Abstract
Autologous stem cell transplantation, in the setting of hematologic malignancies such as lymphoma, improves disease-free survival if the graft has undergone tumor purging. Here we show that flowing hematopoietic cells through pulsed electric fields (PEFs) effectively purges myeloma cells without sacrificing functional stem cells. Electric fields can induce irreversible cell membrane pores in direct relation to cell diameter, an effect we exploit in a flowing system appropriate for clinical scale. Multiple myeloma (MM) cell lines admixed with human bone marrow (BM) or peripheral blood (PB) cells were passed through PEFs at 1.35 kV/cm to 1.4 kV/cm, resulting in 3- to 4-log tumor cell depletion by flow cytometry and 4.5- to 6-log depletion by tumor regrowth cultures. Samples from patients with MM gave similar results by cytometry. Stem cell engraftment into nonobese diabetic–severe combined immunodeficient (NOD/SCID)/β2m-/- mice was unperturbed by PEFs. Flowing cells through PEFs is a promising technology for rapid tumor cell purging of clinical progenitor cell preparations.
Introduction
Contaminant cancer cells in bone marrow (BM) or mobilized peripheral blood (PB) transfusions have been shown to correlate with disease relapse in the setting of some hematologic malignancies.1-4 In recent years, several technologies have been employed to purge tumor cells from autologous transfusions, including antibody-mediated selection for progenitor cells,2,5-8 depletion of tumor cells,9 genetic modification of tumor cells,10,11 selective chemical or physical means of tumor depletion,12,13 and in vitro expansion of hematopoietic cells.14 Clinical studies have employed tumor purging from stem cell preparations in non-Hodgkin lymphoma (NHL) with recent evidence of clinical benefit.1,15 Whereas no studies on a similar scale or with similar results exist for multiple myeloma (MM), we selected this hematologic disease as a test case for our purging technology since BM samples from patients with myeloma are consistently infiltrated with tumor, the tumor cells are readily quantified, and autologous transplantation is used to treat patients with this disorder. It should be emphasized however, that the data presented here simply use myeloma as an example and do not argue for or against the clinical utility of autologous transplantation with purging for MM.
The NHL studies that did use purging employed CD34 selection that is time-, cost-, and labor-intensive, and may not efficiently preserve hematopoietic stem cells. We sought to test a different method based on prior studies showing that defined electric field pulses applied to static, small volume samples selectively depleted breast cancer and megakaryocyte cell lines by 2 to 2.5 logs from mixtures with blood cells, and preserved small cells including lymphocytes and CD34+ cells.16 The principle behind selective pulsed electric field (PEF) purging is that a cell's cytosol is largely conductive, but the lipid cell membrane does not conduct electricity.17,18 The voltage developed across each cell is proportional to the cell's diameter. Under defined electric field conditions, larger cells are killed without altering the viability of smaller cells, including hematopoietic stem cells (HSCs). Whereas HSCs and resting lymphocytes are generally 6 μm to 8 μm in diameter,19,20 myeloma cells and other tumor cells are generally more than 10 μm in diameter.
We have now applied this technology in a modified format that permits continuous and rapid pulsing of clinically relevant numbers of cells (> 109 cells in 30 minutes) at controlled flow rates that negate the effects of cell concealment or cell settling (manuscript in preparation).22 Applying this technology to myeloma, we define tumor cell depletion by 3 to 6 orders of magnitude without sacrificing functional stem cells.
Study design
Preparation of primary cells and cell lines
PB was obtained from donors at Massachusetts General Hospital (MGH). BM was obtained from NDRI (Philadelphia, PA), and 2 mL to 5 mL of discard BM aspirates were obtained from patients with MM at the Dana-Farber/Harvard Cancer Center (institutional review board approval no. 1999-P008 401/4). Bone marrow or peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque centrifugation. Cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA).
Flowing PEF apparatus
A prototype flowing PEF apparatus was designed and constructed by Science Research Laboratory (SRL). Components include the flowing treatment chamber, Cytopulse (Rockville, MD) electric pulse driver system, Tektronix oscilloscope (Beaverton, OR), PC-based control system, syringe pumps (Harvard Apparatus, Framingham, MA), and 4-way valve and outlet for purging air (manuscript in preparation).23
In vitro assays and staining
For tumor regrowth assays, cells were serially diluted in triplicate in conditioned medium/RPMI/fetal calf serum (FCS) in 96-well plates. After 2 weeks, plates were scored for colony formation (> 1 colony of > 4 viable tumor cells).
Colony-forming cell (CFC) and long-term culture-initiating cell (LTC-IC) assays were performed as described.1
All antibodies and staining reagents were used according to company protocols (BDPharmingen, San Diego, CA, and Molecular Probes, Eugene, OR). Flow cytometry was performed as described in Figure 1.
PEF selectively purges myeloma cell lines from mixtures with blood or bone marrow cells. (A) RPMI8226 myeloma cells were mixed 1:1 with PBMCs, and were PEF-treated in standard pulsing buffer (90% isotonic [282 mM] dextrose/10% PBS, pH 7.2) at 0 kV/cm or 1.4 kV/cm, using the flowing PEF apparatus. After ficolling, cells were photographed at ×50 magnification. The distinct large myeloma cells seen at 0 kV/cm (top) are not present at 1.4 kV/cm (bottom). Photographs were taken with a Nikon Diaphot 300 microscope, ×50 magnification, and a Nikon 6006 camera and using IP Labview Macintosh software (National Instruments, Austin, TX). (B) Raw flow cytometry data for PEF-treated and control mixed cells. Flow cytometry was performed on a Becton Dickinson FACSVantage flow cytometer (San Diego, CA), and data were analyzed using CellQuest software. Mixtures of PBMCs and CFDA-SE–labeled RPMI8226 myeloma cells were PEF-treated at 0 kV/cm or 1.4 kV/cm. PBMC suspensions from whole blood or cord blood were seeded with a CFDA-SE–labeled myeloma cell line, RPMI8226 or U266. Mixed cells were resuspended in the high resistivity pulsing buffer (500 Ω-cm) at between 106 and 2 × 106/mL. A quantity of 5 mL to 10 mL of cells was run through the flowing apparatus at a 4 mL/min flow rate, exposing cells to 250 20-μs pulses. Cell samples were treated at different electric field strengths, or pumped through the flowing apparatus without pulsing (0 kV/cm, control group). Cells were stained with anti-CD3/CD19–phycoerythrin (PE), anti-CD14–allophycocyanin (APC), and the nuclear exclusion dye 7-aminoactinomycin (7-AAD). Dot plots represent cells gated as 7-AAD–negative. Dot plots for forward and side scatter (left), CD3/CD19 and CFDA-SE (center), and CD14 and CFDA-SE (right) are shown for stained cells that were PEF-treated at 0 kV/cm (top row) or 1.35 kV/cm (bottom row). The results of this experiment are representative of more than 20 experiments using several different tumor cell lines. (C) Percent survival of RPMI8226 myeloma cells (▴), CD3+/CD19+ lymphocytes ( ), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.
), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.
PEF selectively purges myeloma cell lines from mixtures with blood or bone marrow cells. (A) RPMI8226 myeloma cells were mixed 1:1 with PBMCs, and were PEF-treated in standard pulsing buffer (90% isotonic [282 mM] dextrose/10% PBS, pH 7.2) at 0 kV/cm or 1.4 kV/cm, using the flowing PEF apparatus. After ficolling, cells were photographed at ×50 magnification. The distinct large myeloma cells seen at 0 kV/cm (top) are not present at 1.4 kV/cm (bottom). Photographs were taken with a Nikon Diaphot 300 microscope, ×50 magnification, and a Nikon 6006 camera and using IP Labview Macintosh software (National Instruments, Austin, TX). (B) Raw flow cytometry data for PEF-treated and control mixed cells. Flow cytometry was performed on a Becton Dickinson FACSVantage flow cytometer (San Diego, CA), and data were analyzed using CellQuest software. Mixtures of PBMCs and CFDA-SE–labeled RPMI8226 myeloma cells were PEF-treated at 0 kV/cm or 1.4 kV/cm. PBMC suspensions from whole blood or cord blood were seeded with a CFDA-SE–labeled myeloma cell line, RPMI8226 or U266. Mixed cells were resuspended in the high resistivity pulsing buffer (500 Ω-cm) at between 106 and 2 × 106/mL. A quantity of 5 mL to 10 mL of cells was run through the flowing apparatus at a 4 mL/min flow rate, exposing cells to 250 20-μs pulses. Cell samples were treated at different electric field strengths, or pumped through the flowing apparatus without pulsing (0 kV/cm, control group). Cells were stained with anti-CD3/CD19–phycoerythrin (PE), anti-CD14–allophycocyanin (APC), and the nuclear exclusion dye 7-aminoactinomycin (7-AAD). Dot plots represent cells gated as 7-AAD–negative. Dot plots for forward and side scatter (left), CD3/CD19 and CFDA-SE (center), and CD14 and CFDA-SE (right) are shown for stained cells that were PEF-treated at 0 kV/cm (top row) or 1.35 kV/cm (bottom row). The results of this experiment are representative of more than 20 experiments using several different tumor cell lines. (C) Percent survival of RPMI8226 myeloma cells (▴), CD3+/CD19+ lymphocytes ( ), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.
), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.
Engraftment of NOD/SCID/β2m-/- mice
Six- to 8-week-old nonobese diabetic–severe combined immunodeficient (NOD/SCID)/β2m-/- mice were obtained from the Jackson Laboratory (Bar Harbor, ME), handled, maintained, and underwent transplantation as described.21
Results and discussion
Selective PEF purging of carboxyfluoroscein diacetate succinimidyl ester (CFSE)–labeled myeloma cell lines (RPMI8226), mixed with PBMCs or bone marrow cells, was achieved at 1.4 kV/cm (Figure 1A-B). After PEF, viable cells were small and confined largely to the “lymphocyte gate” of a forward- and side-scatter plot, known to contain stem and progenitor cells, while the high forward- and side-scatter myeloma cells largely disappeared (Figure 1B, left). Purging of myeloma cells (CFDA-SE+) as well as monocytes (CD14+), with preservation of lymphocytes (CD3+/CD19+), was also evident.
Dose response to PEFs was evaluated. We noted purging of tumor cells (4 logs) with preservation of small lymphocytes (Figure 1C). While a representative experiment is shown, PEF consistently purged cancer cells by 3 to 4 logs, (4- to 5-log limit of detection) with preservation of small blood cells. Monocytes (CD14+), larger than lymphocytes and stem cells, were also purged by about 2 logs at 1.35 kV/cm. Experiments involving higher cell densities, up to 107 cell/mL, demonstrated similar tumor purging with preservation of small cells (data not shown), validating the utility of this system for large-scale tumor purging.
In order to quantify the purging of truly viable, proliferation-competent myeloma cells, tumor regrowth cultures were established (Figure 1D). Comparison between unpulsed and pulsed tumor cell regrowth showed 5 to 6 logs or greater purging (limit of detection for < 106 starting tumor cells). The mean log purging using RPMI8226 cells seeded into PBMCs, assessed by flow cytometry or tumor regrowth assay, is shown in Figure 1E (n = 6). These data suggest that flow cytometry enumeration, unable to distinguish between replication-competent cells and osmotically damaged but morphologically intact cells, underestimates the extent of tumor purging. Further, the lysing of cells by the PEF process releases large amounts of genomic DNA that cannot be effectively removed even with DNAse treatment. Therefore, DNA polymerase chain reaction for detection of MM cells after PEF purging could not be effectively applied.
After PEF purging, the capacity of human BM cells to engraft irradiated NOD/SCID/β2m-/- BM was preserved, with more than 5% human CD45 cells after 7 weeks (Figure 1F). Preservation of engraftment capacity has also been demonstrated in NOD/SCID mice. Furthermore, CFC and LTC-IC frequency in vitro were similar or slightly enriched after PEF purging at 1.40 kV/cm, the upper limit and current standard field strength for most efficient tumor purging and progenitor cell preservation, and frequency decreased at and above 1.45 kV/cm (Figure 1G). These results demonstrate the functional and differentiation competence of the PEF-treated progenitor cells in vitro and in vivo.
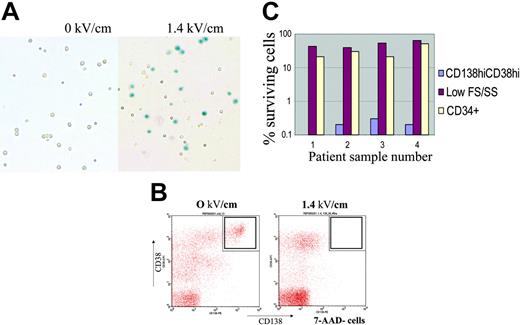
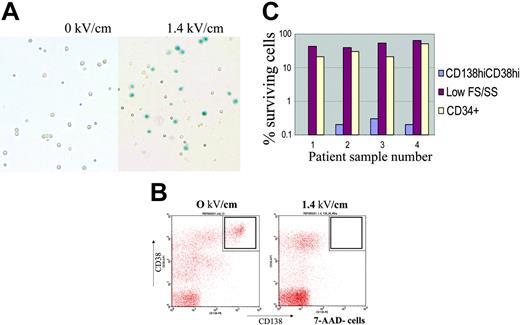
Myeloma cells from the BM of patients with MM were killed at 1.40 kV/cm, demonstrated by trypan blue staining (Figure 2A) and flow cytometry (CD45dimCD138+CD38hi cells; Figure 2B). Percent survival of myeloma or small CD34+ cells after PEF treatment is summarized in Figure 2C (n = 4). In each case, the CD45dimCD38hiCD138+ population was purged at or near the limit of detection of 3 logs (due to small starting cell numbers); CD34+ cells were also affected at that pulse dose but with lower cell kill, preserving 50% ± 16%.
PEF selectively purges myeloma cells from primary multiple myeloma bone marrow. (A) Small volumes (2 mL to 4 mL) of BM from patients with MM, some of which contained more than 3% myeloma plasma cells, by CD38CD138 staining, were processed and PEF-treated at 0 kV/cm (left) or 1.4 kV/cm (right). Representative photographs of cells stained with trypan blue after PEF treatment are shown. Dead cells were not separated by centrifugation over ficoll (unlike Figure 1A) and lysed cells appear as trypan blue–positive, on the right, whereas small cells including progenitor cells are preserved. (B) After PEF treatment cells were stained with anti–CD38-PerCP, anti–CD138-PE, anti–CD45–fluorescein isothyocyanate (FITC), or anti–CD34-FITC, and the nuclear exclusion dye 7-AAD. Cells were analyzed by Cellquest software. Cells gated as 7-AAD–negative and CD45low were analyzed for the presence of CD38high, CD138+ myeloma plasma cells. This population is noticeably absent in the dot plot on the right for cells pulsed at 1.4 kV/cm group. (C) Percent survival of CD38highCD45lowCD138+ myeloma cells (blue bars), CD34+ cells (cream bars), and other small cells (red) after PEF treatment, at 0 kV/cm or 1.4 kV/cm, of 4 independent multiple myeloma patient bone marrow specimens. The limit of detection based on input bone marrow cell number, and percent myeloma cells within bone marrow specimens, is approximately 3 logs (or 0.1% of starting number). Cells staining positive for the CD38highCD45lowCD138+ phenotype constituted 2% to 7% of total bone marrow mononuclear cells (45% for sample 4) from MM samples analyzed. Primary myeloma cells do not proliferate reproducibly in vitro; therefore, serial dilution tumor regrowth assays cannot be routinely performed to enumerate patient myeloma cells after PEF.
PEF selectively purges myeloma cells from primary multiple myeloma bone marrow. (A) Small volumes (2 mL to 4 mL) of BM from patients with MM, some of which contained more than 3% myeloma plasma cells, by CD38CD138 staining, were processed and PEF-treated at 0 kV/cm (left) or 1.4 kV/cm (right). Representative photographs of cells stained with trypan blue after PEF treatment are shown. Dead cells were not separated by centrifugation over ficoll (unlike Figure 1A) and lysed cells appear as trypan blue–positive, on the right, whereas small cells including progenitor cells are preserved. (B) After PEF treatment cells were stained with anti–CD38-PerCP, anti–CD138-PE, anti–CD45–fluorescein isothyocyanate (FITC), or anti–CD34-FITC, and the nuclear exclusion dye 7-AAD. Cells were analyzed by Cellquest software. Cells gated as 7-AAD–negative and CD45low were analyzed for the presence of CD38high, CD138+ myeloma plasma cells. This population is noticeably absent in the dot plot on the right for cells pulsed at 1.4 kV/cm group. (C) Percent survival of CD38highCD45lowCD138+ myeloma cells (blue bars), CD34+ cells (cream bars), and other small cells (red) after PEF treatment, at 0 kV/cm or 1.4 kV/cm, of 4 independent multiple myeloma patient bone marrow specimens. The limit of detection based on input bone marrow cell number, and percent myeloma cells within bone marrow specimens, is approximately 3 logs (or 0.1% of starting number). Cells staining positive for the CD38highCD45lowCD138+ phenotype constituted 2% to 7% of total bone marrow mononuclear cells (45% for sample 4) from MM samples analyzed. Primary myeloma cells do not proliferate reproducibly in vitro; therefore, serial dilution tumor regrowth assays cannot be routinely performed to enumerate patient myeloma cells after PEF.
The data presented here confirm the principle of size selectivity using PEF technology, and demonstrate the utility of the flowing PEF technique for rapid purging of myeloma cells from progenitor and stem cell specimens. This method is appropriate for clinical sample volumes, and may provide a useful strategy for tumor cell purging from autologous BM or mobilized PB stem cell preparations. Overall purging efficacy, volume of specimen, and procedure time are significantly improved compared with our prior report.17 The patient sample data presented here emphasize the potential clinical utility of this approach.
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2003-12-4399.
Supported by National Institutes of Health grant no. R44CA079364-02 and NASA grant no. NAS800009.
A.C. and Y.S. contributed equally to this work.
Several of the authors (J.A.M., A.C., A.L., and J.F.) are employed by, and 2 authors (H.M.E. and J.A.M.) have declared a financial interest in, Science Research Laboratory Inc, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Ai Hong Li at the Dana-Farber Cancer Institute and Aron Chiang and Meagan Kilbride at MGH for technical assistance.

![Figure 1. PEF selectively purges myeloma cell lines from mixtures with blood or bone marrow cells. (A) RPMI8226 myeloma cells were mixed 1:1 with PBMCs, and were PEF-treated in standard pulsing buffer (90% isotonic [282 mM] dextrose/10% PBS, pH 7.2) at 0 kV/cm or 1.4 kV/cm, using the flowing PEF apparatus. After ficolling, cells were photographed at ×50 magnification. The distinct large myeloma cells seen at 0 kV/cm (top) are not present at 1.4 kV/cm (bottom). Photographs were taken with a Nikon Diaphot 300 microscope, ×50 magnification, and a Nikon 6006 camera and using IP Labview Macintosh software (National Instruments, Austin, TX). (B) Raw flow cytometry data for PEF-treated and control mixed cells. Flow cytometry was performed on a Becton Dickinson FACSVantage flow cytometer (San Diego, CA), and data were analyzed using CellQuest software. Mixtures of PBMCs and CFDA-SE–labeled RPMI8226 myeloma cells were PEF-treated at 0 kV/cm or 1.4 kV/cm. PBMC suspensions from whole blood or cord blood were seeded with a CFDA-SE–labeled myeloma cell line, RPMI8226 or U266. Mixed cells were resuspended in the high resistivity pulsing buffer (500 Ω-cm) at between 106 and 2 × 106/mL. A quantity of 5 mL to 10 mL of cells was run through the flowing apparatus at a 4 mL/min flow rate, exposing cells to 250 20-μs pulses. Cell samples were treated at different electric field strengths, or pumped through the flowing apparatus without pulsing (0 kV/cm, control group). Cells were stained with anti-CD3/CD19–phycoerythrin (PE), anti-CD14–allophycocyanin (APC), and the nuclear exclusion dye 7-aminoactinomycin (7-AAD). Dot plots represent cells gated as 7-AAD–negative. Dot plots for forward and side scatter (left), CD3/CD19 and CFDA-SE (center), and CD14 and CFDA-SE (right) are shown for stained cells that were PEF-treated at 0 kV/cm (top row) or 1.35 kV/cm (bottom row). The results of this experiment are representative of more than 20 experiments using several different tumor cell lines. (C) Percent survival of RPMI8226 myeloma cells (▴), CD3+/CD19+ lymphocytes (), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2003-12-4399/6/m_zh80050574880001.jpeg?Expires=1768507590&Signature=u82ufK1xG7Z8eDpF5IOUufH80wTOLzNiKAHG~akEUSxC1a2K5vXtDuoDJnS30GebgMVvg-zQUwndHweSpntOEQTg94WGYgxpYlwUTMHmqIAvEjBQ1o4K4LSEGqKD~eObR32gR5FkxCWtrNhBpofCbUIjUWylx55ALNkT6w-u2xxGv3EEo7K3b-2azEcKF6Jg6HVhQJRMxP0Q4DyZ4WueSfqEmFHGSBBmfxKTVHjfBtuL6C-k9PNcHMVehHc7l0o3LP~3T7lalwVYd008uX62qGm8Z4mv1MttMV6EatQ1vXd4Jsf9SZn83nBrqQVDv7sqkOnzLZMjTHwb3bL2VV9Yug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. PEF selectively purges myeloma cell lines from mixtures with blood or bone marrow cells. (A) RPMI8226 myeloma cells were mixed 1:1 with PBMCs, and were PEF-treated in standard pulsing buffer (90% isotonic [282 mM] dextrose/10% PBS, pH 7.2) at 0 kV/cm or 1.4 kV/cm, using the flowing PEF apparatus. After ficolling, cells were photographed at ×50 magnification. The distinct large myeloma cells seen at 0 kV/cm (top) are not present at 1.4 kV/cm (bottom). Photographs were taken with a Nikon Diaphot 300 microscope, ×50 magnification, and a Nikon 6006 camera and using IP Labview Macintosh software (National Instruments, Austin, TX). (B) Raw flow cytometry data for PEF-treated and control mixed cells. Flow cytometry was performed on a Becton Dickinson FACSVantage flow cytometer (San Diego, CA), and data were analyzed using CellQuest software. Mixtures of PBMCs and CFDA-SE–labeled RPMI8226 myeloma cells were PEF-treated at 0 kV/cm or 1.4 kV/cm. PBMC suspensions from whole blood or cord blood were seeded with a CFDA-SE–labeled myeloma cell line, RPMI8226 or U266. Mixed cells were resuspended in the high resistivity pulsing buffer (500 Ω-cm) at between 106 and 2 × 106/mL. A quantity of 5 mL to 10 mL of cells was run through the flowing apparatus at a 4 mL/min flow rate, exposing cells to 250 20-μs pulses. Cell samples were treated at different electric field strengths, or pumped through the flowing apparatus without pulsing (0 kV/cm, control group). Cells were stained with anti-CD3/CD19–phycoerythrin (PE), anti-CD14–allophycocyanin (APC), and the nuclear exclusion dye 7-aminoactinomycin (7-AAD). Dot plots represent cells gated as 7-AAD–negative. Dot plots for forward and side scatter (left), CD3/CD19 and CFDA-SE (center), and CD14 and CFDA-SE (right) are shown for stained cells that were PEF-treated at 0 kV/cm (top row) or 1.35 kV/cm (bottom row). The results of this experiment are representative of more than 20 experiments using several different tumor cell lines. (C) Percent survival of RPMI8226 myeloma cells (▴), CD3+/CD19+ lymphocytes (), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2003-12-4399/6/m_zh80050574880001.jpeg?Expires=1768507591&Signature=kjajTwedj-dGRGniL2BziwnucfXxzYH1zdGmQmSpA2YKWWbxtRB~jBRd0lOO8QyLIyTXnpA2pkI5QZQUNFvvvXfrD0km~ER2CPS5~1lTa4dcxRVfezWN800wuXm5zzBsWmsBKmHlRM9LBBXaR809~76aCxs-fF4Iw8BpLzajv5sGppD-cySZdN~ZRrnz~fXBhj~X13rv6qAvTtZmY35N8Fke6wMMdaJ9L2ZQlfSJTJUTxFjfDOdDZdhhup~BOVHBEOOQqohWyL9z0qjjr~8qL2WZ3oF9HHZNm44OG6-M-VcHer3kYv~0u6w3kUnYxFlFLGvIY~L12-IgDYyiRbVuSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.
), and CD14+ monocytes (▪) for mixtures of tumor cells and PBMCs PEF-treated at the indicated electric field strengths. Percent survival for tumor cells and other blood cells was determined as follows: (number of immunostained viable cells in the PEF-treated group)/(number of immunostained viable cells in the control [0 kV/cm] group) × 100. Results are representative of more than 5 experiments using RPMI8226, U266, and other tumor cell lines. (D) Survival of viable PEF-treated myeloma cells, as assayed by serial dilution tumor regrowth. Tumor regrowth assays were performed using triplicate serial dilutions of PEF-treated and 0 kV/cm aliquots of cells in tumor-conditioned medium, then assessing tumor colony formation after 2 weeks at 37°C. Percent survival is determined by comparing regrowth of PEF-treated and 0 kV/cm groups. Results from 1 of 5 representative experiments performed using RPMI8226 tumor cells is shown. (E) Average RPMI8226 tumor purging and standard errors, assessed by different methodologies, for 6 independent experiments is shown in the bar chart. (F) Human bone marrow mononuclear cells were treated with PEF at 0 kV/cm (control) or at 1.4 kV/cm. Cells (2 × 106) from each group were injected into the tail veins of NOD/SCID/β2m-/- mice. After 7 weeks, mice were killed and bone marrow was removed. Mononuclear cells were isolated and stained with antihuman CD45 and antimurine CD45, then analyzed by flow cytometry (BD FACSVantage). Summary of percent human CD45 cells in mice that did not undergo transplantation (control), mice that received a transplant of 0 kV/cm human bone marrow (n = 5), and mice that received a transplant of PEF-treated (1.4 kV/cm) human bone marrow cells. (G) Representative data from CFC and LTC-IC assays performed after PEF at indicated electric field strengths.24 Blue bars indicate LTC-IC; red bars, erythroid blast-forming units/colony-forming units (BFU/CFU-E); and cream bars, granulocyte/macrophage colony-forming units (CFU-GM) + granulocyte/erythroid/monocyte/megakaryocyte colony-forming units (CFU-GEMM). Error bars indicate standard error for means.