Abstract
The Notch signaling pathway plays a key role at several stages of T-lymphocyte differentiation. However, it remained unclear whether signals induced by the Notch ligand Delta-like 1 could support full T-cell differentiation from a defined source of human hematopoietic stem cells (HSCs) in vitro. Here, we show that human cord blood–derived HSCs cultured on Delta-like 1–expressing OP9 stromal cells undergo efficient T-cell lineage commitment and sustained T-cell differentiation. A normal stage-specific program of T-cell development was observed, including the generation of CD4 and CD8 αβ–T-cell receptor (TCR)–bearing cells. Induction of T-cell differentiation was dependent on the expression of Delta-like 1 by the OP9 cells. Stimulation of the in vitro–differentiated T cells by TCR engagement induced the expression of T-cell activation markers and costimulatory receptors. These results establish an efficient in vitro coculture system for the generation of T cells from human HSCs, providing a new avenue for the study of early T-cell differentiation and function.
Introduction
Hematopoietic stem cells (HSCs), which give rise to erythroid, myeloid, and lymphoid lineages, can be identified on the basis of the expression of CD34 and the absence of lineage-specific markers (termed Lin—).1 Human umbilical cord blood (CB)–derived cells provide a rich source of HSCs, which are comparable to bone marrow–derived HSCs.2-11 T cells of the αβ lineage differentiate in the thymus via discrete developmentally regulated steps that involve a series of commitment events and developmental checkpoints including T-cell receptor (TCR) V(D)J rearrangement, TCR-β-selection, and positive/negative selection of developing thymocytes.12 The earliest intrathymic progenitors express high levels of CD34 and CD7, do not express CD1a, and are triplenegative (TN) for mature T-cell markers CD4, CD8, and CD3.8 Commitment to the T-cell lineage is strongly associated with the expression of CD1a by CD7-expressing thymocytes.12,13
Several studies have implicated the Notch pathway in promoting HSC expansion, self-renewal,14 survival,15,16 and the induction of T-cell–lineage commitment.15,17-21 In humans there are 4 Notch receptors,22-26 which can pair with 2 serrate-like ligands (Jagged 1 and 2)27,28 or 3 Delta-like ligands (Dll1, 3, and 4).29,30 Notch signaling acts at multiple stages during T-cell differentiation, influencing the choice to become an αβ-versus γδ-T cell,31-34 as well as the decision to become a CD4 versus CD8 T cell.35-37 The strongest evidence for the role of Notch signaling in T-cell development comes from gain-of-function and loss-of-function studies,17,20,38-42 in which signaling through Notch-1 was shown to play a crucial role in determining B-versus T-cell–lineage choice.20,21
HSCs express multiple Notch receptors,22,43 but the expression patterns of the various Notch ligands have been reported to be distinct between bone marrow stromal cells29,44-47 and thymic epithelial cells.48 Taken together, these results suggest that different Notch receptors and ligands may control different aspects of hematopoiesis, depending on the microenvironment, allowing for self-renewal in the bone marrow and influencing cell fate decisions in the thymus.46
This led us to hypothesize that bone marrow stromal lines, such as OP9 cells,49 that support B-cell differentiation may do so because the appropriate Notch ligand to induce T-cell commitment and differentiation is absent. We recently demonstrated that the OP9 bone marrow stromal cell line, which does not express the Delta-like 1 (Dll1) Notch ligand, when retrovirally transduced to express Dll1 (OP9-DL1), inhibited the development of B cells and rather favored the development of T cells from fetal liver–derived HSCs50 or mouse embryonic stem (ES) cells.51 Given the high level of homology between mouse and human Dll1 molecules and the observation that mouse stromal lines can support the differentiation of human HSCs,29,52-54 we sought to determine whether human CB-derived HSCs (CD34+CD38–) cultured on OP9-DL1 cells could initiate and support T-cell differentiation in vitro.
Here, we show that human CD34+CD38– HSCs cultured on OP9-DL1 cells rapidly express CD38 and initiate lymphocyte differentiation, as compared to HSCs cultured on OP9-control cells. Strikingly, HSCs cultured on OP9-DL1 cells display many of the early differentiation steps associated with normal human T-cell differentiation, including the sustained generation of pre-T CD7+CD1a+ cells as well as the efficient generation of CD4+ CD8+ double-positive (DP) αβ–T-lineage cells. Furthermore, stimulation of in vitro–generated T cells with anti-CD3 plus anti-CD28 induced the expression of CD69 and CD27 and led to the up-regulation of the CD28 and CTLA-4 costimulatory receptors. These results establish an efficient and versatile in vitro system for the generation of large numbers of human T-lineage cells from CB-HSCs and offer a model system to investigate the factors that influence human T-cell–lineage commitment and differentiation.
Materials and methods
OP9 cells
Generation of the mouse OP9 stromal cells engineered to express the green fluorescent protein (GFP) alone (OP9-control) or GFP and the mouse Delta-like 1 gene (OP9-DL1) has been described previously.50
Cord blood cells: collection, cryopreservation, and separation
Human cord blood samples (approximately 50 mL/sample) were obtained by syringe extraction and collected in heparinized tubes from consenting mothers following birth on the delivery floor of the Women's College Ambulatory Care Centre in accordance to the guidelines established by the Research Ethics Board of Sunnybrook and Women's Health Sciences Center (Toronto, ON, Canada). Within 6 hours of collection, cord blood was separated by Ficoll-Hypaque (1.070 g/cm3) density gradient centrifugation into a low-density (< 1.070 g/cm3) mononuclear cell fraction, which was subsequently washed 3 times with Hanks balanced salt solution (HBSS) (Sigma, St Louis, MO) and then frozen in 10% dimethyl sulfoxide (DMSO) and 90% fetal bovine serum (FBS) for later use. Debulked frozen CB was thawed and washed twice with HBSS, and human hematopoietic progenitors were enriched using the StemSep human CD34-positive selection cocktail (Stem Cell Technologies, Vancouver, BC, Canada) and then separated into CD34+ and CD34– fractions on an AutoMACS machine (Miltenyi Biotec, Auburn, CA). The CD34-positive fraction underwent a second passage on the AutoMACS, and aliquots of both the positive and negative fractions were saved to determine fold enrichment. Human CD34+ cells underwent a more than 70 × enrichment from presorted to post-CD34+ fraction (1.2% +/– 3.8% to 85.4% +/– 12.6%, respectively). To further isolate the HSC-containing fraction from AutoMACS-sorted CB samples, the CD34-positive fraction was blocked with anti–human CD32 (FcRII) antibody (Stem Cell Technologies) and stained with allophycocyanin (APC)–conjugated anti-CD38 (clone HIT2) and subsequently sorted for CD34+CD38– cells using a Coulter Elite cytometer (Hialeah, FL). Sorted cells were more than 99% pure, as determined by postsort analysis.
Human hematopoietic stem cells and OP9 stromal cell coculture
The 10 × 103, 5 × 103, or 2.5 × 103 sorted human hematopoietic stem cells (CD34+CD38–) were added per individual well of a 6-well plate confluent with either OP9-DL1 cells or OP9-control cells in α-minimal essential medium (αMEM) media supplemented with 20% coculturecharacterized FBS plus 50 U/mL penicillin and 50 μg/mL streptomycin. Recombinant human cytokines Flt-3L (5 ng/mL) and interleukin-7 (IL-7) (5 ng/mL) (Peprotech, Rocky Hill, NJ) were added at the initiation of coculture and every 2 days during media changes. Cocultures were disaggregated by vigorous pipetting and passaged through a 70-μm filter to reduce stromal cell line aggregates and eliminate contaminating OP9 cells at the time points indicated. Each experiment was repeated at least 3 times, with results similar to those presented in Figure 1A.
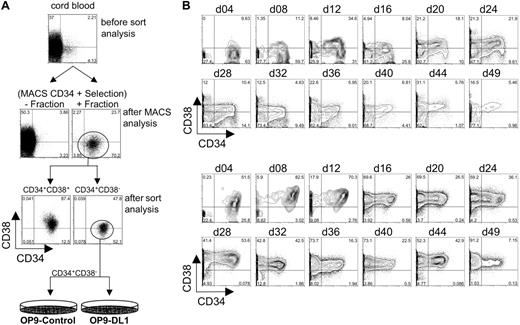
Flow cytometric analysis of CD34 and CD38 expression following isolation from human cord blood and long-term coculture with OP9-control cells or OP9-DL1 cells. (A) Human HSCs were isolated into CD34+ and CD34– fractions from human cord blood following a CD34-positive selection protocol on AutoMACS. As a reference for fold enrichment, we show CD34 by CD38 expression on Ficolled cord blood before and after AutoMACS enrichment. The CD34+ fraction obtained following AutoMACS was then sorted for the expression of CD34+CD38+ and CD34+CD38– cells. The CD34+CD38– cell population was then seeded onto OP9-control cells or OP9-DL1 cells. (B) CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (top 2 rows of histograms) or OP9-DL1 (bottom 2 rows of histograms) stromal cells for 49 days. Initiation of differentiation and maintenance of hematopoietic potential was evaluated approximately every 4 days of coculture through the loss and gain of CD34 and CD38 expression, respectively.
Flow cytometric analysis of CD34 and CD38 expression following isolation from human cord blood and long-term coculture with OP9-control cells or OP9-DL1 cells. (A) Human HSCs were isolated into CD34+ and CD34– fractions from human cord blood following a CD34-positive selection protocol on AutoMACS. As a reference for fold enrichment, we show CD34 by CD38 expression on Ficolled cord blood before and after AutoMACS enrichment. The CD34+ fraction obtained following AutoMACS was then sorted for the expression of CD34+CD38+ and CD34+CD38– cells. The CD34+CD38– cell population was then seeded onto OP9-control cells or OP9-DL1 cells. (B) CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (top 2 rows of histograms) or OP9-DL1 (bottom 2 rows of histograms) stromal cells for 49 days. Initiation of differentiation and maintenance of hematopoietic potential was evaluated approximately every 4 days of coculture through the loss and gain of CD34 and CD38 expression, respectively.
Human T-cell activation
Experiments to induce T-cell activation markers and costimulatory receptors were carried out on day 52 of coculture. Briefly, cocultures maintained with recombinant human Flt-3L and IL-7 were treated with exogenous human IL-2 (6 ng/mL) (Peprotech) and stimulated with anti-CD3 (1 μg/mL) plus anti-CD28 (1 μg/mL) monoclonal antibodies (mAbs) for 5 days. The expression of T-cell activation markers and costimulatory receptors was analyzed before stimulation and 2 and 5 days after stimulation. Each experiment was repeated at least 3 times with similar results.
Flow cytometry
Fluorescein isothiocyanate (FITC)–, R-phycoerythrin (PE)–, allophycocyanin (APC)–, and biotin-conjugated antibodies and streptavidin-allophycocyanin (SAv-APC) were obtained from PharMingen, BD-Biosciences (San Diego, CA). They include FITC-conjugated: anti–major histocompatibility complex II (MH-CII) (clone Tü39), anti-CD8 (clone RPA-T8), anti-TCRαβ (clone T10B9.1A-31), anti-TCRγδ (clone B1.1), anti-CD33 (clone HIM3-4), anti-CD27(M-T271), and anti-CD45 (clone H130); PE-conjugated: anti-CD34 (clone 581), anti-CD4 (clone RPA-T4), anti-CD7 (clone M-T701), anti-CD19 (clone H1B19); APC-conjugated: anti-CD38 (clone HIT2), anti-CD40 (clone 5C3), anti-CD3 (clone UCHT1), anti-CD1a (clone HI149), anti-CD14 (clone M5E2), anti-CD19 (clone 1D3), anti-CD69 (FN50), anti-Vβ3 (clone JOVI.3), anti-Vβ5 (clone MH3.2), anti-Vβ8 (clone JR2), anti-Vβ23 (clone AHUT7), anti–mouse IgG2a, κ (G155-178), anti–mouse IgG2b, κ (G155-178), anti–mouse IgG1, κ (MOPC-21); and biotin-conjugated: anti-CD3 (clone UCHT1), anti-CD28 (clone CD28.2), anti-CD152 (clone BNI3.1), anti-IgM (clone G20-127). Cell suspensions obtained from cocultures were FcRII blocked, stained in the dark in 50 μL fluorescence activated cell sorter (FACS) buffer (HBSS without phenol red, plus 1% bovine serum albumin [BSA], and 0.05% NaN3) for 20 minutes on ice and washed twice before analysis. Stained cells were analyzed with a FACSCalibur flow cytometer (BD-Biosciences) using FlowJo software (Tree Star, Ashland, OR); data were live-gated by forward/side light scatter and lack of propidium iodide uptake. GFP-expressing OP9 stromal cells were gated out of the analysis through GFP expression and side scatter exclusion. This procedure eliminated 99% of contaminating GFP-expressing OP9 stromal cells. Frequencies in quadrant corners are given as percent of gated cells.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was isolated using the Trizol reagent (Invitrogen, Burlington, ON). Random hexamer-primed cDNA was generated with the Omniscript RT kit (Qiagen, Mississauga, ON). All PCR reactions were carried out using the same serially diluted cDNA samples normalized to a β-actin–specific signal. Gene-specific primer sequences were as follows: (5′ → 3′): human β-actin (forward primer [F]) AAG ACC TGT ACG CCA ACA CAG T, (reverse primer [R]) AGA AGC ATT TGC GGT GGA CGA T; human Deltex-1 (F) TGT GCC GCA AGA CCA AGA AGA A, (R) TGC CAT CCT TGT TGC CAT TGG A; and human HES-1 (F) AAG ATA GCT CGC GGC ATT CCA A, (R) AGG TCA TGG CAT TGA TCT GGG T. PCR products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. All PCR products shown correspond to the expected molecular size.
Results
Initiation and maintenance of hematopoietic differentiation from human CB-HSCs cultured on OP9-DL1 cells
To determine the potential of human HSCs to differentiate into lymphocytes in vitro, CB samples were obtained from consenting mothers, and HSCs were isolated by magnetic-assisted cell sorting for the expression of CD34, followed by a secondary purification step by flow cytometric cell sorting for the expression of CD34 and the absence of CD38 expression (Figure 1A). This approach typically yielded more than 99% pure CD34+CD38– cells. CD34+CD38– cells were cultured on OP9-control cells or OP9 cells retrovirally transduced to express the mouse Delta-like 1 gene (OP9-DL1). The differentiation of HSCs can be temporally followed by the loss of CD34 expression and the gain of CD38 expression, which typically coincides with the expression of lineage-specific markers. To this end, the expression of CD34 and CD38 was followed over 7 weeks of culture (Figure 1B)
Our findings show that CD34+CD38– cells cultured on OP9-control cells did not readily differentiate into CD34+CD38+ cells during the first 8 days of culture (Figure 1B), although by day 12 of culture, a modest population (∼ 35%) of CD34+CD38+ cells was present. In contrast, CD34+CD38– cells cocultured with OP9-DL1 cells rapidly differentiated into CD34+CD38+ cells during the first 4 days of culture. In particular, a clear population of approximately 50% CD34+CD38+ cells were present on day 4, and this population increased to approximately 80% by day 8 (Figure 1B).
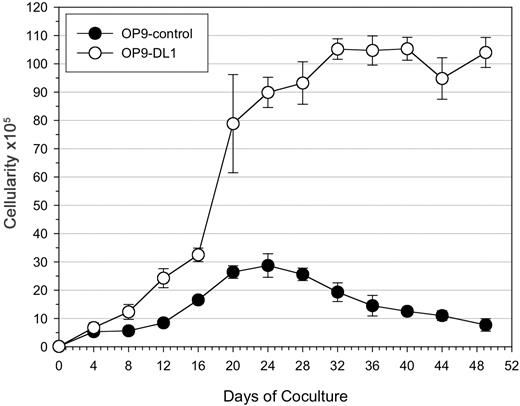
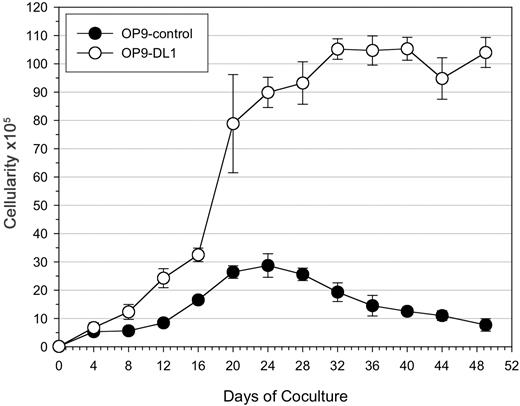
Induction of hematopoiesis was evident in CD34+CD38– cells cultured on OP9 cells, as the number of viable cells increased during the first 3 weeks of culture (Figure 2). However, CD34+CD38– cells cultured on OP9-DL1 cells showed a greater increase in cellularity (> 3-fold) over the OP9-control cultures. Following the initial 3-week culture, there was an apparent plateau in the number of cells generated in OP9-DL1 cultures, while a decline was observed in OP9-control cultures. The reduced ability of OP9-control cells to support hematopoietic differentiation was independent of the number of CD34+CD38– cells used at the start of the culture (2.5 × 103, 5 × 103, and 10 × 103; data not shown).
Expansion potential of cord blood–derived HSCs following long-term coculture on OP9 cells. 1 × 104 CD34+CD38– cells sorted from human cord blood were seeded onto OP9-control or OP9-DL1 stromal cells and cultured for 7 weeks. Total cellularity from OP9 control (•) or OP9-DL1 (○) cocultures was determined during the course of coculture at the indicated time points. The data are presented as the mean ± standard deviation of 3 independent experiments.
Expansion potential of cord blood–derived HSCs following long-term coculture on OP9 cells. 1 × 104 CD34+CD38– cells sorted from human cord blood were seeded onto OP9-control or OP9-DL1 stromal cells and cultured for 7 weeks. Total cellularity from OP9 control (•) or OP9-DL1 (○) cocultures was determined during the course of coculture at the indicated time points. The data are presented as the mean ± standard deviation of 3 independent experiments.
Flow cytometric analysis at later time points revealed the presence of a larger percentage of differentiated CD34–CD38+ cells in OP9-DL1 cocultures than was observed on OP9-control cocultures, even as late as day 49 of culture (∼ 90% vs ∼ 15%, respectively). Moreover, the percentage of CD34+CD38+ precursor–like cells present in the OP9-DL1 cocultures dropped sharply between days 12 and 16, 32 and 36, and 44 and 49, as more differentiated cells (CD34–CD38+) emerged and dominated the cultures. Notably, this rapid change in population dynamics coincided with the passaging of robustly differentiating cocultures.
Induction of T-cell lineage commitment and T-cell development from human CB-HSCs cultured on OP9-DL1 cells
T-cell development occurs in the thymus through a series of discrete steps that are defined based on the expression of various cell surface markers. Specifically, commitment to the T-cell lineage is associated with the expression of CD1a on CD34+CD7++ cells (CD7++CD1a+), which are derived from incoming CD34+ thymic progenitor cells (CD7++CD1a–). Progression to the next stage of T-cell development, the pre-T1 stage, is characterized by a lower level of CD7 expression on CD34+CD1a+ cells (CD7+CD1a+).12
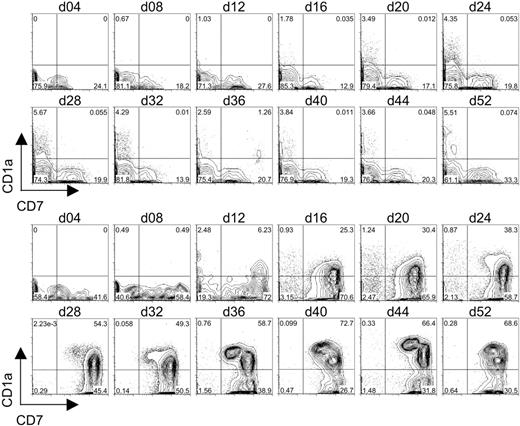
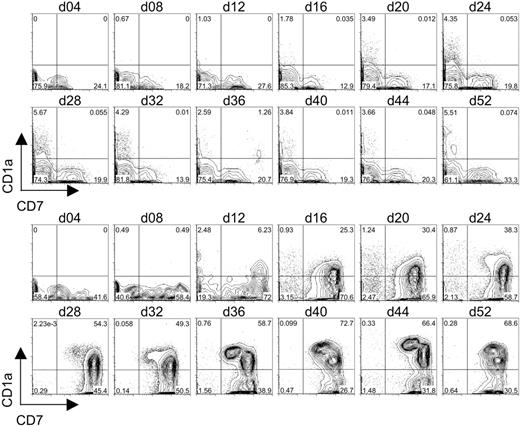
To determine whether OP9-DL1 cells can induce the generation of T-lineage cells from CB-derived HSCs in vitro, CD34+CD38– cells were cultured on OP9-DL1 cells or OP9-control cells, and the expression of early T-cell–lineage markers CD7 and CD1a was examined by flow cytometry. As shown in Figure 3, CD34+CD38– cells cultured on OP9-control cells gave rise to a population of CD7+CD1a– cells that could be detected within 8 days of culture. However, expression of CD1a on the CD7+ cells was not observed in the OP9-control cocultures. Additionally, these cultures failed to give rise to significant numbers of CD7++ cells that either did or did not express CD1a on the cell surface.
Flow cytometric analysis of CD7 and CD1a expression on differentiating human cord blood HSCs following long-term coculture with OP9-control cells or OP9-DL1 cells. CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (top 2 rows of histograms) or OP9-DL1 (bottom 2 rows of histograms) stromal cells for 52 days. Induction of the T-cell phenotype was evaluated approximately every 4 days of coculture through the co-expression of CD7 and CD1a.
Flow cytometric analysis of CD7 and CD1a expression on differentiating human cord blood HSCs following long-term coculture with OP9-control cells or OP9-DL1 cells. CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (top 2 rows of histograms) or OP9-DL1 (bottom 2 rows of histograms) stromal cells for 52 days. Induction of the T-cell phenotype was evaluated approximately every 4 days of coculture through the co-expression of CD7 and CD1a.
In contrast, CB-derived CD34+CD38– cells cultured on OP9-DL1 cells led to a rapid emergence of approximately 60% CD7++CD1a– cells within 8 days of culture. Moreover, a population of CD7++ cells expressing CD1a was first observed on day 12 of OP9-DL1 coculture and steadily increased throughout the culture period. In addition, a discernible population of CD7+CD1a+ cells began to emerge by day 28 of coculture, and this subset continued to increase. Notably, the temporal induction of the different subsets of CD7- and/or CD1a-expressing cells derived from CD34+CD38– cells effectively recapitulated the known stages of early T-cell differentiation.
Generation of CD4+CD8+ T cells from human CB-HSCs cultured on OP9-DL1 cells
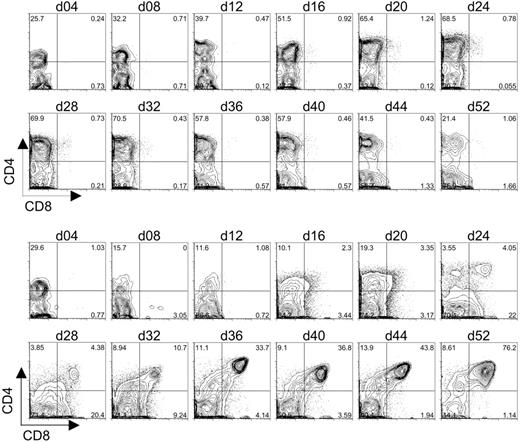
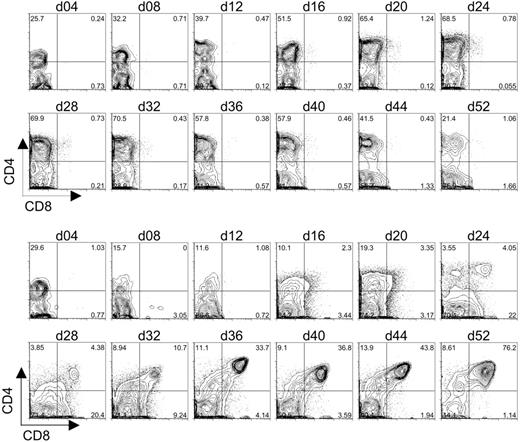
The differentiation of CD7+CD1a+ pre-T1 cells from human CB-HSCs cultured on OP9-DL1 cells indicated that Dll1-expressing stromal cells could support the induction of early T-cell development in vitro. To determine whether T-lymphocyte differentiation could proceed further toward the DP stage, CB-derived HSCs cultured on OP9-DL1 or OP9-control cells were examined for the expression of CD4 and CD8. As shown in Figure 4, CD34+CD38– cells seeded onto either OP9-control or OP9-DL1 cells rapidly gave rise to a large subset of CD4+ cells by day 4 of culture. Moreover, in the case of OP9-DL1 cocultures, the percentage of CD4+CD8– cells dropped over time from 30% on day 4 to 10% by day 16; while the percentage of the population of CD4+CD8– cells in OP9-control cultures increased from 25% on day 4 to 50% by day 16. Importantly, a discernable shift in the population of CD4+ cells that expressed low/dull levels of CD8 could be detected as early as day 16 of OP9-DL1 cocultures and continued to develop until a definitive population of CD4+CD8+ DP cells could be detected by day 24. Strikingly, this population of DP cells continued to increase until it reached more than 75% by day 52. Interestingly, the DP cell population first detected at day 24 of culture (Figure 4) corresponded temporally to the appearance of CD7+CD1a+ pre-T1 cells (Figure 3), suggesting that T-cell differentiation steps observed in our in vitro system progress similarly as those observed in vivo.
Flow cytometric analysis of CD4 and CD8 expression on differentiating human cord blood HSCs following long-term coculture with OP9-control cells or OP9-DL1 cells. CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (top 2 rows of histograms) or OP9-DL1 (bottom 2 rows of histograms) stromal cells for 52 days. The generation of DP T cells was evaluated approximately every 4 days of coculture through the co-expression of CD4 and CD8.
Flow cytometric analysis of CD4 and CD8 expression on differentiating human cord blood HSCs following long-term coculture with OP9-control cells or OP9-DL1 cells. CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (top 2 rows of histograms) or OP9-DL1 (bottom 2 rows of histograms) stromal cells for 52 days. The generation of DP T cells was evaluated approximately every 4 days of coculture through the co-expression of CD4 and CD8.
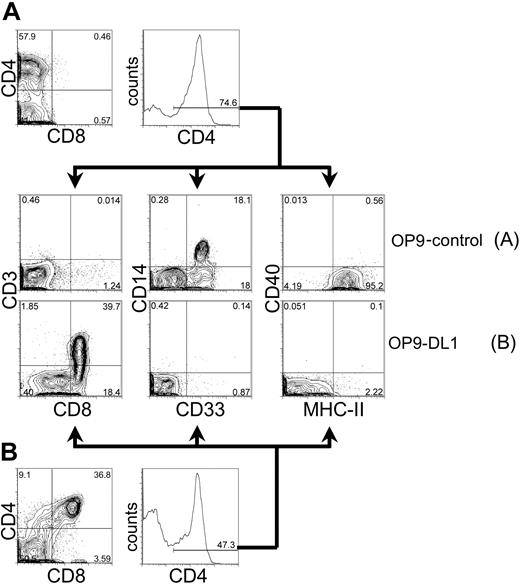
Characterization of CD4+ cells generated from cord blood progenitors cultured on OP9-control or OP9-DL1 cells
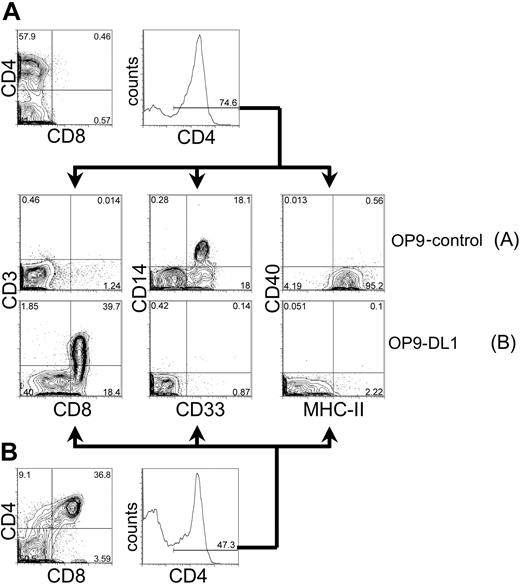
CD4 is expressed not only on developing thymocytes and mature T lymphocytes, but also in cells of the myeloid lineage. To further characterize the population of CD4+ cells generated from HSC cultured on OP9-control or OP9-DL1 cells (Figure 4), we analyzed these cells for the expression of markers associated with either T-lineage or myeloid-lineage differentiation (Figure 5). As shown in Figure 5A, CD4+ cells derived from day-40 OP9-control cocultures did not express the T-cell lineage markers CD3 or CD8, but rather expressed the myeloid markers CD14, CD33, and MHC class II. In striking contrast, CD4+ cells from OP9-DL1 cocultures did not express the myeloid lineage markers CD14, CD33, and MHC-II, but rather expressed the T-lineage makers CD3 and CD8 (Figure 5B). Interestingly, myeloid markers could be detected on the CD4+ cells as early as day 4 of OP9-control or OP9-DL1 cocultures (data not shown). These findings imply that the development of myeloid cells is efficiently supported by OP9 cells, but expression of Dll1 on OP9 cells induces the development of T-lineage cells at the apparent expense of myeloid differentiation. This is evidenced by the decline of myeloid differentiation (days 4-12; CD4+ CD8–CD1a– MHC-II+ cells) followed by the initiation of T-cell–lineage commitment (days 16-24; CD4+ CD8+ CD1a+ MHC-II– cells) (Figures 4, 5, and data not shown).
Phenotypic analysis of CD4-expressing cells derived from human cord blood HSCs cocultured on OP9 control cells or OP9-DL1 cells. CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (A) or OP9-DL1 (B) stromal cells for 40 days. Cells derived from coculture with either OP9-control or OP9-DL1 stromal cells were gated for CD4+ expression and subsequently examined for the expression of T-cell markers CD8 by CD3, or myeloid markers CD33 by CD14 and MHC class II. The percentage of gated cells is given within the histogram and contour plots. As a reference, the expression of CD4 by CD8 is presented from day 40 cocultures on OP9-control (A, top) or OP9-DL1 (B, bottom) stromal cells.
Phenotypic analysis of CD4-expressing cells derived from human cord blood HSCs cocultured on OP9 control cells or OP9-DL1 cells. CD34+CD38– cells sorted from human cord blood were cocultured with OP9-control (A) or OP9-DL1 (B) stromal cells for 40 days. Cells derived from coculture with either OP9-control or OP9-DL1 stromal cells were gated for CD4+ expression and subsequently examined for the expression of T-cell markers CD8 by CD3, or myeloid markers CD33 by CD14 and MHC class II. The percentage of gated cells is given within the histogram and contour plots. As a reference, the expression of CD4 by CD8 is presented from day 40 cocultures on OP9-control (A, top) or OP9-DL1 (B, bottom) stromal cells.
Generation of αβ-TCR–bearing T cells from human CB-HSCs cultured on OP9-DL1 cells
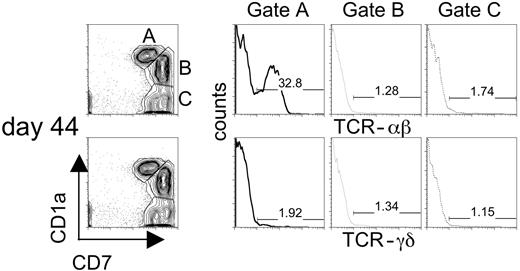
To determine whether Notch signaling induced by Dll1 favored the development of αβ and/or γδ T cells, CD34+CD38– cells cultured on OP9-DL1 cells were analyzed for the expression of TCR αβ and TCR γδ by flow cytometry. Although CD7-expressing cells arise following coculture of CB-derived HSCs on OP9-control stromal cells, these cells fail to express CD1a (Figure 3) or αβ-TCR (data not shown). In contrast, expression of αβ-TCR on CD1a+CD7+ cells could be detected as early as day 16 and reached a maximum by day 36 of culture on OP9-DL1 cells (data not shown). Figure 6 shows that αβ-TCR+ T cells could be observed from CD34+CD38– cells cultured on OP9-DL1 cells (day 44 culture is shown). To delineate which subset of developing T cells in these cultures expressed TCR, we looked for γδ- and αβ-TCR expression on CD7+CD1a+ (gate A), CD7++CD1a+ (gate B), and CD7++CD1a– (gate C) cells (Figure 6). Our results show that αβ-TCR expression was detected mainly on CD7+CD1a+ cells (gate A) and accounted for approximately 33% of this population. In contrast, γδ-TCR expression was detected on fewer than 2% of CD7+CD1a+ cells. Analysis of the other 2 subsets (gates B and C) indicated that few αβ-TCR or γδ-TCR cells were present among these populations. Furthermore, a limited analysis of TCR-Vβ use among CD3+ αβ-TCR+ T cells revealed that a diverse TCR repertoire is generated from these cultures (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Although a small but reproducible number of γδ-TCR+ cells could be detected on cocultures with OP9-DL1 cells, this population was consistently absent on cocultures with OP9-control cells (data not shown). These results indicate that while OP9-DL1 cells efficiently support the differentiation of αβ-T cells, the differentiation γδ-T cells is not favored.
Flow cytometric analysis of the expression of TCR-αβ and TCR-γδ on progenitor T cells arising following coculture of human CB–derived HSCs with OP9-DL1 cells. Human CB–derived HSCs give rise to 3 populations of progenitor T cells that can be discriminated based on the level of expression of CD7 and CD1a. Gated populations of cells CD7+CD1a+ (gate A), CD7++CD1a+ (gate B), CD7++CD1a– (gate C) from day 44 cocultures were stained for the expression of TCR-αβ (gated histograms presented in top panel) or TCR-γδ (gated histograms presented in bottom panel). The percentage of gated T progenitor populations of TCR-positive cells is displayed within the histogram.
Flow cytometric analysis of the expression of TCR-αβ and TCR-γδ on progenitor T cells arising following coculture of human CB–derived HSCs with OP9-DL1 cells. Human CB–derived HSCs give rise to 3 populations of progenitor T cells that can be discriminated based on the level of expression of CD7 and CD1a. Gated populations of cells CD7+CD1a+ (gate A), CD7++CD1a+ (gate B), CD7++CD1a– (gate C) from day 44 cocultures were stained for the expression of TCR-αβ (gated histograms presented in top panel) or TCR-γδ (gated histograms presented in bottom panel). The percentage of gated T progenitor populations of TCR-positive cells is displayed within the histogram.
Activation of T cells generated from human CB–HSCs cultured on OP9-DL1 cells by TCR/CD3 stimulation
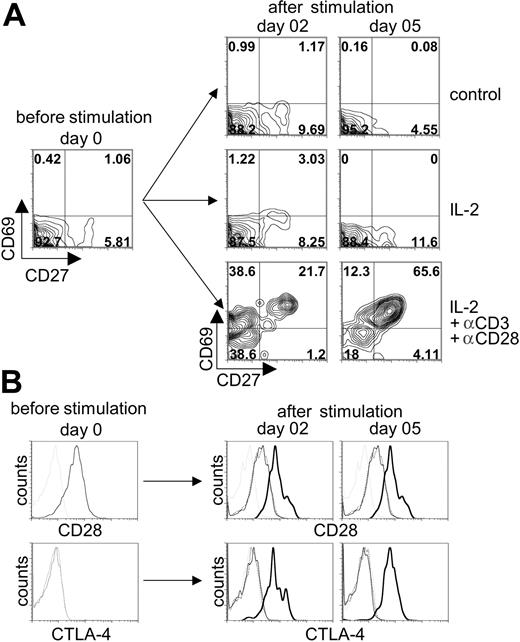
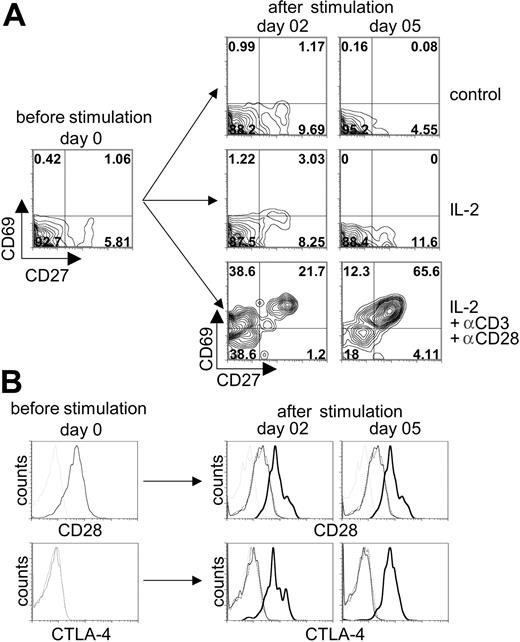
To determine whether αβ-TCR/CD3+ T cells were responsive to TCR-mediated activation, cells from day-54 cocultures were stimulated with anti-CD3 plus anti-CD28 mAbs in the presence of exogenous IL-2, and the expression of T-cell activation markers (CD27, CD69, CD28, and CTLA-4 [CD152]) was analyzed by flow cytometry (Figure 7). Nonstimulated control or cultures treated with IL-2 alone did not show an increase in the expression of the activation markers: CD69 or CD27 (Figure 7A) and CD28 or CTLA-4 (Figure 7B). In contrast, stimulation with anti-CD3/CD28 mAbs led to a robust induction of CD69 expression by day 2 of stimulation, followed with an increase in CD27 coexpression by day 5 after stimulation (Figure 7A). Likewise, the expression of the T-cell activation markers CD28 and CTLA-4 also were specifically up-regulated following anti-CD3/CD28 stimulation (Figure 7B). Taken together, anti-TCR/CD3 stimulation of T cells generated from OP9-DL1 cocultures led to a clear up-regulation of T-cell activation markers, demonstrating that this simple culture system supports the differentiation of CB-derived HSCs into functionally responsive DP human T cells.
Induction of T-cell activation markers following stimulation of human cord blood–derived HSCs induced to differentiate on OP9-DL1 cells. CB-derived HSCs cocultured with OP9-DL1 cells for 52 days were stimulated with anti-CD3 plus anti-CD28 mAbs in the presence or absence of recombinant human IL-2 added to the media. (A) The expression of CD27 and CD69 was analyzed before stimulation and 2 and 5 days after stimulation on CD3+-gated cells. Anti-CD3 plus anti-CD28 mAb in IL-2–enriched cocultures are compared to IL-2–enriched or control (no IL-2 added) cocultures alone. (B) The expression of CD28 (histograms presented in top row) and CTLA-4 (histograms presented in bottom row) was analyzed before stimulation and 2 and 5 days after stimulation on CD3+-gated cells from cocultures treated without IL-2 (dotted thin black line) or with IL-2 (solid thin black line), or with IL-2 and anti-CD3 plus anti-CD28 (solid thick black line) added and then compared to the appropriate isotype control (thin dotted gray line).
Induction of T-cell activation markers following stimulation of human cord blood–derived HSCs induced to differentiate on OP9-DL1 cells. CB-derived HSCs cocultured with OP9-DL1 cells for 52 days were stimulated with anti-CD3 plus anti-CD28 mAbs in the presence or absence of recombinant human IL-2 added to the media. (A) The expression of CD27 and CD69 was analyzed before stimulation and 2 and 5 days after stimulation on CD3+-gated cells. Anti-CD3 plus anti-CD28 mAb in IL-2–enriched cocultures are compared to IL-2–enriched or control (no IL-2 added) cocultures alone. (B) The expression of CD28 (histograms presented in top row) and CTLA-4 (histograms presented in bottom row) was analyzed before stimulation and 2 and 5 days after stimulation on CD3+-gated cells from cocultures treated without IL-2 (dotted thin black line) or with IL-2 (solid thin black line), or with IL-2 and anti-CD3 plus anti-CD28 (solid thick black line) added and then compared to the appropriate isotype control (thin dotted gray line).
Discussion
Lymphocyte development occurs in distinct microenvironments in vivo, with B cells developing in the bone marrow and T cells developing in the thymus. In vitro systems for the differentiation of lymphocytes from HSCs have relied on recreating the appropriate microenvironment (bone marrow or thymus) to induce and support lineage-specific lymphocyte differentiation through the use of bone marrow–derived stromal cells or fetal thymus organ cultures (FTOCs), respectively. While large numbers of B cells could be generated in relatively simple 2-dimensional culture techniques using bone marrow stromal cell lines, T cells appeared to require the use of a 3-dimensional FTOC approach.55 The inherent dependency on thymic tissue for T-cell development had been ascribed to the complex structure of the thymus, soluble factors, or specific cell-cell interactions.56
The molecular rationale for this thymic dependence has begun to be elucidated. Reports showing that Notch signals play a key role at multiple cell fate decisions,15,17-21 and in particular the decision of a lymphoid progenitor to proceed along a B- or T-cell lineage,20,21 suggested that in vitro approaches may support T-cell differentiation, provided that the appropriate Notch ligands are present. Recently, we demonstrated that the OP9 stromal cell genetically engineered to express the Notch ligand Delta-like 1 (OP9-DL1) efficiently supports the generation of T cells, at the expense of B cells, from mouse HSCs and mouse embryonic stem cells (ESCs).50,51 Here we demonstrate that OP9-DL1 cells effectively support the generation human T cells from CD34+CD38– HSCs isolated from CB samples, providing a novel and efficient approach for the generation of human T cells in vitro from a defined source of stem cells.
Our results show that culturing CB-derived HSCs with OP9-DL1 cells, but not OP9-control cells, allows for an initial expansion of hematopoietic progenitor cells (CD34+CD38+), consistent with the reported role of Notch signaling in the survival and maintenance of hematopoietic progenitors.14-16 Human CD34+ CB cells retrovirally transduced to express a constitutively activated form of Notch (ICN) displayed a delayed progenitor cell differentiation,57 and exposure of mouse or human HSCs to Notch ligands promoted increased expansion and cell survival of primitive hematopoietic precursor cells.46,58,59 In our system, CB-derived HSCs, when cocultured with OP9-DL1 cells, appeared capable of undergoing continuous waves of T-cell differentiation, maintaining a stable number of short-lived CD4+CD8+ DP cells. Although it is not clear whether self-renewing progenitors persist in these cultures, the sustained maintenance of CD4+CD8+ DP cells in these cultures suggests that T-cell development is likely derived from T-cell progenitors, such as CD34+ prothymocytes,55,60 which are present in long-term cultures (data not shown).
In contrast, human CB-derived HSCs, when cultured on OP9-control cells, did not appear capable of expanding or renewing CD34+CD38+ cells as CD34 expression rapidly declined with time, as CD19+IgM– B cells emerged and then sharply declined over the same period of time (Figure S2). Furthermore, analysis of HSCs differentiating on OP9-control cells showed a marked increase in cell death as indicated by forward scatter and propidium iodide uptake as compared to progenitors cultured on OP9-DL1 cells (data not shown). This observation suggests that Notch signaling through Dll1 not only promotes T-lineage commitment, but also supports progenitor cell renewal and survival.
Our results show that human CB-derived HSCs cultured on OP9-DL1 cells can be induced to follow a normal program of early T-cell differentiation and give rise to CD4+CD8+ DP T cells, suggesting that a Dll1-mediated Notch signals, such as the induction of HES-1 and Deltex-1 expression (Figure S3), in the context of the lymphopoietic environment provided by the OP9 cells, is sufficient to allow for the generation of human T cells. Furthermore, our findings show that the TCR/CD3+ T cells generated on OP9-DL1 cells were indeed functional and responsive to TCR stimulation. Thus, the present approach provides a powerful new tool for the study of human T-cell development at the molecular level.
Several studies have evaluated the role of Notch ligands in promoting the induction of human T-cell development.54,59 Recently, Ohishi et al59 reported the induction of early stages of T-cell development in vitro from CB-derived HSCs cultured in serum-free media with recombinant immobilized Delta-like-1, and, importantly, demonstrated enhanced in vivo T-cell engraftment of immunodeficient mice. In comparing the effectiveness of the Notch ligands Jagged 1 and Delta-like 1 to induce human T-cell differentiation, Jaleco et al54 reported that the S17 bone marrow stromal line retrovirally transduced with Delta-like 1, but not Jagged 1, promoted the initiation of T-cell differentiation within 6 weeks of coculture. Our present findings confirm their initial observation, in that the percentage of CD7+ cells increases upon initiation of coculture with OP9-DL1 cells, but also extend their observations by demonstrating that CD7+ cells in our system also express CD1a, a phenotype consistent with the pre-T1 stage of human T-cell differentiation. In this regard, the findings reported by Jaleco et al are consistent with the ability of stromal cells expressing Dll1 to support the induction of T-cell development at the expense of B-cell development.50,51 However, it was unclear whether Dll1-expressing S17 cells promoted de novo T-cell differentiation from HSCs or merely supported the expansion of mature and/or immature T cells present within the unfractionated CD34+ CB-derived sample used in their cultures. Nevertheless, the report by Jaleco et al showed that CD34+ CB progenitors after 6 weeks of culture on S17-DL1 cells could give rise to a small number (0%-3.0%) of CD4+CD8+ DP cells. In contrast, our results demonstrate a clear induction and promotion of sustained T-cell differentiation following culture of CD34+CD38– HSCs on OP9-DL1 cells. This induction of T-cell differentiation is evident by the clear expression of CD7++CD1a+ (pre-T1) cells on day 20 of culture, followed by the robust appearance of CD7+CD1a+ and CD4+CD8+ cells by 4 weeks of culture (Figures 3-4). Moreover, the efficiency of this system is further highlighted by the fact that the generation of pro-T and DP T cells was consistently observed with as few as 50 HSCs seeded onto OP9-DL1 cells.
Importantly, CD7+CD1a+ cells were shown to express αβ-TCR, but curiously few belonged to the γδ-TCR T-cell lineage. Additionally, few, if any, CD4+ or CD8+ single-positive (SP) cells arose from CD4+CD8+ DP cells. Given that OP9 cells, like S17 cells, lack expression of human MHC class I and II molecules, it is perhaps not surprising that few CD4+ or CD8+ SP cells would emerge during the culture period. Our observation that the human pre-T1 (CD7+CD1a+) cells that emerged from OP9-DL1 cocultures favored the αβ–T-lineage choice, but not the γδ fate, is consistent with reports showing that Notch-1 signals induce αβ T-cell differentiation rather than γδ T cells.33 However, this is in contrast to the findings of other groups showing that Notch-1 signaling favored a γδ over αβ T-cell fate.31,32,34 Nevertheless, we recently reported that OP9-DL1 cell-induced Notch signaling can support the differentiation of both mouse αβ and γδ T cells derived from fetal liver and bone marrow HSCs.50,51 Several hypotheses, which are not necessarily mutually exclusive, can be advanced to explain the contradictory results among published reports. It is conceivable that nonredundant functions of individual Notch receptors and ligands may impart or fail to impart T-cell–specific signals, which along with the level61,62 or pattern29,44-48 of expression of Notch receptors and their ligands may impart additional strengths or weaknesses to the overall signal determining binary cell fate decisions. These observations also may help to explain how Notch signaling can function both in the maintenance/self-renewal of HSCs as well as influence differentiation along alternative cell fate pathways.46
It also is unclear how in some experimental systems the overexpression of Notch ligands or constitutively active forms of Notch (ICN) by hematopoietic progenitors determine cell fate choice in the context of the normal physiologic setting. Additionally, the source of progenitor cells used, as well as the particular microenvironment, stromal cell line (S17 versus OP9), or experimental approach, also may affect the experimental outcome observed. It is likely that several or all of these factors may in fact play a role in the ability of the system we describe here to efficiently and successfully allow for the generation of human T cells from CB-derived HSCs.
Taken together, the data presented in this report offer a novel and efficient in vitro coculture system for the differentiation of human CD4+CD8+ DP T cells from a defined source of stem cells. It also demonstrates that mouse Dll1 can initiate and support T-cell differentiation from human cord blood progenitors. Further development of the coculture system through the transfection of human MHC class I and II molecules may be desirable to promote the positive and negative selection of DP cells into CD8 and CD4 SP cells, respectively. Given the sustained and high percentage of CD4+CD8+ DP cells that emerge, it is tempting to envision the adaptation of this coculture system for the differentiation of T cells from human embryonic stem cells. Such a system, developed thus far with mouse ES cells,51 would have obvious advantages in allowing for a potentially unlimited source of human T cells that could be genetically engineered resistant to HIV infection or chemotherapy for the treatment of human immunodeficiencies.
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-04-1293.
Supported by funds from the Ontario HIV Treatment Network and the Canadian Institutes of Health Research (CIHR); a postdoctoral fellowship (R.N.L.M.-M.) from the CIHR; and an investigator award (J.C.Z.P.) from the CIHR.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Renée de Pooter, Thomas M. Schmitt, and María Luisa Toribio for their critical review of the manuscript. We thank Hergen Spits for his comments on the results prior to submission. We gratefully acknowledge Cheryl Smith and Giselle Knowles for their expert assistance in cell sorting.