Abstract
Bcr-Abl–expressing primary or cultured leukemia cells display high levels of the antiapoptotic heat shock protein (hsp) 70 and are resistant to cytarabine (Ara-C), etoposide, or Apo-2L/TRAIL (TNF-related apoptosis-inducing ligand)–induced apoptosis. Conversely, a stable expression of the cDNA of hsp70 in the reverse orientation attenuated not only hsp70 but also signal transducers and activators of transcription 5 (STAT5) and Bcl-xL levels. This increased apoptosis induced by cytarabine, etoposide, or Apo-2L/TRAIL. Ectopic expression of hsp70 in HL-60 cells (HL-60/hsp70) inhibited Ara-C and etoposide-induced Bax conformation change and translocation to the mitochondria; attenuated the accumulation of cytochrome c, Smac, and Omi/HtrA2 in the cytosol; and inhibited the processing and activity of caspase-9 and caspase-3. Hsp70 was bound to death receptors 4 and 5 (DR4 and DR5) and inhibited Apo-2L/TRAIL-induced assembly and activity of the death-inducing signaling complex (DISC). HL-60/hsp70 cells exhibited increased levels and DNA binding activity of STAT5, which was associated with high levels of Pim-2 and Bcl-xL and resistance to apoptosis. Expression of the dominant negative (DN) STAT5 resensitized HL-60/hsp70 cells to cytarabine, etoposide, and Apo-2L/TRAIL–induced apoptosis. Collectively, these findings suggest that hsp70 inhibits apoptosis upstream and downstream of the mitochondria and is a promising therapeutic target for reversing drug-resistance in chronic myeloid leukemia-blast crisis and acute myeloid leukemia cells. (Blood. 2005;105:1246-1255)
Introduction
The deregulated activity of the tyrosine kinase (TK) encoded by the bcr-abl, a human fusion oncogene, is a hallmark of chronic myeloid leukemia (CML).1 Bcr-Abl contributes to the aggressive leukemia behavior of CML in blast crisis (CML-BC) and of acute lymphoblastic leukemia (ALL) cells.1,2 Endogenous or ectopic expression of Bcr-Abl in leukemia cells (as in K562 or HL-60/Bcr-Abl cells, respectively) induces resistance to apoptosis due to antileukemia drugs, for example, Ara-C and etoposide.3,4 Bcr-Abl also confers resistance against Apo-2L/TRAIL (TNF-related apoptosis-inducing ligand)–induced cytosolic accumulation of cytochrome c (cyt c), processing and activation of caspase-9 and -3, and apoptosis.5 Bcr-Abl activates diverse molecular mechanisms known to inhibit apoptosis in CML-BC and Bcr-Abl–positive ALL cells.6 For example, Bcr-Abl TK activity leads to the phosphorylation and increased transactivation by STAT-5 (signal transducer and activator of transcription 5), resulting in increased expression of the antiapoptotic Bcl-xL and Pim-2.7-9 Bcr-Abl TK activity also leads to the phosphorylation and activity of AKT, which results in increased phosphorylation of caspase-9, Bad, and Forkhead transcription factor, all contributing to the down-regulation of prodeath signaling in CML-BC and Bcr-Abl–positive ALL cells.10-12,16 Imatinib mesylate (IM, Gleevec) is a relatively specific, adenosine triphosphate (ATP)–binding site antagonist of Bcr-Abl.13 At clinically achievable concentrations (∼ 0.5 μM), IM inhibits Bcr-Abl TK activity and induces growth arrest and apoptosis of Bcr-Abl–positive HL-60/Bcr-Abl and Jurkat/Bcr-Abl (both with ectopic expression of Bcr-Abl) and LAMA-84 and K562 cells (with endogenous expression of Bcr-Abl).14-16 This was shown to be associated with down-regulation of Bcl-xL levels and AKT kinase activity.16 Cotreatment with IM also sensitizes HL60/Bcr-Abl and K562 cells to Ara-C, etoposide, or Apo-2L/TRAIL–induced apoptosis of HL-60/Bcr-Abl and K562 cells.5,16-18 Treatment with IM, which yields approximately 90% complete cytogenetic responses (CCRs) in the chronic phase of CML, induces CCR only in a minority of patients and produces only short-term complete hematologic and cytogenetic responses in CML-BC and ALL.19,20
Recent studies from our laboratory have shown that Bcr-Abl expression in acute leukemia cells is associated with increased expression of the heat shock protein 70 (hsp70).21 Hsp70 is one of the ATP-dependent molecular chaperones.22 These interact with a large number of non-native newly synthesized polypeptides and assist in their folding into native proteins, promote the formation of multiprotein complexes, and assist in the transport of proteins across cellular membranes.23,24 By binding to the surface-exposed hydrophobic regions, ATP-dependent chaperones prevent aggregation of proteins into insoluble nonfunctional inclusions. The misfolded, abnormal proteins due to mutations or errors in transcription or translation are either rescued by ATP-dependent chaperones or degraded by ATP-dependent proteases (eg, 26S proteasome) or undergo aggregation.23,24 Hsp70 is induced by cellular stress due to misfolded and denatured proteins. Facilitated by ATP binding and hydrolysis, hsp70 binds and releases small hydrophobic regions of misfolded proteins, thereby enabling the damaged proteins to refold into their native state.22-24 In normal nontransformed cells, the expression of hsp70 is low and largely stress-inducible.25 However, hsp70 is abundantly expressed in most cancer cells.25 Hsp70 has been shown to play an active role in oncogenic transformation, and turning off the hsp70 expression was shown to reverse the transformed phenotype of Rat-1 fibroblasts.26,27 Ectopic overexpression or induced endogenous levels of hsp70 potently inhibits apoptosis.28,29 Several reports have documented that hsp70 inhibits the mitochondrial pathway of apoptosis by blocking Apaf-1–mediated activation of caspases-9 and -3, as well as by repressing the activity of caspase-3.30-33 Additionally, hsp70 can also inhibit caspase-independent apoptosis by directly interacting with apoptosis-inducing factor (AIF), thereby preventing nuclear import and DNA fragmentation by AIF.34,35 Conversely, hsp70 depletion by antisense oligonucleotides or ectopic transfection and expression of a fragment of hsp70 DNA in the antisense orientation has been shown to induce apoptosis.36,37 Our observation that hsp70 is overexpressed in Bcr-Abl–positive leukemia cells raised the issue whether hsp70 plays a mechanistic role in mediating the antiapoptotic effects of Bcr-Abl. Present studies confirm that hsp70 contributes to Bcr-Abl–mediated resistance to apoptosis due to antileukemia agents, for example, Ara-C, etoposide, and Apo-2L/TRAIL. Our findings demonstrate that hsp70 interdicts apoptosis signaling upstream to mitochondria by inhibiting Bax conformational change and localization to mitochondria. Also, by up-regulating STAT5 levels and activity hsp70 induces Bcl-xL and Pim-2 levels, thereby augmenting resistance to apoptosis exerted at the level of the mitochondria. Additionally, present studies show that down-modulation of hsp70 and STAT5 can sensitize Bcr-Abl–expressing human leukemia cells to Ara-C, etoposide, and Apo-2L/TRAIL-induced apoptosis.
Materials and methods
Reagents and antibodies
Gleevec was a gift from Dr Elisabeth Buchdunger of Novartis Pharma AG (Basel, Switzerland). The recombinant homotrimeric form of Apo-2L/TRAIL was produced in E coli and was a gift from Genentech (San Francisco, CA).38 Ara-C and etoposide were purchased from Sigma Chemical (St Louis, MO) and prepared as a 10-mM stock solution in sterile phosphate-buffered saline (PBS) and diluted in RPMI 1640 medium before use. Anti-hsp70, anti–HSF-1, anti-hsp90, anti-hsp27, and anti-XIAP antibodies were purchased from StressGen Biotechnologies (Victoria, BC, Canada). Anti-Bid and anti-Smac/DIABLO antibodies were kindly provided by Dr Xiaodong Wang of the University of Texas, Southwestern School of Medicine (Dallas, TX).38 The monoclonal anticytochrome oxidase-2 antibody was purchased from Molecular Probe (Eugene, OR). Anti–Bcl-2 antibody was purchased from DAKO (Carpenteria, CA), the monoclonal anti–cyt c and anti–Bcl-xL was purchased from Pharmingen (San Diego, CA), and anti-Abl antibody was purchased from Santa Cruz (Santa Cruz, CA). Polyclonal anti-Omi antibody was kindly provided by Dr Emad Alnemri (Thomas Jefferson University, Philadelphia, PA). Monoclonal anti-oncostatin M antibody was purchased from R&D System (Minneapolis, MN). Polyclonal anti–poly-adenosine-diphosphate-ribose polymerase (PARP), caspase-9, anti–caspase-3, and monoclonal anti–caspase-8 antibody were purchased from Cell Signaling Technology (Beverly, MA). Polyclonal antisurvivin was purchased from Alpha Diagnostic (San Antonio, TX). Polyclonal anti-DR5 was purchased from Cayman Chemicals (Ann Arbor, MI). Polyclonal anti–STAT 5 antibody, anti-DR4 antibody, and goat polyclonal anti–Pim-2 and monoclonal anti–c-Myc antibodies were purchased from Santa Cruz Biotechnology. Monoclonal anti–p-STAT5 antibody was purchased from Upstate Biotechnology (Lake Placid, NY).
Cultured cells
Human AML HL-60/Neo, HL-60/Bcr-Abl, Jurkat/Neo, and Jurkat/Bcr-Abl, as well as mouse myeloid 32D/Neo and 32D/Bcr-Abl cells, were created and maintained in culture as previously described.16,21,39 K562 and LAMA-84 cells were passaged as previously reported.21 Logarithmically growing cells were used for the studies described below.
Creation and culture of HL-60/hsp70 and K562/hsp70AS cells
pcDNA3-hsp70 plasmid was kindly provided by Dr Richard Morimoto (Northwestern University, Urbana, IL). This construct was stably transfected into HL-60 cells, and the stable clones were selected, subcloned by limiting dilution, and maintained in 500 μg/mL of G418. To create the pcDNA3-hsp70AS construct, pcDNA3-hsp70 was used as a template for polymerase chain reaction (PCR) amplification using forward primer containing Xho1 restriction site (5-′CCC TCG AGA GGA TGC GGG TGT GAT CG-3′) and a reverse primer containing HindIII restriction site (5′ CCC AAG CTT CTT GGC GTC GCG CAG CAG AGA 3′).36 The PCR product was digested with XhoI and HindIII and subcloned into pcDNA3.1 vector. K562 cells were stably transfected with the pcDNA3-hsp70AS construct and maintained in 500 μg/mL of G418.
Leukemia blasts from patients with CML-BC
Leukemia blasts were procured from the peripheral blood of 4 patients who had met the clinical criteria of Ph chromosome–positive CML-BC. Sample 1 was from a patient with lymphoid blast crisis of CML, while samples 2, 3, and 4 were from patients with myeloid blast crisis of CML. Informed consents were signed by all patients to allow use of their cells for these experiments as part of a clinical protocol approved by the University of South Florida institutional review board. Bone marrow aspirate was obtained in heparinized tubes, and mononuclear cells (BMMCs) were used to isolate CD34+ leukemia blasts of 95% purity from BMMCs, as previously described.21
Preparation of S100 and Western analysis of cytosolic cytochrome c, Smac, and Omi
Western analyses of proteins
Western analyses of Bcl-2, Bcl-xL, Bcr-Abl, AKT, DR4, DR5, Apo-2L, Fas-associated death domain (FADD), caspase-8, c-FLIPL and FLIPS, BID, caspase-9, caspase-3, PARP, XIAP, c-Myc, oncostatin M, Pim-2, and β-actin were performed using specific antisera or monoclonal antibodies according to previously reported protocols.4,16,41
Bax conformation change analysis
Cells were lysed in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid lysis buffer containing 150 mM NaCl, 10 mM HEPES (pH 7.4) (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid] containing protease inhibitors. Immunoprecipitation was performed in lysis buffer by using 500 μg of total cell lysate and 2.5 μg of anti-Bax 6A7 monoclonal antibody (Sigma Chemical). The resulting immune complexes were subjected to immunoblotting analysis with anti-Bax polyclonal antibody, as described previously.42
Apo-2L/TRAIL–induced death-inducing signaling complex (DISC) analysis
Untreated or Apo-2L/TRAIL–treated HL-60/Neo or HL-60/hsp70 cells were suspended at a final concentration of 106 cells/mL in a prewarmed, complete RPMI media. Cells were treated with 200 ng/mL Apo-2L/TRAIL for 4 hours at 37°C, followed by washing with 1.0 mL of ice-cold PBS. Cells were lysed in 500 μL lysis buffer (25 mM Tris [tris(hydroxymethyl) aminomethane]-HCl, pH 7.2, 150 mM NaCl, 25 mM NaF, 1 mM benzamidine, 1.0% Triton X-100, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstin-A, and 0.1 μg/mL phenylmethylsulfonyl fluoride [PMSF]) for 30 minutes on ice.38,43 In the untreated controls, 200 ng/mL Apo-2L/TRAIL was added after lysis of cells to immunoprecipitate nonstimulated Apo-2L/TRAIL receptors. Five-hundred micrograms of the lysates were incubated at 4°C for 2 hours with 2 μg each of anti–Apo-2L/TRAIL receptor 1 and 2 (DR4 and DR5) antibodies. The immune complexes were incubated overnight at 4°C with 20 μL protein A-agarose beads (Roche, Indianapolis, IN). The beads were recovered by centrifugation and washed twice with the lysis buffer. The pellet was resuspended in the sample buffer, and immunoblot analysis using antibodies against hsp70, caspase-8, DR5, DR4, and FADD were performed.16,21
Autophosphorylation of Bcr-Abl
Cells were lysed in the lysis buffer, and the immunoprecipitates of Bcr-Abl were obtained by using anti-Abl monoclonal antibody, as previously described.16,21 Immunoprecipitates were examined by Western blot analysis with anti-phosphotyrosine antibody and anti-Abl antibody to control for equal loading.21
AKT kinase assay
AKT kinase activity was determined by using an immunoprecipitation-kinase assay with reagents provided in a commercially available kit (Cell Signaling).23 Briefly, AKT was immunoprecipitated from cell lysates, using a polyclonal anti-AKT antibody. Immunoprecipitates were then incubated with glycogen synthase kinase (GSK)-α/β fusion protein in the presence of ATP and the kinase buffer, allowing AKT to phosphorylate GSK-α/β, which was analyzed by Western blotting using an anti–phospho-GSK-3α/β (serine 21/9) antibody.16
Electrophoretic mobility shift analysis (EMSA) for STAT-5
A previously described method was employed.21 The cells were lysed in buffer A containing 10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), and 0.5 mM PMSF. Cell lysates were left on ice for 10 minutes and then centrifuged at 12 000g for 30 seconds. Pellets were resuspended in buffer A containing 0.05% NP40. After 20 strokes of B pestle-tight fit to release the nuclei, these were then pelleted by centrifugation at 12 000g for 10 minutes. Nuclei were resuspended in buffer C containing 5 mM HEPES, pH 7.9, 26% glycerol, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM DTT, 0.5 mM PMSF, and 0.3 M NaCl, and mixed well (∼ 10 strokes) and left on ice for 30 minutes. Finally, nuclear extracts were collected as the supernatant after centrifugation at 12 000g for 30 minutes at 4°C. The nuclear extracts (4 to 50 μg) were incubated with 2.0 μg of poly(dI-dC) for 15 minutes on ice, followed by 15 minutes of incubation with 1.0 ng Klenow-labeled DNA harboring the STAT-5 optimal double-stranded DNA binding sequence 5′-GATCCGAATTCCAGGAATTCA-3′. For supershift analysis, prior to the addition of poly(dI-dC) the nuclear extracts were incubated with anti–STAT-5 antibody (Santa Cruz) or mouse IgG as a control for 30 minutes on ice. DNA-protein complexes were separated on a 5% nondenaturing polyacrylamide gel in 0.25 × Tris borate EDTA (TBE) (1 × TBE = 50 mM Tris-borate, 1.0 mM EDTA) and analyzed by autoradiography.
Infection with recombinant vaccinia virus encoding the dominant negative STAT5
Recombinant vaccinia virus encoding dominant-negative STAT5 (mutation at the transactivation domain) was constructed using the pSP11 vector in recombination with the Western Reserve (WR) strain of vaccinia.44 As a control, vaccinia virus expressing CD56, a large granular lymphocyte-specific surface marker, were used. The procedure for generating vaccinia virus for infection has been previously described.44 Briefly, HL-60/hsp70 cells (5 × 106 cells per treatment group) were incubated with the vaccinia virus constructs for 2 hours at 37°C in the infection media (serum free) at a multiplicity of infection (MOI) of 6. Cells then were washed once with infection media and then incubated in 4 mL of the infection media for an additional 2 hours. The cells were washed again and further incubated in media containing 10% fetal bovine serum (FBS) with or without the Ara-C or etoposide for 24 hours prior to the assessment of cell lethality or obtaining total cell lysates for the immunoblot analyses.44
Apoptosis assessment by annexin-V staining
Morphology of apoptotic cells
After drug treatment, 50 × 103 cells were washed and resuspended in 1 × PBS (pH 7.4). Cytospin preparations of the cell suspensions were fixed and stained with Wright stain. Cell morphology was determined, and the percentage of apoptotic cells was calculated for each experiment, as described previously.16
RT-PCR assay for hsp70 or STAT5 mRNA levels
Total RNA was isolated from cells using a TRIZOL LS reagent (Invitrogen Carlsbad, CA). Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis was performed, as previously described.43 For the hsp70 PCR, the primer sequences were as follows: forward primer, 5′-CCTGATCGGCCGCAAGTTCG-3′; reverse primer, 5′-TGCCCCCGCCCAGGTCAAAGAT-3′. For the STAT5 PCR, the primer sequences were as follows: forward primer, 5′-CCCGGAACGCAACCTGTGGAACC-3′; reverse primer, 5′-GGGGCGAGAGGCGGGAGTCAAGA-3′. For β-actin, the forward primer was 5′-CTA CAA TGA GCT GCG TGT GG-3′, and the reverse primer was 3′-AAG GAA GGC TGG AAG AGT GC. The PCR products were separated on a 2% agarose/ethidium bromide gel. The size of the amplified hsp70 product was 394 bases pairs, the STAT5 product was 439 base pairs, and the β-actin product was 527 base pairs.
Statistical analysis
Significant differences between values obtained in a population of leukemic cells treated with different experimental conditions were determined using the Student t test.
Results
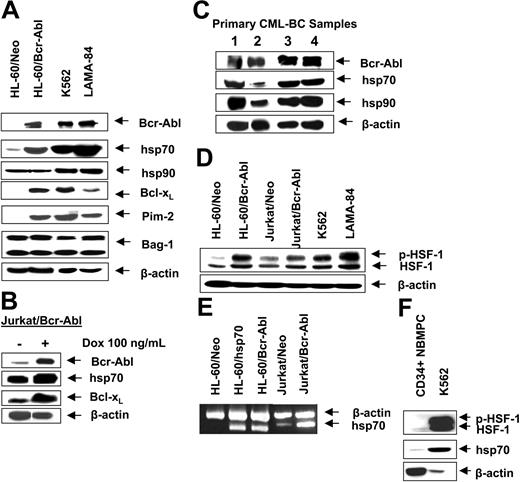
Bcr-Abl up-regulates p-HSF-1 and hsp70 levels in acute leukemia cells
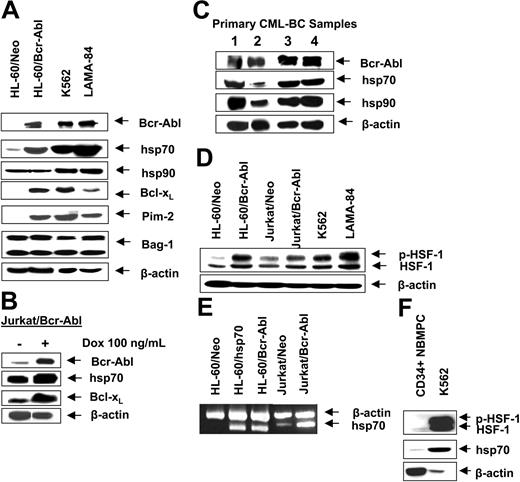
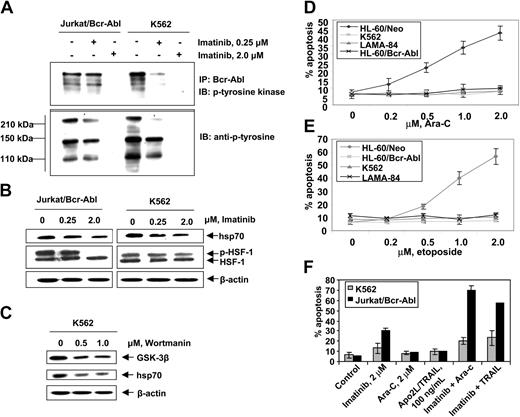
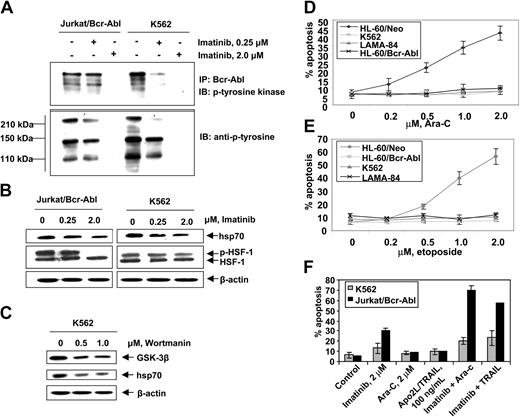
First, we confirmed that, as compared to the control (HL-60/Neo), HL-60/Bcr-Abl cells with ectopic expression of Bcr-Abl or K562 and LAMA-84 cells with the endogenous expression of Bcr-Abl possess markedly higher expression of hsp70 but not hsp90 (Figure 1A). BAG-1 is an antiapoptotic protein that interacts with Bcl-2.45 BAG-1 also is a cofactor of hsp70 and modulates its chaperone function.46 Increased levels of hsp70 did not significantly affect the levels of BAG-1 isoforms (depicted as the higher or lower molecular weight form, BAG-1L and BAG-1S, respectively) in HL-60/Bcr-Abl, K562, and LAMA-84 cells (Figure 1A). As previously reported, Bcr-Abl expression was associated with increased Bcl-xL levels (Figure 1A), without alterations in Bax levels (data not shown). In the T-cell leukemia Jurkat cells engineered to display tetracycline-inducible expression of Bcr-Abl, exposure to doxycycline for 48 hours was associated with not only the inducible expression of Bcr-Abl but also hsp70 and Bcl-xL (Figure 1B). As has been previously reported for a variety of other cancer cell types, hsp70 was expressed in all of the 4 primary CML-BC samples examined, albeit to a variable extent (Figure 1C). In human cells, expression of hsp70 is dependent on the phosphorylation, oligomerization, nuclear localization, and binding of heat shock factor 1 (HSF-1) to the heat shock elements present upstream of the hsp70 promoter.47,48 Consistent with this, higher levels of the phosphorylated form of HSF-1 (p-HSF-1) (Figure 1D), as well as more hsp70 mRNA transcript levels (Figure 1E), were detected in HL-60/Bcr-Abl, Jurkat/Bcr-Abl, K562, and LAMA-84, as compared to HL-60/Neo or Jurkat/Neo cells. Collectively, these studies suggest that Bcr-Abl TK activity is associated with increased mRNA and protein levels of hsp70, potentially through a mechanism that may involve increased activity of HSF-1. Conversely, as compared to the Bcr-Abl–expressing K562 cells, in a representative sample of CD34+ normal bone marrow progenitor cells (NBMPCs) p-HSF-1, HSF-1, and hsp70 levels were barely detectable (Figure 1F). Next, we determined whether inhibition of Bcr-Abl TK would inhibit p-HSF-1 and hsp70 levels. Figure 2A shows that exposure to 0.25 or 2.0 μM of imatinib mesylate for 24 hours in a dose-dependent manner inhibited the tyrosine phosphorylation of Bcr-Abl. It also inhibited Bcr-Abl–mediated tyrosine phosphorylation of other proteins in Jurkat/Bcr-Abl and K562 cells. This was associated with down-modulation of the levels of p-HSF-1 (serine phosphorylated form of HSF-1 detected by the immunoblot analysis) and hsp70 (Figure 2B). Although not shown, a similar effect of imatinib mesylate also was observed in HL-60/Bcr-Abl and LAMA-84 cells (data not shown). To evaluate whether the activity of PI3K/AKT was involved in mediating this, we determined the effect of wortmannin on hsp70 and p-HSF-1 levels. Figure 2C demonstrates that treatment with 0.5 or 1.0 μM wortmannin for 24 hours inhibited the activity of AKT, as determined by its ability to phosphorylate GSK-α/β (a substrate of AKT), as well as down-modulated the levels of hsp70 and p-HSF-1 (not shown) in K562 cells. A similar effect of wortmannin also was observed in LAMA-84 and HL-60/Bcr-Abl cells (data not shown).
Bcr-Abl expression is associated with higher expression of hsp70 in human leukemia cells. (A) Western analyses of Bcr-Abl, hsp70, hsp90, Bcl-xL, Pim-2, Bag-1, and Bcl-2 in cell lines with expression of Bcr-Abl (K562, LAMA-84, Jurkat/Bcr-abl, HL-60/Bcr-Abl) and cell lines without expression of Bcr-Abl (HL-60/Neo, Jurkat). β-actin levels were used as the loading control. (B) Western analyses of Bcr-Abl, hsp70, and Bcl-xL in Jurkat/Bcr-Abl cells with doxycycline-inducible expression of Bcr-Abl. β-actin levels were used as the loading control. (C) Protein expression of Bcr-Abl, hsp70, and hsp90 in four samples of primary CML-BC leukemia cells. Cell lysates were immunoblotted with anti–Bcr-Abl, hsp70, and hsp90 antibodies. β-actin levels were used as loading control. (D) Western analysis of the heat shock factor-1 (HSF-1) and p-HSF-1 in cell lines with expression of Bcr-Abl (K562, LAMA-84, Jurkat/Bcr-abl, HL-60/Bcr-Abl) and without expression of Bcr-Abl (HL-60Neo, Jurkat/Neo). β-actin levels serve as the loading control. (E) RT-PCR analysis of mRNA expression of hsp70 in HL-60/Neo, HL-60/hsp70 (with ectopic overexpression of hsp70), Jurkat/Neo Jurkat/Bcr-abl, and HL-60/Bcr-Abl cells.β-actin mRNA served as the control. (F) Western analysis of p-HSF-1, HSF-1, and hsp70 performed on the cell lysates obtained from a representative sample of primary normal bone marrow progenitor cells (NBMPCs). β-actin levels were used as a loading control.
Bcr-Abl expression is associated with higher expression of hsp70 in human leukemia cells. (A) Western analyses of Bcr-Abl, hsp70, hsp90, Bcl-xL, Pim-2, Bag-1, and Bcl-2 in cell lines with expression of Bcr-Abl (K562, LAMA-84, Jurkat/Bcr-abl, HL-60/Bcr-Abl) and cell lines without expression of Bcr-Abl (HL-60/Neo, Jurkat). β-actin levels were used as the loading control. (B) Western analyses of Bcr-Abl, hsp70, and Bcl-xL in Jurkat/Bcr-Abl cells with doxycycline-inducible expression of Bcr-Abl. β-actin levels were used as the loading control. (C) Protein expression of Bcr-Abl, hsp70, and hsp90 in four samples of primary CML-BC leukemia cells. Cell lysates were immunoblotted with anti–Bcr-Abl, hsp70, and hsp90 antibodies. β-actin levels were used as loading control. (D) Western analysis of the heat shock factor-1 (HSF-1) and p-HSF-1 in cell lines with expression of Bcr-Abl (K562, LAMA-84, Jurkat/Bcr-abl, HL-60/Bcr-Abl) and without expression of Bcr-Abl (HL-60Neo, Jurkat/Neo). β-actin levels serve as the loading control. (E) RT-PCR analysis of mRNA expression of hsp70 in HL-60/Neo, HL-60/hsp70 (with ectopic overexpression of hsp70), Jurkat/Neo Jurkat/Bcr-abl, and HL-60/Bcr-Abl cells.β-actin mRNA served as the control. (F) Western analysis of p-HSF-1, HSF-1, and hsp70 performed on the cell lysates obtained from a representative sample of primary normal bone marrow progenitor cells (NBMPCs). β-actin levels were used as a loading control.
Imatinib mesylate inhibits autophosphorylation of Bcr-Abl tyrosine kinase and depletes the levels of p-HSF-1 and hsp70 levels in K562 and Jurkat/Bcr-abl cells. (A) Jurkat/Bcr-Abl cells and K562 cells were treated with 0.25 μM or 2.0 μM imatinib for 24 hours. Cell lysates were collected and immunoprecipitated with Bcr-abl antibody and immunoblotted with anti–p-tyrosine antibody. (B) Following treatment with total cell lysates were immunoblotted with p-tyrosine, HSF-1, p-HSF-1, and hsp70 antibodies. (C) Following treatment with 0.5 or 1.0 μM wortmannin, AKT was immunoprecipitated from cell lysates and allowed to phosphorylate GSK-α/β, which was analyzed by Western blotting using an anti–phospho-GSK-3α/β (serine 21/9) antibody. Cell lysates also were immunoblotted with anti-hsp70 antibody. β-actin levels were used as loading control. (D, E) Indicated cell types were treated with Ara-C (D) and etoposide (E) at the indicated concentrations for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. Values represent the mean ± SE of 3 experiments. (F) K562 and Jurkat/Bcr-Abl cells were incubated with the indicated levels of imatinib and/or Ara-C or Apo-2L/TRAIL for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. Values represented by the bars indicate the mean ± SE of 3 experiments.
Imatinib mesylate inhibits autophosphorylation of Bcr-Abl tyrosine kinase and depletes the levels of p-HSF-1 and hsp70 levels in K562 and Jurkat/Bcr-abl cells. (A) Jurkat/Bcr-Abl cells and K562 cells were treated with 0.25 μM or 2.0 μM imatinib for 24 hours. Cell lysates were collected and immunoprecipitated with Bcr-abl antibody and immunoblotted with anti–p-tyrosine antibody. (B) Following treatment with total cell lysates were immunoblotted with p-tyrosine, HSF-1, p-HSF-1, and hsp70 antibodies. (C) Following treatment with 0.5 or 1.0 μM wortmannin, AKT was immunoprecipitated from cell lysates and allowed to phosphorylate GSK-α/β, which was analyzed by Western blotting using an anti–phospho-GSK-3α/β (serine 21/9) antibody. Cell lysates also were immunoblotted with anti-hsp70 antibody. β-actin levels were used as loading control. (D, E) Indicated cell types were treated with Ara-C (D) and etoposide (E) at the indicated concentrations for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. Values represent the mean ± SE of 3 experiments. (F) K562 and Jurkat/Bcr-Abl cells were incubated with the indicated levels of imatinib and/or Ara-C or Apo-2L/TRAIL for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. Values represented by the bars indicate the mean ± SE of 3 experiments.
Hsp70 confers resistance against cytarabine or etoposide-induced apoptosis of Bcr-Abl–expressing acute leukemia cells
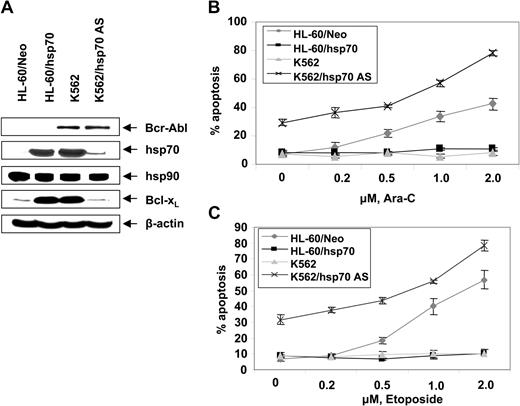
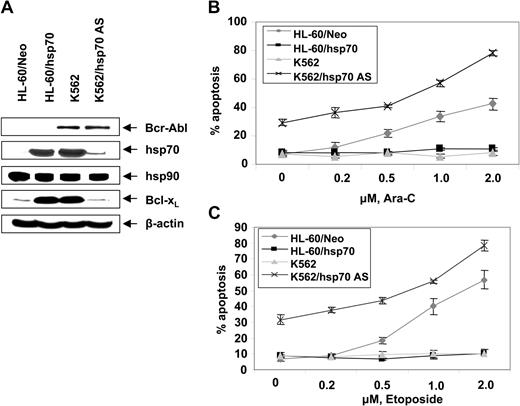
Figure 2D,E show that exposure to relatively high but clinically achievable levels of Ara-C or etoposide induced apoptosis of HL-60/Neo and Jurkat/Neo (not shown) cells in a dose-dependent manner. In contrast, Bcr-Abl–expressing HL-60/Bcr-Abl, K562, LAMA-84, and Jurkat/Bcr-Abl (not shown) that express high levels of hsp70 were markedly resistant to Ara-C or etoposide-induced apoptosis. Figure 2F demonstrates that treatment with imatinib (2 μM for 24 hours) alone induced more apoptosis of Jurkat/Bcr-Abl versus K562 cells. Additionally, cotreatment with imatinib-sensitized Jurkat/Bcr-Abl more than K562 cells to apoptosis induced by 2 μM Ara-C or 100 ng/mL Apo-2L/TRAIL for 24 hours (Figure 2F). These findings are consistent with greater decline in p-HSF-1 and hsp70 levels caused by imatinib mesylate treatment in Jurkat/Bcr-Abl versus K562 cells. Stable overexpression of hsp70 in HL-60/hsp70 cells did not increase hsp90 levels but resulted in marked increase in Bcl-xL (Figure 3A) and Pim-2 levels (vide infra), which are known to confer resistance to apoptosis.49,50 HL-60/hsp70 cells were resistant to Ara-C or etoposide-induced apoptosis (Figure 3B,C). The direct role of hsp70 in mediating Bcr-Abl–mediated resistance to apoptosis was confirmed by the finding that stable transfection of the cDNA of hsp70 in the reversed orientation resulted in significant restoration of the sensitivity to Ara-C or etoposide-induced apoptosis of K562/hsp70AS cells (Figure 3B,C). This was associated with reduction in Bcl-xL and Pim-2 levels in K562/hsp70AS cells (Figure 3A). As compared to K562, the untreated K562/hsp70AS cells also demonstrated significantly higher baseline levels of apoptosis (Figure 3B,C). In K562/hsp70AS cells the transfection of pcDNA-3-hsp70AS may not only abrogate hsp70-mediated resistance to apoptotic signaling but also trigger a modest level of apoptosis.
Ectopic expression of hsp70 cDNA in the reverse orientation depletes hsp70 levels and sensitizes CML-BC cells to Ara-C or etoposide-induced apoptosis. (A) Western analyses of the expression of hsp70, hsp90, Bcl-xL, Bcl-2, and XIAP in cells with high (K562, HL-60/hsp70) or low or depleted expression of hsp70 (HL-60, K562/hsp70 AS). (B, C) HL-60/Neo, HL-60/hsp70, K562, and K562/hsp70AS cell were treated with the indicated levels of Ara-C (B) or etoposide (C) for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining and flow cytometry. Values represent the mean ± SE of 3 experiments.
Ectopic expression of hsp70 cDNA in the reverse orientation depletes hsp70 levels and sensitizes CML-BC cells to Ara-C or etoposide-induced apoptosis. (A) Western analyses of the expression of hsp70, hsp90, Bcl-xL, Bcl-2, and XIAP in cells with high (K562, HL-60/hsp70) or low or depleted expression of hsp70 (HL-60, K562/hsp70 AS). (B, C) HL-60/Neo, HL-60/hsp70, K562, and K562/hsp70AS cell were treated with the indicated levels of Ara-C (B) or etoposide (C) for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining and flow cytometry. Values represent the mean ± SE of 3 experiments.
Additionally, K562/hsp70AS cells were more sensitive than HL-60/Neo cells to Ara-C or etoposide-induced apoptosis (Figure 3B,C).
Hsp70 inhibits Bax conformation change and mitochondria localization, thereby inhibiting release of pro-death molecules from mitochondria
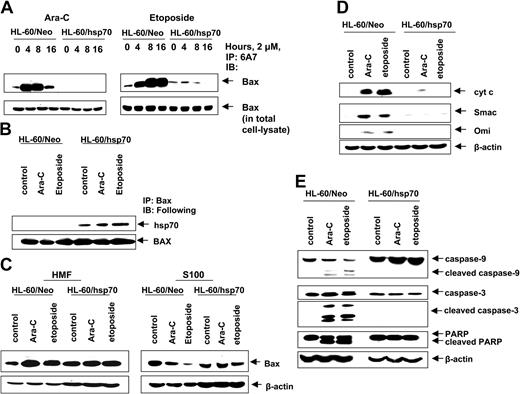
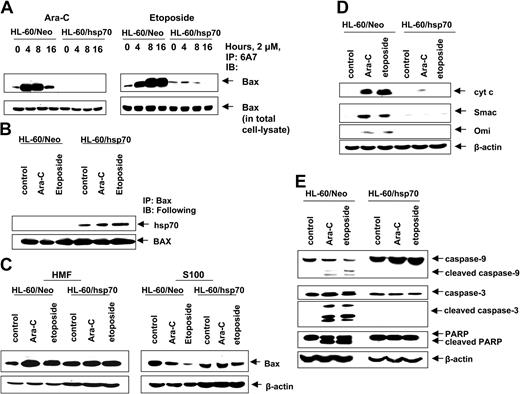
We next determined where hsp70 interdicts the pathway to apoptosis. In unperturbed cells the multi-BH domain proapoptotic molecule Bax is predominantly localized in the cytosol.50 Following exposure to an apoptotic stimulus, Bax undergoes a conformational change, leading to exposure of its N- and C-termini and to its mitochondrial targeting, which results in mitochondrial permeabilization and release of pro-death molecules cytochrome c, Smac, Omi, and AIF.50-52 Figure 4A demonstrates that in the control HL-60/Neo cells, treatment with Ara-C or etoposide induced Bax conformation change, detected by the 6A7 antibody, and subsequent localization of Bax from the cytosol (S100 fraction) to the mitochondria in the heavy membrane fraction (Figure 4C).42,53 In contrast, etoposide and Ara-C–induced Bax conformation change was markedly inhibited in hsp70 overexpressing HL-60/hsp70 cells (Figure 4A). Moreover, in HL-60/hsp70 cells, Bax could be coimmunoprecipitated with hsp70 (Figure 4B), which inhibited the localization of Bax from the cytosol to mitochondria, following treatment of HL-60/hsp70 cells with 2 μM Ara-C or etoposide (Figure 4C). These results are consistent with a recent report where hsp70 was shown to inhibit endoplasmic reticulum (ER) stress-induced apoptosis by binding to Bax and preventing its translocation to the mitochondria.54 Figure 4D,E demonstrates that in the control HL-60/Neo cells, treatment with 2 μM Ara-C or etoposide induced the release of the pro-death molecules cyt c, Omi, and Smac, which was associated with the processing of caspase-9 and -3, along with the PARP cleavage activity of caspase-3. In contrast, these events were not observed following treatment of HL-60/hsp70 cells with Ara-C or etoposide (Figure 4D,E).
Hsp70 binds to Bax, blocks Bax conformational change and its translocation to the mitochondria, and inhibits initiation of mitochondria pathway. (A) HL-60/Neo cells and HL-60/hsp70 cells were treated with 2.0 μM Ara-C or 2.0 μM etoposide for 4, 8, or 16 hours. After this treatment, cell lysates were first immunoprecipitated with the 6A7 antibody that detects the conformationally changed Bax, then immunoblotted with a polyclonal anti-Bax antibody. (B) HL-60/Neo cells and HL-60/hsp70 cells were treated with either 2.0 μM of Ara-C or etoposide for 8 hours. Following this, the cell lysates were immunoprecipitated with anti-Bax antibody and immunoblotted with either anti-hsp70 or anti-Bax antibody. (C) HL-60/Neo cells and HL-60/hsp70 cells were treated with 2.0 μM Ara-C or 2.0 μM of etoposide for 24 hours. Following this, cytosolic (S100) and heavy membrane fractions were obtained and immunoblotted with anti-Bax antibody. β-actin levels were used as loading control. (D) HL-60/Neo and HL60/hsp70 were treated with 2.0 μM of Ara-C or etoposide for 24 hours. Following this treatment, the S100 fractions were obtained from the cells and used for the immunoblot analysis of cyto c, Smac/DIABLO, and Omi. (E) Total cell lysates also were immunoblotted with antibodies to caspase-9, caspase-3, and PARP. β-actin levels were used as loading control.
Hsp70 binds to Bax, blocks Bax conformational change and its translocation to the mitochondria, and inhibits initiation of mitochondria pathway. (A) HL-60/Neo cells and HL-60/hsp70 cells were treated with 2.0 μM Ara-C or 2.0 μM etoposide for 4, 8, or 16 hours. After this treatment, cell lysates were first immunoprecipitated with the 6A7 antibody that detects the conformationally changed Bax, then immunoblotted with a polyclonal anti-Bax antibody. (B) HL-60/Neo cells and HL-60/hsp70 cells were treated with either 2.0 μM of Ara-C or etoposide for 8 hours. Following this, the cell lysates were immunoprecipitated with anti-Bax antibody and immunoblotted with either anti-hsp70 or anti-Bax antibody. (C) HL-60/Neo cells and HL-60/hsp70 cells were treated with 2.0 μM Ara-C or 2.0 μM of etoposide for 24 hours. Following this, cytosolic (S100) and heavy membrane fractions were obtained and immunoblotted with anti-Bax antibody. β-actin levels were used as loading control. (D) HL-60/Neo and HL60/hsp70 were treated with 2.0 μM of Ara-C or etoposide for 24 hours. Following this treatment, the S100 fractions were obtained from the cells and used for the immunoblot analysis of cyto c, Smac/DIABLO, and Omi. (E) Total cell lysates also were immunoblotted with antibodies to caspase-9, caspase-3, and PARP. β-actin levels were used as loading control.
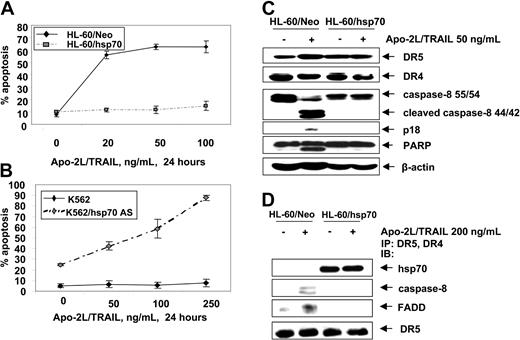
Hsp70 is incorporated into and inhibits Apo-2L/TRAIL–induced DISC and apoptosis
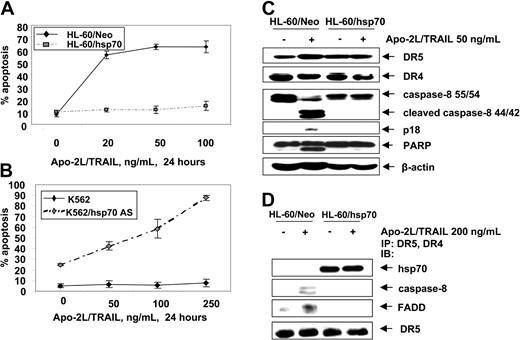
Next we determined whether hsp70 also would inhibit the extrinsic pathway of apoptosis triggered by Apo-2L/TRAIL. Figure 5A shows that, as compared to HL-60/Neo, HL-60/hsp70 cells were resistant to Apo-2L/TRAIL–induced apoptosis. Conversely, while K562 cells were resistant, K562/hsp70AS cells displayed restored sensitivity to Apo-2L/TRAIL–induced apoptosis (Figure 5B). Apo-2L/TRAIL–induced apoptosis of HL-60/Neo cells was associated with the processing of caspase-8 into 42/44-kDa and 18-kDa cleavage products, as well as with PARP-cleavage activity of the effector caspases (Figure 5C). Treatment with Apo-2L/TRAIL was unable to trigger these events in HL-60/hsp70 cells. Concomitantly, treatment of HL-60/Neo cells with Apo-2L/TRAIL resulted in the recruitment of FADD and caspase-8 to DR4 and DR5, which was associated with the processing of caspase-8 (Figure 5D). In contrast, in Apo-2L/TRAIL–treated HL-60/hsp70 cells, while hsp70 was present in the immunoprecipitates of DR4 and DR5, FADD and caspase-8 were absent, with concomitant lack of the processing of caspase-8 (Figure 5D). It is noteworthy that, in HL-60/Neo versus HL-60/hsp70 cells, DR5 and DR4 levels were neither different in the total cell lysates nor in Apo-2L/TRAIL–assembled DISC (Figure 5C,D). These findings suggest that hsp70 may inhibit Apo-2L/TRAIL–induced DISC assemblyand activity and resulting apoptosis.
Hsp70 blocks Apo-2L/TRAIL–induced apoptosis. (A) HL-60/Neo and HL-60/hsp70 cells were treated with Apo-2L/TRAIL at the indicated concentrations for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. Values represent the mean ± SE of 3 experiments. (B) K562 and K562/hsp70 AS cells were treated with Apo-2L/TRAIL at the indicated concentrations for 24 hours, and the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. (C) Cell lysates of untreated and Apo-2L/TRAIL–treated HL-60/Neo and HL-60/hsp70 cells also were immunoblotted with antibodies to DR4, DR5, caspase-8, FLIPL, and PARP. β-actin levels were used as loading control. (D) HL-60/Neo and HL-60/hsp70 cells were treated with Apo-2L/TRAIL at the indicated concentration for 4 hours. After this, from the cell lysates, immunoprecipitates with anti-DR5 and anti-DR4 antibodies were immunoblotted with anti-hsp70, anti–caspase-8, anti-FADD, or anti-DR5 antibody.
Hsp70 blocks Apo-2L/TRAIL–induced apoptosis. (A) HL-60/Neo and HL-60/hsp70 cells were treated with Apo-2L/TRAIL at the indicated concentrations for 24 hours. Following these treatments, the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. Values represent the mean ± SE of 3 experiments. (B) K562 and K562/hsp70 AS cells were treated with Apo-2L/TRAIL at the indicated concentrations for 24 hours, and the percentages of apoptotic cells were determined by annexin-V staining followed by flow cytometry. (C) Cell lysates of untreated and Apo-2L/TRAIL–treated HL-60/Neo and HL-60/hsp70 cells also were immunoblotted with antibodies to DR4, DR5, caspase-8, FLIPL, and PARP. β-actin levels were used as loading control. (D) HL-60/Neo and HL-60/hsp70 cells were treated with Apo-2L/TRAIL at the indicated concentration for 4 hours. After this, from the cell lysates, immunoprecipitates with anti-DR5 and anti-DR4 antibodies were immunoblotted with anti-hsp70, anti–caspase-8, anti-FADD, or anti-DR5 antibody.
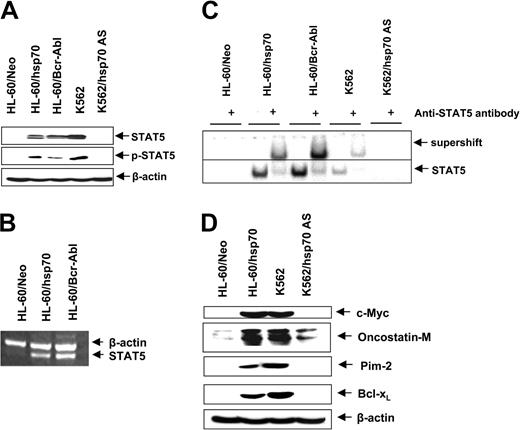
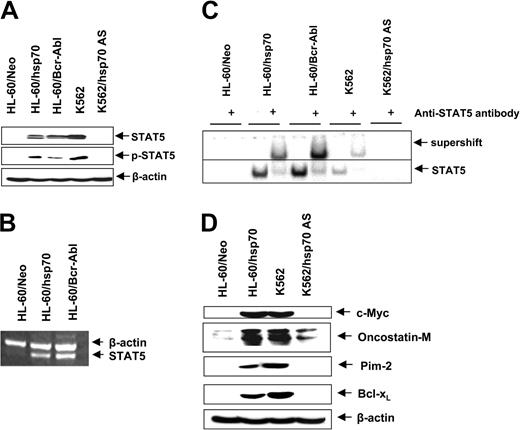
Ectopic expression of hsp70 and/or Bcr-Abl up-regulates STAT5 levels and activity
In HL-60 cells ectopic expression of hsp70, similar to Bcr-Abl (Figure 1A), resulted in increased levels of Bcl-xL and Pim-2 (Figures 3A and 6E). Since these genes are transactivated by STAT5, we determined the levels and activity of STAT5 in HL-60/Neo versus HL-60/hsp70 cells. Figure 6A,B demonstrates that, as compared to HL-60/Neo, HL-60/hsp70 cells expressed increased levels of STAT5 mRNA transcripts and p-STAT5. HL-60/hsp70 and HL60/Bcr-Abl also possessed increased DNA binding activity of STAT5, as determined by EMSA for STAT5 (Figure 6C). Conversely, as compared to K562, K562/hsp70AS cells displayed marked attenuation of STAT5 levels and DNA binding activity. In addition to Pim-2 and Bcl-xL, c-Myc and oncostatin M also are among the genes transactivated by STAT5.55,56 Figure 6D shows that compared to HL-60/Neo, increased STAT5 DNA binding activity in HL-60/hsp70 cells is also associated with increased levels of c-Myc and oncostatin M. In contrast, concomitantly with reduced STAT5 DNA binding activity, the expression levels of these genes are attenuated in K562/hsp70AS versus K562 cells (Figure 6D).
Increased hsp70 levels directly correlate with STAT5 levels and transcriptional activity. (A) Immunoblot analyses of total cell lysate from HL-60/Neo, HL-60/hsp70, HL-60/Bcr-abl, K562, and K562AS HSP70 cells, using anti-STAT5 or p-STAT5 antibodies. β-actin levels were used as loading control. (B) In the indicated cell types, the mRNA level of STAT5 was determined by reverse-transcription PCR, with β-actin mRNA as the control. (C) Nuclear extracts from the indicated cell types also were subjected to EMSA to estimate levels of STAT 5 DNA binding activity. Supershift of the STAT5 band was demonstrated by using anti-STAT5 antibody. (D) Immunoblot analyses of c-Myc, oncostatin-M, Bcl-xL, and β-actin in the cell lysates obtained from the indicated cell lines.
Increased hsp70 levels directly correlate with STAT5 levels and transcriptional activity. (A) Immunoblot analyses of total cell lysate from HL-60/Neo, HL-60/hsp70, HL-60/Bcr-abl, K562, and K562AS HSP70 cells, using anti-STAT5 or p-STAT5 antibodies. β-actin levels were used as loading control. (B) In the indicated cell types, the mRNA level of STAT5 was determined by reverse-transcription PCR, with β-actin mRNA as the control. (C) Nuclear extracts from the indicated cell types also were subjected to EMSA to estimate levels of STAT 5 DNA binding activity. Supershift of the STAT5 band was demonstrated by using anti-STAT5 antibody. (D) Immunoblot analyses of c-Myc, oncostatin-M, Bcl-xL, and β-actin in the cell lysates obtained from the indicated cell lines.
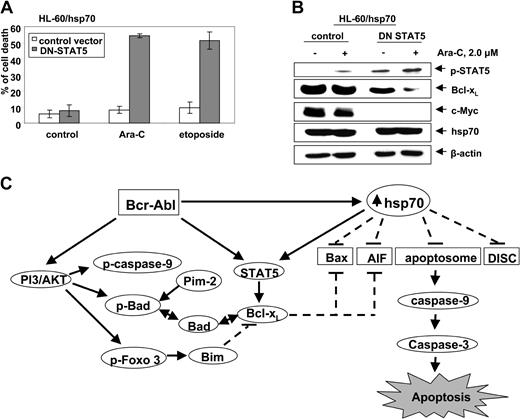
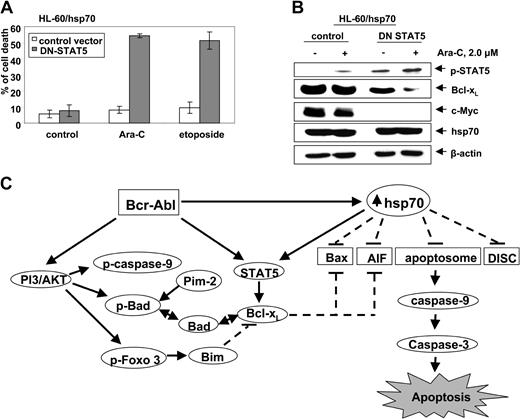
Abrogation of hsp70-induced STAT5 resensitizes hsp70 overexpressing HL-60 cells
To determine whether hsp70-induced STAT5 plays a mechanistic role in conferring resistance to apoptosis, we investigated the effect of abrogating STAT5 activity in HL-60/hsp70 cells. For this, HL-60/hsp70 cells were infected with the vaccinia virus encoding dominant-negative (DN) STAT5 (with mutation in the transactivation domain) or the control vaccinia virus, prior to further cotreatment with 2.0 μM Ara-C or etoposide for 24 hours. Following this, the percent of apoptotic cells was determined. Figure 7A demonstrates that HL-60/hsp70 cells infected with DN STAT5-containing virus but not with the control vaccinia virus were significantly resensitized to Ara-C or etoposide-induced apoptosis. HL-60/hsp70 cells infected with DN STAT5 also were resensitized to Apo-2L/TRAIL–induced apoptosis (data not shown). As compared to the control, HL-60/hsp70 cells infected with DN STAT5 containing vaccinia virus expressed higher levels of p-STAT5, as previously described,44 but lower levels of Bcl-xL and c-Myc (Figure 7B). Although in HL-60/hsp70 cells the ectopically expressed DN STAT5 is inactive as a transcription factor, it can be phosphorylated and detected as increased levels of p-STAT5 (Figure 7B). The expression of hsp70 was similar in the control cells as compared to the cells infected with the vaccinia virus containing DN STAT5. It is noteworthy that treatment with Ara-C further attenuated Bcl-xL levels in HL-60/hsp70 cells infected with DN STAT5 without affecting the pSTAT5 or hsp70 levels (Figure 7B).
Infection with dominant negative (DN) STAT 5 sensitizes HL-60/hsp70 cells to Ara-C and etoposide-induced cell death. (A) HL-60/hsp70 cells were incubated with the media containing the vaccinia virus encoding DN-STAT5 cDNA for 4 hours (the mutant DN-STAT5 construct is phosphorylated and able to bind STAT5 DNA binding elements). Following this, cells were washed and exposed to Ara-C or etoposide for 24 hours. Subsequently, the control cells (infected with the control vaccinia virus or DN-STAT5 containing vaccinia virus) and the drug-treated cells were harvested to determine percent of dead cells (PI uptake by flow cytometry). Values represent the mean ± SE of 3 experiments. (B) Total cell lysates from the HL-60/hsp70 cells treated as above with the control virus or DN-STAT5 containing vaccinia virus followed by Ara-C were immunoblotted with antibodies specific for p-STAT5, Bcl-xL, c-Myc, and hsp70. β-actin levels were used as loading control. (C) Molecular consequence of Bcr-Abl–mediated up-regulation of the levels and/or activity of AKT, STAT5, and hsp70 on the apoptotic signaling through the Apo-2L/TRAIL–induced DISC or the “apoptosome” downstream of the mitochondria.
Infection with dominant negative (DN) STAT 5 sensitizes HL-60/hsp70 cells to Ara-C and etoposide-induced cell death. (A) HL-60/hsp70 cells were incubated with the media containing the vaccinia virus encoding DN-STAT5 cDNA for 4 hours (the mutant DN-STAT5 construct is phosphorylated and able to bind STAT5 DNA binding elements). Following this, cells were washed and exposed to Ara-C or etoposide for 24 hours. Subsequently, the control cells (infected with the control vaccinia virus or DN-STAT5 containing vaccinia virus) and the drug-treated cells were harvested to determine percent of dead cells (PI uptake by flow cytometry). Values represent the mean ± SE of 3 experiments. (B) Total cell lysates from the HL-60/hsp70 cells treated as above with the control virus or DN-STAT5 containing vaccinia virus followed by Ara-C were immunoblotted with antibodies specific for p-STAT5, Bcl-xL, c-Myc, and hsp70. β-actin levels were used as loading control. (C) Molecular consequence of Bcr-Abl–mediated up-regulation of the levels and/or activity of AKT, STAT5, and hsp70 on the apoptotic signaling through the Apo-2L/TRAIL–induced DISC or the “apoptosome” downstream of the mitochondria.
Discussion
Previous reports have clearly established that ectopic or endogenous expression of Bcr-Abl results in increased phosphorylation and activity of AKT, a potent mediator of antiapoptosis signaling (Figure 7C). AKT phosphorylates and inactivates Bad, caspase-9, and Forkhead transcription factor.10-12 Bcr-Abl–expressing leukemia cells also show increased phosphorylation and activity of STAT5, which results in increased levels of Bcl-xL and confers resistance to apoptosis due to antileukemia agents, for example,Ara-C, etoposide, and Apo-2L/TRAIL.4,5,7,8 Present studies demonstrate for the first time that ectopic or endogenous expression of Bcr-Abl also results in up-regulation of the antiapoptotic hsp70, which in turn induced the levels and activity of STAT5, as well as interfered with apoptosis signaling at multiple levels in human leukemia cells (Figure 7C). Conversely, attenuation of hsp70 levels down-regulated STAT5 levels and activity, as well as sensitized K562 cells to Ara-C, etoposide, or Apo-2L/TRAIL–induced apoptosis. Collectively, these findings demonstrate that the increased hsp70 levels contribute to the resistance to apoptosis in Bcr-Abl–expressing human leukemia cells.
Our findings also indicate that Bcr-Abl–expressing acute leukemia cells exhibited increased levels of serine-phosphorylated p-HSF-1, the main heat shock factor responsible for the induction of hsp70 in human cells.28 Under physiologic stress, including heat shock, the serine phosphorylated and monomeric HSF-1 is translocated into the nucleus.47,48,57 Here, HSF-1 forms trimers, is hyperphosphorylated, binds to heat shock elements, and transactivates hsp70.57 Therefore, Bcr-Abl expression leads to increased mRNA and protein levels of hsp70, which significantly contributes to the resistance to apoptosis observed in Bcr-Abl–positive CML-BC cells.28 Notably, and consistent with this, inhibition of Bcr-Abl tyrosine kinase activity with imatinib resulted in attenuation of p-HSF and hsp70 levels. This also was seen when phosphoinositide 3-kinase (PI3K) and resulting AKT activities were inhibited by treatment with wortmannin, an inhibitor of PI3K. Under physiologic and stress conditions, multiple protein kinases are likely to be involved in phosphorylating HSF-1, which may positively or negatively affect its nuclear localization and transactivation of heat shock proteins.28,57 Our findings, however, do not establish a direct mechanistic link between Bcr-Abl and the downstream PI3K/AKT, and the phosphorylation of HSF and induction of hsp70 in Bcr-Abl–expressing acute leukemia cells. Such a relationship remains to be established.
Previous reports had highlighted the direct interaction of hsp70 with Apaf-1, leading to inhibition of Apaf-1–mediated “apoptosome,” as well as repression of caspase-3.31,32 Indeed, hsp70 also was shown to inhibit apoptosis due to enforced expression of caspase-3.33 These reports clearly established the antiapoptotic function of hsp70 at or downstream of the Apaf-1–assembled “apoptosome.” In contrast, present studies in human leukemia cells show for the first time that, following treatment with antileukemia agents, hsp70 binds to Bax and inhibits its conformation change and mitochondrial localization, thereby inhibiting the initiation of the mitochondrial pathway of apoptosis. This conclusion is further supported by the observation that Ara-C or etoposide-induced mitochondrial release and cytosolic accumulation of the pro-death molecules cyt c, Omi, and Smac were blocked in HL-60/hsp70 but not in HL-60/Neo cells. These observations are consistent with a recent report that in RAW 264.7 macrophages, hsp70 binds to Bax and prevents nitric oxide–induced apoptosis.54 Taken together with present findings, these reports highlight that hsp70 inhibits caspase-dependent apoptosis in human leukemia cells. However, it is noteworthy that hsp70 also has been shown to bind AIF and abrogate the execution of the noncaspase-dependent nuclear DNA fragmentation triggered by the release of AIF from the mitochondria.34,35
Present findings also show that hsp70 inhibits the assembly and activity of the Apo-2L/TRAIL–induced DISC and apoptosis. The recruitment of hsp70 to DR4 and DR5 in cells treated with Apo-2L/TRAIL inhibited the recruitment of FADD and caspase-8. This inhibited Apo-2L/TRAIL–induced processing and activity of caspase-8 that results in the generation of the truncated tBid in the type II and the direct activation of caspase-3 in type I cells.38,58,59 Therefore, high hsp70 levels can block apoptosis triggered by Apo-2L/TRAIL treatment both upstream and downstream of the mitochondria. Recent reports have shown that TNFα–mediated activation of JNK (c-Jun N-terminus kinase) induces caspase-8–independent cleavage of Bid into jBid at a distinct site, which results in the translocation of jBid to the mitochondria with the selective release of Smac from the mitochondria into the cytosol.60 Although overexpression of hsp70 inhibits JNK activation and Bid cleavage, JNK inhibition has been shown to be insufficient for the antiapoptotic function of hsp70.30,61,62 Apoptosis signal-regulating kinase1 (ASK1) is a serine threonine kinase, which functions in apoptosis induced by TNFα and agonistic antibody to Fas.63 Hsp70 also has been shown to inhibit the homo-oligomerization of ASK1 and ASK1-dependent apoptosis.64 Conversely, antisense oligonucleotides to hsp70 were demonstrated to de-repress JNK and ASK1-induced apoptosis.61,64 However, the role of JNK and ASK1 in regulating Apo-2L/TRAIL–induced apoptosis has not been confirmed. Regardless, present studies clearly show that attenuation of hsp70 levels sensitized human acute leukemia cells to Apo-2L/TRAIL–induced apoptosis.
While Bcr-Abl expression is known to be associated with increased phosphorylation and activity of STAT5, present studies show for the first time that ectopic expression of hsp70 in HL-60 cells results in increased levels and DNA binding and transcriptional activity of STAT5. This is associated with up-regulation of the genes known to be transactivated by STAT5, including c-Myc, oncostatin M, Bcl-xL, and Pim-2.56 A recent report has demonstrated that the serine threonine kinase Pim-2 is a potent antiapoptotic protein whose substrates include Bad and 4EBP-1.49 Ectopic and increased expression of Pim-2 confers a prolonged and superior survival advantage than Bcl-xL in circumstances where apoptosis is induced by cytokine withdrawal.49 Therefore, by inducing Bad phosphorylation, Pim-2 would reduce Bad-mediated antagonism of Bcl-xL, thereby enhancing the antiapoptotic effects of
Prepublished online as Blood First Edition Paper, September 23, 2004; DOI 10.1182/blood-2004-05-2041.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.