level MCs and very low-level MCs were found only in the RIC group. However, at last follow-up the MCs remain stable.
Chimerism in the 2 groups at engraftment, at one year after transplantation, and at last follow-up. At one year after transplantation, 45% of children in the RIC group and 36% of children in the MAT group developed MC. The low-level MCs and very low-level MCs were found only in the RIC group. However, at last follow-up the MCs remain stable.
MAT group
The median time to neutrophil recovery was 15 days (range, 10-23 days) and to an unsupported platelet count of more than 20 × 109/L was 22.5 days (range, 16-40 days). At one month after transplantation, 14 of 19 children were alive. All of these children had engrafted with 100% donor chimerism in whole blood. At one year after transplantation, 11 of 19 children were alive. Of these, 64% (n = 7) retained 100% donor chimerism, and 36% (n = 4) had high-level MC. At last follow-up, 10 of 19 children were alive. Of these, 50% (n = 5) still retained 100% donor chimerism; the other 50% had high-level MC. There were no children with low-level or very low-level MC.
There was no statistical difference in the speed of neutrophil or platelet recovery between the 2 groups.
Viral reactivations
There were more viral reactivations in the RIC group compared with the MAT group (P = .02). Of the children, 3 had CMV reactivation; 5, adeno viremia; and 10, EBV reactivations. The CMV and adenovirus reactivations were picked up on prospective monitoring, and none of these children had evidence of disease. There were 6 children with EBV reactivation who were classified as having EBV disease, defined as the presence of the virus together with the appropriate symptoms in the absence of any other cause. All of them were successfully treated, and there were no deaths due to EBV. There were 3 children with EBV reactivation who also had simultaneous reactivation of CMV or adenovirus.
In the MAT group, 3 children had CMV reactivation. None of them had evidence of CMV disease. One child died of EBV lymphoproliferative disease on day 28.
Graft-versus-host disease
As shown in Table 4, the incidence of acute GvHD more than grade 2 was equal and low in both groups (9% in the RIC group and 10.5% in the MAT group). No child in the RIC group had limited chronic GvHD compared with 3 of 19 children in the MAT group. One child in each group had extensive chronic GVHD, and both of these children died.
Immune reconstitution
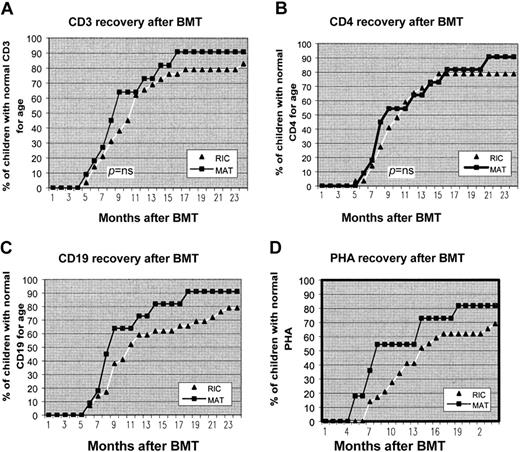
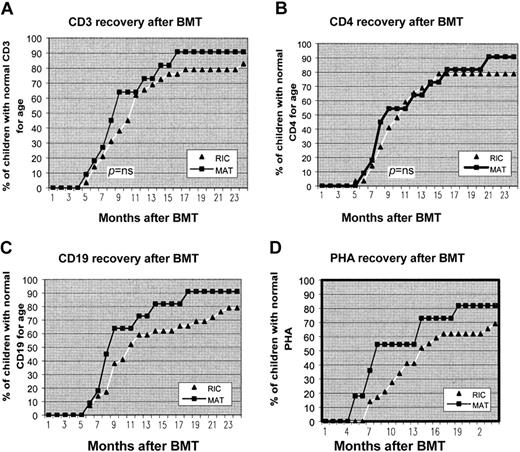
Figure 2 shows the recovery of CD3+ and CD4+ T cells and CD19+ B-cell counts to normal age-related counts after transplantation and return to normal T-cell function as assessed by PHA stimulation index. From 6 months to 2 years after BMT an increasing number of children in both groups developed normal T- and B-cell function. At 12 months after transplantation 73%, 64%, 54%, and 73% of the MAT group had age-related normal levels of CD3, CD4, PHA, and CD19, respectively, compared with 66%, 65%, 41%, and 59% in the RIC group, respectively. There was no statistically significant difference in the percentage of children achieving normal age-related values at 12 months for any of these parameters. Of surviving children in the MAT group, 100% had normal production of immunoglobulins and are off IVIg. There are 5 patients in the RIC group who remain on IVIg for incomplete B-cell reconstitution. Of these children, 2 received rituximab for EBV disease during transplantation. Table 5 shows the vaccine responses to Haemophilus influenza and tetanus. Nearly all children in both groups achieved good antibody titres after vaccination, indicating good functional B-cell recovery.
Recovery of CD3, CD4, CD19, and PHA to age-related normal levels after transplantation. From 6 months to 2 years after BMT, an increasing number of children in both groups developed normal T- and B-cell function. There was no statistical difference in speed of immune reconstitution between the 2 groups.
Recovery of CD3, CD4, CD19, and PHA to age-related normal levels after transplantation. From 6 months to 2 years after BMT, an increasing number of children in both groups developed normal T- and B-cell function. There was no statistical difference in speed of immune reconstitution between the 2 groups.
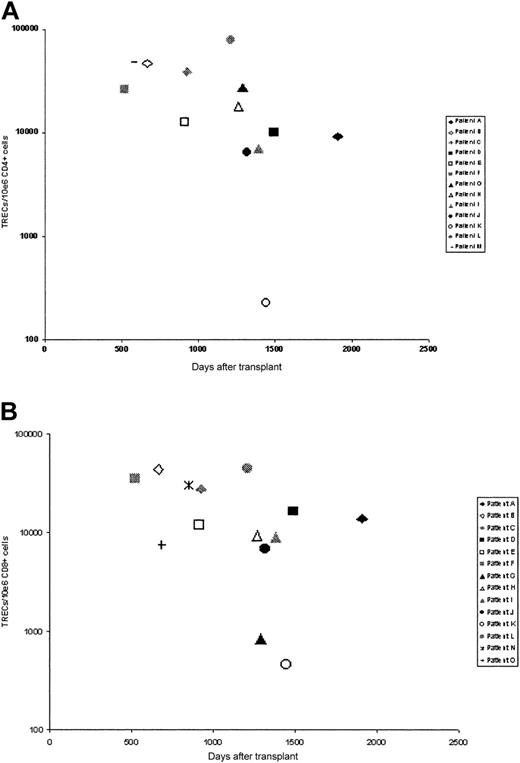
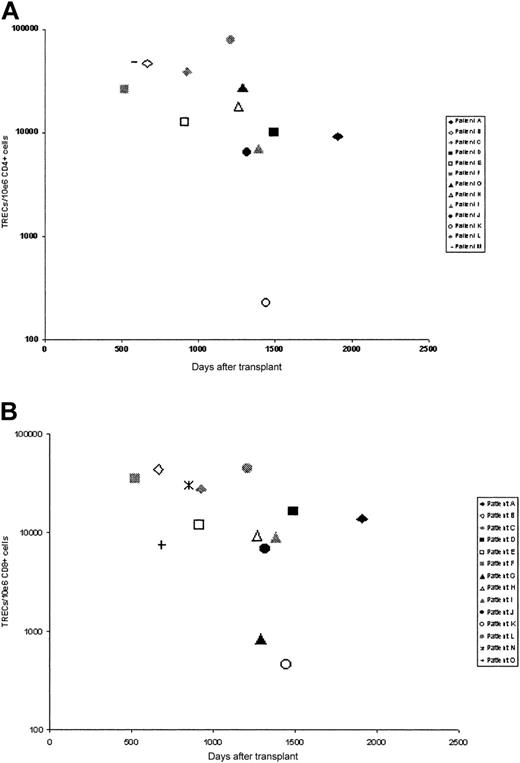
Thymic activity after transplantation was assessed by measurement of T-cell receptor excision circle (TREC) numbers in separated CD4 and CD8 populations using a quantitative PCR assay (Figure 3). Samples were obtained from 15 patients at a mean of 1076 days after transplantation. In 12 patients (patients A-L), TREC numbers in both CD4 and CD8 populations were determined, but in a few patients, values in only CD4+ (patient M) or CD8+ (patients N-O) cells were obtained. In the large majority of patients, TREC numbers in both CD4+ and CD8+ populations were more than 5000 copies/106 cells for both CD4+ (mean, 25 443 TRECs/106 CD4+ cells) and CD8+ (mean, 17 179 TRECs/106 CD8+ cells) cells. These values are comparable with TREC values in healthy young donors,17 suggesting that in the majority of cases thymic output after transplantation is within normal limits. In one individual, patient K, TREC numbers in both CD4+ (228 TRECs/106 CD4+ cells) and CD8+ (462 cells TRECs/106 CD8+) cells were low, and in another, patient G, levels in CD8+ cells were low although CD4 levels were normal. In one other individual, TRECs were not detectable, that is, they were beyond the sensitivity of the assay (data not shown). Interestingly, all 3 of these patients show poor immune recovery in terms of quantitative lymphocyte recovery, and all 3 remain on immunoglobulin replacement therapy.
TREC numbers in selected T-cell populations. TREC numbers in (A) CD4+ cells and (B) CD8+ cells.
TREC numbers in selected T-cell populations. TREC numbers in (A) CD4+ cells and (B) CD8+ cells.
Survival
RIC group. SCT with RIC conditioning was extremely well tolerated. Of 33 children, 31 (94%) are alive and well at the time of writing. One child died of respiratory syncitial virus (RSV) pneumonitis during conditioning, and one child died of chronic GvHD.
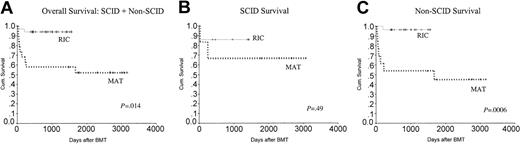
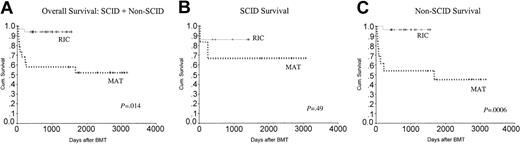
As is evident in the survival curve in Figure 4A, there was a significantly better overall survival (OS) in the RIC group compared with the MAT group (P = .014). As there were only 2 deaths in the RIC group, it was not possible to analyze the influence of different variables on mortality. However in the MAT group, there were more deaths in the non-SCID patients (54%) compared with SCID patients (30%). This is shown in Figure 4B-C.
Kaplan-Meier analysis showing the overall survival in the 2 groups. OS was significantly better in the RIC group at 94% compared with 53% in the MAT group. Non-SCID patients in the MAT group did worse, with an overall survival of 46% compared with 96% in the RIC group. CyA indicates cyclosporin A..
Kaplan-Meier analysis showing the overall survival in the 2 groups. OS was significantly better in the RIC group at 94% compared with 53% in the MAT group. Non-SCID patients in the MAT group did worse, with an overall survival of 46% compared with 96% in the RIC group. CyA indicates cyclosporin A..
MAT group. Of 19 children, 10 are alive and well in this group. Overall mortality was 47% (9/19). TRM was 26% (5/19; 3 regimen-related toxicity, 2 infection) at a median of 38 days after transplantation. One child died of disseminated cryptosporidiosis at day 130; and 2 children, of chronic GvHD at days 217 and 255 after transplantation. One child died 4.6 years after transplantation due to ongoing respiratory problems after transplantation for dyskeratosis congenita.
Quality of life. The mean Lansky score for survivors in the RIC group is 97 compared with 94 in the MAT group.
Discussion
In this paper, we report the outcome of a large series of unrelated donor transplantations in patients with PID using an RIC regimen and have compared these outcomes with a retrospective control cohort that underwent transplantation with a MAT regimen.
The most striking aspect of our study is the difference in survival between the 2 groups. In the MAT group, 47% of children died, largely due to toxicity and infection. Although it may be argued that the MAT transplantations were performed between 1994 and 1998 and there has been significant improvement in supportive care, early detection of viral problems, and GvHD treatment since then, these factors alone are unlikely to account for the superior survival of the RIC group. Hitherto, many groups have shown an improvement in survival over time for the SCID patients who received transplants from family or unrelated donors.6,9 This has not been the case with the non-SCID patients. In this group, there was an initial improvement in survival between 1973 and 1985, but since then there has been no further improvement.9,18 Among the non-SCID immunodeficiencies, the T-cell deficiencies had a particularly poor prognosis, with a 43% survival at 3 years.9 We too noted a particularly poor outcome for the non-SCID patients in the MAT group who had a mortality of 58% (7/12) compared with 28% (2/7) in the SCID group; P = .0006 (Figure 4C). In this respect, it is noteworthy that 27 (82%) of 33 patients in the RIC group were non-SCID and half of these, 14 of 27, were T-cell immunodeficiencies, making them an extremely high-risk group of children. They were also significantly older at transplantation than the MAT group. It has been shown in multivariate analysis that age older than 12 months was the single most important factor determining poor outcome after transplantation in patients with SCID.9,18,19 These data imply that the RIC group would be predicted to have a higher mortality from SCT.
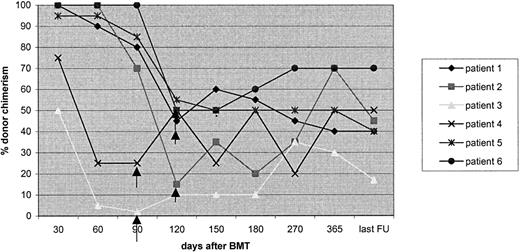
Chimerism data on the RIC patients show that initially all children engrafted with donor hematopoiesis, and subsequently there was a significant incidence of MC, approximating that previously reported after RIC conditioning.11,13 However, it is well established that MC is sufficient to cure the disease phenotype in PIDs. In our study, all children with high- and low-level MC remain well and free of disease. All patients with phagocytic disorders in the RIC cohort achieved full donor chimerism in the myeloid lineage and would therefore be expected to have normal neutrophil function. In the children with low-level MC who have had a longer follow-up, it has been possible to demonstrate that this has been maintained, and many children have moved from being low-level MC to high level or full chimeras on withdrawal of immunosuppression (Figure 5). The 2 children with very low-level MC had early onset of falling chimerism following a one-antigen mismatched transplantation procedure that did not respond to withdrawal of immunosuppression and are likely to need a second transplantation procedure. But the RIC procedure was extremely well tolerated even in these high-risk children without a fully matched donor and will allow us to consider a second transplantation procedure at a later date. Since this study closed, 6 patients with one-antigen mismatched UDs have received the same conditioning regimen with mycophenolate mofetil in addition to cyclosporin and peripheral blood (4/6) as the source of stem cells. Of 6 children, 5 survive and are 100% engrafted at a median follow-up of 12.4 months (P. Veys, Great Ormond Street Hospital, personal communication, January 2004).
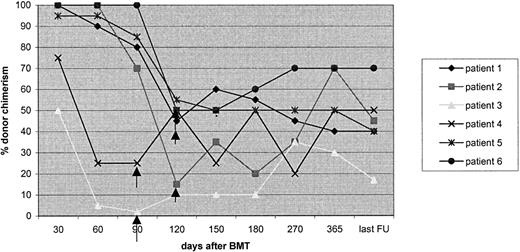
Chimerism stabilizing or improving after cyclosporin withdrawal in 6children with low-levelMC in the RIC group
Chimerism stabilizing or improving after cyclosporin withdrawal in 6children with low-levelMC in the RIC group
We do not as yet have much experience with RIC using umbilical cord blood (UCB) as the source of stem cells. It is possible that the use of UCB could broaden access to an acceptable allogeneic donor and perhaps further reduce the incidence of acute GvHD. The limiting factor for older children may be the cell dose. Trials are being planned in pediatric hematologic malignancies to use 1 or 2 unrelated UCB units using RIC. Similar trials for PID would be very useful.
Patients receiving highly immunosuppressive RIC transplantation regimens may be prone to an increased incidence of viral infections including CMV20 and EBV.21 In our study, we likewise observed a high rate of viral reactivations in the RIC group compared with the MAT group; but with early detection and treatment, there were no deaths due to viral reactivations. The fact that many children had reactivation of more than one virus simultaneously emphasizes the extremely immunosuppressive nature of this protocol. Initially, we speculated that the use of alemtuzumab may cause fewer problems with EBV reactivation, but we did not find a significant difference in EBV incidence with ATG or alemtuzumab.22,23 It is very likely that the children who received rituximab for the treatment of EBV disease will have poor B-cell numbers and need IVIg replacement after transplantation for a longer period of time. Vigilant prospective monitoring and early treatment of infections are crucial to this protocol.
There is no published data comparing immune reconstitution in children with PID using different conditioning regimens. Overall, both groups of patients seem to have an apparently slower immune reconstitution than reported previously after MAT conditioning.24 This may be due to the fact that in our study, we have used age-specific normal ranges for T- and B-cell numbers, which may be a more accurate way of assessing immune recovery. At 2 years after transplantation, the percentage of children achieving T- and B-cell reconstitution is comparable with previous reports.9 Although data on the kinetics of immune reconstitution in immunodeficiency patients is lacking, the pace of T-cell reconstitution observed in our RIC patients was similar to that observed by Eyrich et al in a cohort of pediatric patients with nonimmunodeficiency disorders undergoing haploidentical transplantation.25 Immune reconstitution in the RIC group was comparable with that in the MAT group; although alemtuzumab 1H was used as serotherapy in the RIC group, which may be more immunosuppressive than Campath 1G used in the MAT group.26 More children in the RIC group remain on IVIg, and this may be, as discussed earlier, due to the use of rituximab in this group. However in those children in the RIC group who achieved normal B-cell numbers and normal immunoglobulin production, B-cell function as assessed by specific antibody responses to vaccinations was comparable with the MAT group. Vaccine responses after RIC conditioning have not been previously examined. All vaccinated children in this group had good responses to Haemophilus influenza and to tetanus, as did the children in the MAT group.
The longevity of immune recovery after RIC transplantations is dependent on successful engraftment of multipotent stem cell/progenitor HSC populations and can be accurately determined only by long-term analysis of immune function. Analysis of donor chimerism shows that the majority of patients have donor engraftment in multiple lineages, data that suggest that multipotent cells have been engrafted. Further, TREC frequency analysis on a subgroup of patients also shows that the majority has values comparable with healthy young donors,17 which demonstrates that prethymic lymphoid progenitors have been successfully engrafted in these patients. In 3 individuals, low TREC values correlate with poor immune recovery. Over time, these individuals are at risk of T-cell exhaustion and may require retransplantation or stem cell boosts.
Acute GvHD more than grade 2 has been noted to be an adverse prognostic factor for survival following PID transplantations.9 Previous groups have reported an incidence of acute GVHD in the RIC setting similar to that following conventional transplantation.11 Our patients had a low incidence of GvHD, reflecting our use of in vivo T-cell depletion using alemtuzumab/ATG.27 Similarly there was a low incidence of chronic GVHD, but extensive chronic GVHD was associated with poor prognosis as is well known.
In summary, despite the limitations of a retrospective, nonrandomized study, our study strongly suggests that RIC results in superior survival in children with PID undergoing SCT. Our regimen is well tolerated even in high-risk patients with preexisting organ dysfunction. GvHD and immune reconstitution are comparable with MAT transplantations. The highly immunosuppressive nature of the regimen results in a high incidence of viral reactivations. MC is frequently seen but is not associated with disease relapse. On the basis of these data, we recommend the use of RIC in patients with PID undergoing MUD SCT in non-SCID immunodeficiencies.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-03-0960.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank the Primary Immunodeficiency Association (PiA) and SPARKS for their support.