Abstract
Vascular endothelial growth factor receptor 2 (VEGFR2/Flk-1)–positive cells derived from embryonic stem (ES) cells serve as vascular progenitors, which differentiate into endothelial cells (ECs) in the presence of VEGF-A. VEGFR3/Flt-4 (fms-like tyrosine kinase 4) signaling is known to be important for the development of lymphatic endothelial cells (LECs). To elucidate the roles of VEGFR3 signaling in the differentiation of vascular progenitor cells into ECs, we introduced various types of VEGFR3 cDNAs into mouse ES cells. VEGF-C, a ligand for VEGFR2 and VEGFR3, stimulated the endothelial differentiation of the VEGFR2+ cells transfected with the VEGFR3 cDNA but not those transfected with kinasenegative mutants of VEGFR3. The VEGFR3-transfected ECs exhibited high expression levels of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), one of the markers of LECs, and showed efficient binding of hyaluronan. VEGF-C(C152S), which is able to activate VEGFR3 but not VEGFR2, failed to induce the endothelial differentiation of mock- and VEGFR3-transfected VEGFR2+ cells, suggesting the essential role of VEGFR2 signaling for endothelial differentiation. Furthermore, kinase-negative mutants of VEGFR3 prevented the VEGF-C–mediated endothelial differentiation of the vascular progenitor cells. Thus, VEGFR2 signaling is required for the endothelial differentiation of mouse ES cells induced by VEGF-C, and VEGFR3 signaling may confer lymphatic endothelial-like phenotypes to ECs.
Introduction
Members of the vascular endothelial growth factor (VEGF) family, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor, regulate various aspects of blood vascular and lymphatic growth and function. VEGF-A acts through the tyrosine kinase receptors VEGFR2 (encoded by kinase insert domain-containing receptor/fetal liver kinase-1 [KDR/Flk-1]) and VEGFR1 (encoded by fms-like tyrosine kinase 1 [Flt-1]), which mediate signals that are essential for vasculogenesis and angiogenesis. VEGF-C and VEGF-D bind to VEGFR2 and VEGFR3 (encoded by Flt-4) and mediate signals to vascular and lymphatic endothelial cells (ECs).1 VEGF-C is expressed in various human cancers1-3 and promotes spread of metastases through induction of angiogenesis and lymphangiogenesis.4-6 Metastatic spread of tumors is also observed by overexpression of VEGF-D through induction of lymphangiogenesis.7 In VEGF-C-/- mice, ECs committed to the lymphatic lineage do not sprout to form lymph vessels, resulting in prenatal death because of the accumulation of fluid in tissues.8
Of the 3 VEGF receptors, VEGFR1 is expressed solely on vascular endothelial cells,9 VEGFR2 is found on both vascular and lymphatic endothelial cells,10 and VEGFR3 is expressed mainly on lymphatic endothelial cells.11 Two splice variants of human VEGFR3 have been identified; the short form lacks 65 amino acids found in the cytoplasmic tail of the long form.12-14 Activation of VEGFR3 leads to protein kinase C–dependent activation of extracellular signal-related kinase 1 (Erk-1) and Erk-2 and activation of protein kinase B/Akt, which support cell proliferation and survival, respectively.10 The VEGFR3-/- embryos die at embryonic day 9.5 because of accumulation of fluid in the pericardial cavity resulting in cardiovascular failure.15 Missense point mutations in one VEGFR3 allele result in inactivation of tyrosine kinase activity and chronic lymphedema.16 Moreover, overexpression of a soluble form of VEGFR3 (VEGFR3-immunoglobulin [Ig]) in mouse skin leads to regression of lymph vessels and development of lymphedema, without any apparent effects on the blood vasculature.17
In adult humans, circulating endothelial precursor cells may be recruited from the stem cell reservoirs to the peripheral circulation and play a critical role in postnatal angiogenesis.18 VEGFR2+ cells derived from peripheral blood can differentiate into ECs.19 Furthermore, VEGFR2+ cells derived from embryonic stem (ES) cells can differentiate into both endothelial and mural cells, which can reproduce the organization processes of vascular tissues. Yamashita et al20 reported that cultivation of undifferentiated mouse ES cells on collagen IV–coated dishes with 10% fetal bovine serum (FBS) results in induction of vascular progenitor cells expressing VEGFR2, and that these cells can be purified by cell sorting using anti–Flk-1/ VEGFR2 antibody. After additional culture of the VEGFR2+ cells in the presence of 10% FBS, mural cells expressing α-smooth muscle actin (SMA) can be obtained, whereas addition of VEGF-A in the presence of 10% FBS results in differentiation of the VEGFR2+ cells into both platelet-endothelial cell adhesion molecule-1 (PECAM-1)–positive ECs and mural cells. They also demonstrated that in the absence of FBS, platelet-derived growth factor (PDGF)–BB, but not PDGF-AA, selectively induced SMA+ mural cells from the VEGFR2+ cells.
Although mechanisms of angiogenesis and vasculogenesis have extensively been studied, those of lymphangiogenesis are still largely unknown, and the origin of lymphatic ECs (LECs) has not yet been fully determined. Mice lacking homeobox transcriptional factor Prox-1 are defective in the initiation program of lymphangiogenesis from cardinal vein ECs, supporting the hypothesis that the lymphatic vascular system is originated from embryonic veins.21 However, in postnatal lymphangiogenesis, it is not clear whether lymphangiogenic factors, including VEGF-C and -D, induce the recruitment of LECs from preexisting lymphatic vessels, or recruit LEC precursors from bone marrow. Although the existence of LEC precursors has not been fully studied, a recent report supports the possibility of the existence of these cells; CD34+/VEGFR3+/CD133+ cells derived from fetal liver were able to differentiate into lymphatic or vascular ECs22 and thus could contribute to postnatal lymphangiogenesis and/or angiogenesis.
LECs express not only VEGFR3 but also VEGFR2,10 suggesting that VEGFR2 signaling is required for induction of endothelial features, including PECAM-1 and vascular endothelial (VE)–cadherin expression. Dixelius et al23 reported that VEGF-C induces the formation of VEGFR3-VEGFR2 heterodimers, in which VEGFR3 is phosphorylated at carboxyl-terminal tyrosine residues by VEGFR2. Moreover, a heterodimer between VEGFR1 and VEGFR2 has been reported to be induced by VEGF-A and placental growth factor.24 These observations suggest that the intermolecular and intramolecular VEGFR cross talk is likely to be important in amplification of each signal transduction pathway through phosphorylation of tyrosine residues.
In the present study, we introduced VEGFR3 cDNA into ES cells and investigated the roles of VEGFR3 signaling in the differentiation of VEGFR2+ vascular progenitor cells into ECs using the system established by Yamashita et al.20 Our findings suggest that VEGFR3 heterodimerizes with VEGFR2 in the ECs and stimulates VEGFR2 signaling induced by VEGF-C. In addition, the VEGFR3 kinase-negative mutants inhibit the differentiation of vascular progenitor cells into ECs by VEGF-C, suggesting that the VEGFR3 mutants may affect the VEGFR2 signaling pathway through the formation of a VEGFR2-VEGFR3 mutant heterodimer. Thus, VEGFR3 signaling alone is not sufficient to induce the differentiation of mouse ES cells into ECs, but that the coordinated action of VEGFR2 and VEGFR3 is required for this process. In addition, we found that VEGFR3 signaling induced the expression of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) in ECs derived from mouse ES cells, suggesting that VEGFR3 signaling may confer lymphatic endothelial-like phenotypes to ECs.
Materials and methods
DNA constructs
Human VEGFR3 (long and short forms) cDNAs were cloned from human embryonic kidney (HEK) 293 cells by polymerase chain reaction (PCR). Unless specifically described, the short form of VEGFR3 was used in the present study. Tyrosine kinase–negative mutants of VEGFR3 (R857R and R1041P) reported by Karkkainen et al16 were generated by a PCR-based method. These cDNAs were cloned into the pCAGIP vector, which has a polyoma origin of replication as described previously.25 Murine VEGF-C cDNA cloned into the pCMV-SPORT6 vector was purchased from Invitrogen (Carlsbad, CA). The VEGF-C(C152S) mutant was generated by a PCR-based method. cDNA for VEGFR2 was kindly provided by L. Claesson-Welsh (Department of Genetics and Pathology, Uppsala University, Uppsala, Sweden) and cloned into pcdef3.
Antibodies
Monoclonal antibodies to murine VEGFR2 (Avas 12α1) conjugated with phycoerythrin (PE) and PECAM-1 (Mec13.3) were purchased from BD Pharmingen (San Diego, CA). Monoclonal antibodies to SMA (1A4) and α-tubulin were obtained from Sigma (St Louis, MO). Monoclonal antibody to phospho-tyrosine (PY20) was purchased from BD Biosciences (San Jose, CA). Polyclonal antibody to human VEGFR3 (C-20), VEGFR2 (C-1158), and PECAM-1 (M-20; used for immunobloting) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Stained cells were visualized using an Olympus IX70 microscope (Tokyo, Japan) at × 40 (aperture 0.13) and × 100 (aperture 0.30). All images were imported into Adobe Photoshop as JPEGs for contrast manipulation and figure assembly.
Cell culture
Mouse ES cells, MGZ-5,25 were maintained in Glasgow minimum essential medium (GMEM; Gibco, Grand Island, NY) supplemented with 10% FBS, 1 mM sodium pyruvate, 0.2% 2-mercaptoethanol, 1% nonessential amino acid solution (Gibco), 1% penicillin-streptomycin solution (Gibco), 103 U/mL leukemia inhibitory factor (LIF; Chemicon, Temecula, CA), 10 μg/mL Zeocin (Invitrogen), and 100 μg/mL G418 (Gibco). For generation of VEGFR2+ cells expressing VEGFR3, MGZ-5 cells were transfected with pCAGIP-VEGFR3 and its mutants by FuGENE6 reagent (Roche, Indianapolis, IN). Transfected cells were selected by 0.5 μg/mL puromycin (Sigma) for 4 days and plated on 10-cm dishes coated with collagen IV (Becton Dickinson, Franklin Lakes, NJ) in differentiation medium20 with G418 and puromycin. After 5 days, VEGFR2+ cells were sorted using an anti–murine VEGFR2-PE antibody by the magnetic activated cell sorting (MACS) system (Miltenyi Biotec, Auburn, CA). The sorted cells were plated on collagen IV–coated 24-well dishes (Becton Dickinson) in the differentiation medium. For serum-free culture, MGZ-5 cells were incubated in SFO3 medium (Sanko Junyaku, Tokyo, Japan) as previously described.20 HEK293 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% FBS and antibiotics and transfected using FuGENE6 reagent.
Preparation of VEGFs
Human VEGF-A and rat VEGF-C were purchased from R&D Systems (Flanders, NJ) and Calbiochem (La Jolla, CA), respectively. VEGF-A and VEGF-C were used at the concentrations of 40 ng/mL and 400 ng/mL, respectively, according to the manufacturer's recommendation. Recombinant VEGF-D (500 ng/mL; R&D Systems) did not significantly activate VEGFR3 (see Figure 1E); therefore, VEGF-A and C were used in the present study. For preparation of HEK293 cell supernatants, cells were transfected with mock, VEGF-C(WT), and VEGF-C(C152S) expression vectors by FuGENE6. After 24 hours, cells were washed 3 times with phosphate-buffered saline (PBS) and cultured in the SFO3 medium for an additional 24 hours. The supernatants were centrifuged and used in the experiments.
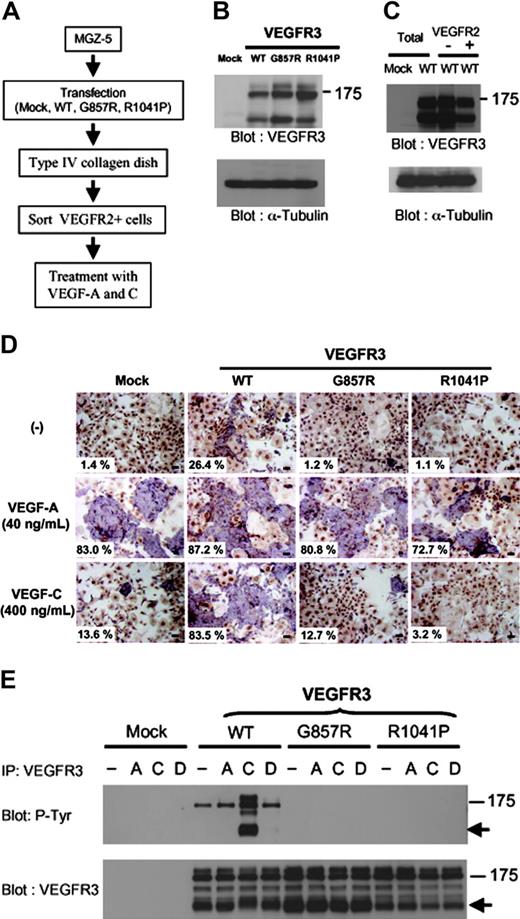
Forced expression of VEGFR3 in VEGFR2+ cells stimulates VEGF-induced endothelial differentiation. (A) A scheme showing transfection and in vitro differentiation of mouse ES cells, MGZ-5. See “Materials and methods” for details. (B-C) MGZ-5 cells were transfected with mock, VEGFR3 wild-type (WT) and kinase-negative mutant (G857R and R1041P) cDNAs. Transfected cells were selected by puromycin and differentiated into mesodermal cells. Expression levels of these constructs were determined by immunoblot analysis using an anti-VEGFR3 antibody (B). The differentiated cells were sorted using an anti-VEGFR2 antibody. Expression levels of VEGFR3(WT) in unsorted (Total), VEGFR2+ and VEGFR2- cells were determined (C). α-tubulin was used as a loading control in panels B and C. (D) MGZ-5 cells transfected with mock, VEGFR3 (WT), and kinase-negative mutants (G857R and R1041P) were differentiated into mesodermal cells and sorted using an anti-VEGFR2 antibody. VEGFR2+ cells were cultured in differentiation medium with or without VEGF-A (40 ng/mL) and VEGF-C (400 ng/mL). After 4 days, cells were doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies. Numbers of ECs and total cells were counted and shown as ECs/total (%). Scale bar, 100 μm. (E) HEK293 cells were transfected with mock, VEGFR3 (WT), and kinase-negative mutant (G857R and R1041P) cDNAs. Transfected cells were treated with or without VEGF-A (40 ng/mL), VEGF-C (400 ng/mL), and VEGF-D (500 ng/mL) for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR3 antibody, followed by immunoblotting using anti-phosphotyrosine (P-Tyr, top panel) and VEGFR3 (bottom panel) antibodies.
Forced expression of VEGFR3 in VEGFR2+ cells stimulates VEGF-induced endothelial differentiation. (A) A scheme showing transfection and in vitro differentiation of mouse ES cells, MGZ-5. See “Materials and methods” for details. (B-C) MGZ-5 cells were transfected with mock, VEGFR3 wild-type (WT) and kinase-negative mutant (G857R and R1041P) cDNAs. Transfected cells were selected by puromycin and differentiated into mesodermal cells. Expression levels of these constructs were determined by immunoblot analysis using an anti-VEGFR3 antibody (B). The differentiated cells were sorted using an anti-VEGFR2 antibody. Expression levels of VEGFR3(WT) in unsorted (Total), VEGFR2+ and VEGFR2- cells were determined (C). α-tubulin was used as a loading control in panels B and C. (D) MGZ-5 cells transfected with mock, VEGFR3 (WT), and kinase-negative mutants (G857R and R1041P) were differentiated into mesodermal cells and sorted using an anti-VEGFR2 antibody. VEGFR2+ cells were cultured in differentiation medium with or without VEGF-A (40 ng/mL) and VEGF-C (400 ng/mL). After 4 days, cells were doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies. Numbers of ECs and total cells were counted and shown as ECs/total (%). Scale bar, 100 μm. (E) HEK293 cells were transfected with mock, VEGFR3 (WT), and kinase-negative mutant (G857R and R1041P) cDNAs. Transfected cells were treated with or without VEGF-A (40 ng/mL), VEGF-C (400 ng/mL), and VEGF-D (500 ng/mL) for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR3 antibody, followed by immunoblotting using anti-phosphotyrosine (P-Tyr, top panel) and VEGFR3 (bottom panel) antibodies.
Immunohistochemistry
For double staining of MGZ-5 cells for PECAM-1 and SMA, cells were harvested on day 4 in the presence of 10% FBS or on day 2 in the absence of serum. The cells were incubated with a mixture of Mec13.3 and 1A4, followed by alkaline phosphatase (ALP)–conjugated anti–rat IgG and horseradish peroxidase (HRP)–conjugated anti–mouse IgG (Zymed Laboratories, San Francisco, CA), as described previously.20
Reverse transcription (RT)–PCR
Total RNA was prepared using Isogen (Nippongene, Toyama, Japan). Reverse transcription was performed with Superscript III (Invitrogen), and PCR was performed using the primers listed in Table 1.
Immunoprecipitation and immunoblotting
Cells were solubilized in a buffer containing 20 mM Tris (tris(hydroxymethyl)-aminomethane)–HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 1.5% Trasylol, and 1 mM phenylmethylsulfonyl fluoride (PMSF). After clearing by centrifugation, cell lysates were directly subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), or immunoprecipitated using anti-VEGFR3 or anti-VEGFR2 antibody. Immunoprecipitates were subjected to SDS-PAGE. Proteins were electrotransferred to polyvinylidene diflouride (PVDF) membranes (ProBlott; Applied Biosystems, Branchburg, NJ) and subjected to immunoblotting. Reacted antibodies were detected using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ).
Hyaluronan (HA) binding assay
HA binding assay was performed as described previously.26 Differentiated cells were incubated in fluorescein isothiocyanate (FITC)–conjugated HA (25 μg/mL; Molecular Probes, Eugene, OR) for 2 hours. Cells were then fixed in 4% formaldehyde, mounted with fluorescent mounting medium, and visualized using a fluorescence microscope. The nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole).
Results
Forced expression of VEGFR3 into ES cells stimulates VEGF-C–induced endothelial differentiation
To investigate the roles of VEGFR3 in the endothelial differentiation of VEGFR2+ vascular progenitor cells, we introduced cDNAs encoding the human short form of VEGFR3 and its kinase-negative mutant (K857R and R1041P) into mouse ES cells, MGZ-5 (Figure 1A). Similar to mouse CCE ES cells,20 MGZ-5 cells differentiate into SMA+ mural cells and ECs expressing PECAM-1, VE-cadherin, and claudin-5 upon stimulation by PDFD-BB and VEGF-A, respectively (Watabe et al27 and T.W., K. Miyazono, unpublished results, June 20, 2003; see Figure 4C). MGZ-5 cells express polyoma virus large T protein and are able to maintain the expression of vectors with a polyoma origin of replication (pCAGIP vector).25 This supertransfection method28 results in efficient episomal propagation of incoming plasmids. In fact, expression of VEGFR3 and its mutants in undifferentiated MGZ-5 cells could be detected by immunoblot analysis for at least 1 month after transfection (data not shown).
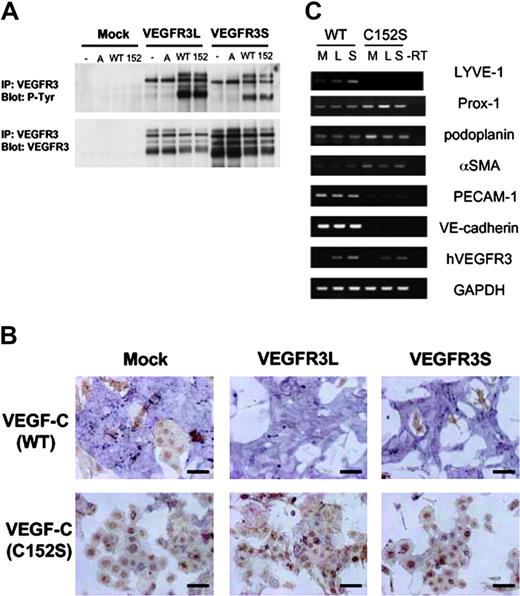
VEGF-C(C152S) did not stimulate the differentiation of VEGFR3-transfected VEGFR2+ cells into ECs. (A) HEK293 cells were transfected with mock and VEGFR3 cDNAs (long and short forms; L and S, respectively). Transfected cells were treated with or without VEGF-A or the supernatant (SFO3) of HEK293 cells transfected with VEGF-C(WT) and (C152S) cDNAs for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR3 antibody, followed by immunoblotting using antiphosphotyrosine (P-Tyr, top) and VEGFR3 (bottom) antibodies. Top blot is phosphorylated VEGFR3; bottom blot, VEGFR3. A indicates VEGF-A; WT, sVEGF-C(WT); 152, sVEGF-C(C152S). (B) VEGFR2+ cells transfected with mock and VEGFR3 (L and S) were cultured in SFO3 serum-free medium in the presence of a supernatant of HEK293 cells as described in panel A. After 2 days, cells were doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies. Scale bar, 100 μm. (C) RT-PCR analysis of vascular and lymphatic endothelial markers in the differentiated ECs described in panel B. M indicates mock; L, long; S, short.
VEGF-C(C152S) did not stimulate the differentiation of VEGFR3-transfected VEGFR2+ cells into ECs. (A) HEK293 cells were transfected with mock and VEGFR3 cDNAs (long and short forms; L and S, respectively). Transfected cells were treated with or without VEGF-A or the supernatant (SFO3) of HEK293 cells transfected with VEGF-C(WT) and (C152S) cDNAs for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR3 antibody, followed by immunoblotting using antiphosphotyrosine (P-Tyr, top) and VEGFR3 (bottom) antibodies. Top blot is phosphorylated VEGFR3; bottom blot, VEGFR3. A indicates VEGF-A; WT, sVEGF-C(WT); 152, sVEGF-C(C152S). (B) VEGFR2+ cells transfected with mock and VEGFR3 (L and S) were cultured in SFO3 serum-free medium in the presence of a supernatant of HEK293 cells as described in panel A. After 2 days, cells were doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies. Scale bar, 100 μm. (C) RT-PCR analysis of vascular and lymphatic endothelial markers in the differentiated ECs described in panel B. M indicates mock; L, long; S, short.
Cells transfected with wild-type (WT) or mutant VEGFR3 cDNAs were selected by puromycin, plated on collagen IV–coated plates, and further cultured without LIF for 5 days. This allowed the differentiation of ES cells into mesodermal cells, including VEGFR2+ cells.20 Expression levels of VEGFR3 and its mutants were equivalent before the sorting (Figure 1B). From the differentiated cells, VEGFR2+ cells were sorted using the anti-VEGFR2 antibody. Expression of VEGFR3 in VEGFR2+ and VEGFR2- cells was confirmed by immunoblot analysis (Figure 1C).
When the VEGFR2+ cells derived from mock-, VEGFR3 WT-, and kinase-negative mutant-transfected ES cells were treated with VEGF-A in the medium containing 10% FBS, they similarly differentiated into ECs (PECAM-1+) and mural cells (SMA+) (Figure 1D). In the absence of VEGF-A, most of the cells differentiated into mural cells, although a small proportion of ECs were observed in the cells transfected with the VEGFR3(WT) cDNA. These results indicate that VEGFR3 and its mutants did not affect the differentiation of ES cell–derived VEGFR2+ cells into ECs in response to VEGF-A. Overexpression of VEGFR3(WT) resulted in activation of its kinase activity, and phosphorylation of the immature form of VEGFR3 (WT) (∼ 175 kDa) was detected in the absence of ligands (Figure 1E). This may explain why VEGFR3(WT)–transfected cells were capable of differentiating into ECs without ligand stimulation.
Next, we treated the VEGFR2+ cells with recombinant VEGF-C, a ligand for VEGFR2 and VEGFR3. VEGFR2+ cells transfected with VEGFR3(WT) differentiated into ECs after treatment with VEGF-C, but those transfected with mock or VEGFR3 mutants did not efficiently do so (Figure 1D). In agreement with this observation, tyrosine phosphorylation of VEGFR3 (mature form; ∼ 125 kDa) was observed after treatment with VEGF-C in HEK293 cells transfected with the VEGFR3(WT) cDNA but not with its mutants (Figure 1E).
FBS contains various growth factors, including PDGFs, which may induce differentiation of the VEGFR2+ cells into mural cells.20 To exclude the effects of other factors derived from serum, we examined the effects of VEGFR3 on cell differentiation under serum-free conditions. VEGF-A induced differentiation of the VEGFR2+ cells mainly into ECs, and only a small number of mural cells were detected under these conditions. Moreover, this phenomenon was not significantly affected by transfection of VEGFR3 WT or mutant cDNAs (Figure 2A-B). When the cells transfected with mock or VEGFR3 kinase mutant cDNAs were treated with VEGF-C, VEGFR2+ cells differentiated mainly into mural cells as judged by the expression of SMA, and only a few small endothelial cells were detected (Figure 2A). In contrast, when VEGFR3(WT) was transfected, some cells differentiated into ECs after treatment with VEGF-C (Figure 2A). Compared with ECs treated with VEGF-A, ECs expressing VEGFR3(WT) cDNA exhibited elongated morphology after VEGF-C treatment (Figure 2B). When the cells were treated with both VEGF-A and VEGF-C, ECs exhibited elongated morphology and sheet formation of ECs was inhibited in VEGFR3 (WT)–transfected cells but not in mock- or mutant-transfected cells (Figure 2A). These results indicate that VEGFR3 stimulates the VEGF-C–induced differentiation of VEGFR2+ progenitor cells into ECs, although sheet formation was affected in VEGFR3(WT)–transfected cells treated with VEGF-C compared with those treated with VEGF-A.
Serum-free culture of sorted VEGFR2+ cells expressing VEGFR3 and its kinase-negative mutants. (A) VEGFR2+ cells transfected with mock, VEGFR3(WT), and its kinase-negative mutant cDNAs were cultured in SFO3 serum-free medium with VEGF-A (40 ng/mL), VEGF-C (400 ng/mL), or VEGF-A and VEGF-C. After 2 days, cells were doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies. (B) Differentiated cells were observed by phasecontrast microscopy. Scale bar, 100 μm.
Serum-free culture of sorted VEGFR2+ cells expressing VEGFR3 and its kinase-negative mutants. (A) VEGFR2+ cells transfected with mock, VEGFR3(WT), and its kinase-negative mutant cDNAs were cultured in SFO3 serum-free medium with VEGF-A (40 ng/mL), VEGF-C (400 ng/mL), or VEGF-A and VEGF-C. After 2 days, cells were doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies. (B) Differentiated cells were observed by phasecontrast microscopy. Scale bar, 100 μm.
sVEGF-C(WT) induces the endothelial differentiation of ES cells
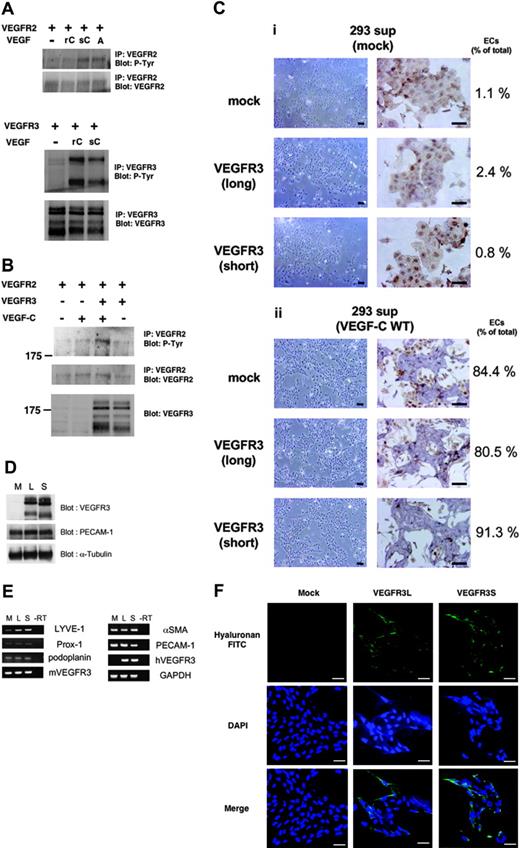
VEGF-C is known to activate both VEGFR2 and VEGFR3; however, 400 ng/mL recombinant VEGF-C failed to phosphorylate VEGFR2 in the present study (Figure 3A). In contrast, VEGF-C (400 ng/mL) induced phosphorylation of VEGFR3, suggesting that higher doses of VEGF-C are required for activation of VEGFR2. We therefore prepared a supernatant of HEK293 cells transfected with wild-type VEGF-C cDNA [sVEGF-C(WT)]. sVEGF-C(WT) appeared to be more potent than recombinant VEGF-C and induced phosphorylation of both VEGFR2 and VEGFR3 (Figure 3A). Interestingly, phosphorylation of VEGFR2 was more strongly induced by VEGF-C in the presence, than in the absence, of VEGFR3 (Figure 3B), suggesting that VEGF-C induced heterodimer formation and intermolecular cross-activation between VEGFR2 and VEGFR3.
Expression of LYVE-1 was induced in VEGFR3-transfected ECs in response to sVEGF-C(WT). (A) HEK293 cells were transfected with VEGFR2 (top) or VEGFR3 (bottom) cDNAs. Transfected cells were treated with or without 400 ng/mL recombinant VEGF-C (rC), sVEGF-C(WT) (sC), and 40 ng/mL VEGF-A (A) for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR2 (top panels) or anti-VEGFR3 (bottom panels) antibodies, followed by immunoblotting using antiphosphotyrosine (P-Tyr) antibody. Expression levels of VEGFR2 and VEGFR3 were confirmed by corresponding antibodies. (B) HEK293 cells were transfected with VEGFR2 and VEGFR3 cDNAs. Transfected cells were treated with or without sVEGF-C(WT) for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR2 antibody, followed by immunoblotting using antiphosphotyrosine (P-Tyr, top) and anti-VEGFR2 (middle) antibodies. Immunoblotting for total cell lysates was performed using anti-VEGFR3 (bottom) antibody. (C) VEGFR2+ cells transfected with mock and VEGFR3 (long and short forms) cDNAs were cultured in SFO3 serum-free medium in the presence of supernatant (SFO3) of HEK293 cells transfected with mock (i) and VEGF-C cDNA (ii). After 2 days, cells were observed by phase-contrast microscopy (left panels) or doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies (right panels). Numbers of ECs and total cells were counted and shown as ECs/total (%). Scale bar, 100 μm. (D) Expression levels of VEGFR3 (long and short forms) in the differentiated ECs were analyzed by immunoblotting using anti-VEGFR3 antibody. Expression levels of PECAM-1 and α-tubulin were confirmed using specific antibodies (lower panels). M indicates mock; L, long; S, short. (E) RT-PCR analysis of lymphatic endothelial markers in the differentiated ECs described in panel C. mVEGFR3 and hVEGFR3 are mouse and human VEGFR3, respectively. (F) Uptake of HA-FITC in the differentiated ECs. Cells on the glass coverslips were treated with 25 μg HA-FITC for 2 hours. After fixation, the coverslips were visualized using a fluorescence microscope. The nuclei were counterstained with DAPI. Scale bar, 100 μm.
Expression of LYVE-1 was induced in VEGFR3-transfected ECs in response to sVEGF-C(WT). (A) HEK293 cells were transfected with VEGFR2 (top) or VEGFR3 (bottom) cDNAs. Transfected cells were treated with or without 400 ng/mL recombinant VEGF-C (rC), sVEGF-C(WT) (sC), and 40 ng/mL VEGF-A (A) for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR2 (top panels) or anti-VEGFR3 (bottom panels) antibodies, followed by immunoblotting using antiphosphotyrosine (P-Tyr) antibody. Expression levels of VEGFR2 and VEGFR3 were confirmed by corresponding antibodies. (B) HEK293 cells were transfected with VEGFR2 and VEGFR3 cDNAs. Transfected cells were treated with or without sVEGF-C(WT) for 20 minutes. Cell lysates were immunoprecipitated by anti-VEGFR2 antibody, followed by immunoblotting using antiphosphotyrosine (P-Tyr, top) and anti-VEGFR2 (middle) antibodies. Immunoblotting for total cell lysates was performed using anti-VEGFR3 (bottom) antibody. (C) VEGFR2+ cells transfected with mock and VEGFR3 (long and short forms) cDNAs were cultured in SFO3 serum-free medium in the presence of supernatant (SFO3) of HEK293 cells transfected with mock (i) and VEGF-C cDNA (ii). After 2 days, cells were observed by phase-contrast microscopy (left panels) or doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies (right panels). Numbers of ECs and total cells were counted and shown as ECs/total (%). Scale bar, 100 μm. (D) Expression levels of VEGFR3 (long and short forms) in the differentiated ECs were analyzed by immunoblotting using anti-VEGFR3 antibody. Expression levels of PECAM-1 and α-tubulin were confirmed using specific antibodies (lower panels). M indicates mock; L, long; S, short. (E) RT-PCR analysis of lymphatic endothelial markers in the differentiated ECs described in panel C. mVEGFR3 and hVEGFR3 are mouse and human VEGFR3, respectively. (F) Uptake of HA-FITC in the differentiated ECs. Cells on the glass coverslips were treated with 25 μg HA-FITC for 2 hours. After fixation, the coverslips were visualized using a fluorescence microscope. The nuclei were counterstained with DAPI. Scale bar, 100 μm.
sVEGF-C(WT) stimulated the differentiation of mock- and VEGFR3 (long and short forms)–transfected VEGFR2+ cells into ECs (> 80%), whereas a supernatant of mock-transfected HEK293 cells did not (Figure 3C). Furthermore, when the sVEGF-C(WT) was diluted 10-fold it stimulated the differentiation of VEGFR3-transfected VEGFR2+ cells into ECs but not that of mock-transfected cells (Supplementa1 Figure S1, available at the Blood website; see the Supplemental Figure link at the top of the online article), similar to the results using recombinant VEGF-C (Figures 1, 2). These results support the findings that sVEGF-C(WT) stimulates VEGFR2 more potently than the recombinant VEGF-C. We also examined the expression of pan-endothelial marker PECAM-1 in the differentiated ECs by sVEGF-C(WT). The expression levels of VEGFR3 (long and short forms; L and S, respectively) were similar in the cells transfected with the corresponding cDNAs. Figure 3D shows that the expression levels of PECAM-1 were similar in the ECs derived from mock- and VEGFR3 (long and short forms)–transfected VEGFR2+ cells.
Increased expression of LYVE-1 in VEGFR3-transfected ECs
VEGFR3 is known to be important for the differentiation, growth, and survival of LECs.10 To examine whether VEGF-C induces differentiation of the VEGFR-2+ cells into LECs, the expression of markers for LECs, LYVE-1, Prox-1, and podoplanin29 was measured by semiquantitative RT-PCR analysis. As shown in Figure 3E, the expression of LYVE-1 was increased in both VEGFR3L- and S-transfected cells, but the expression of Prox-1 and podoplanin was not affected.
LYVE-1 is a receptor for the extracellular matrix mucopolysaccharide HA.30 We next investigated the binding of HA to ECs. As shown in Figure 3F, the binding of HA was increased in VEGFR3L- or VEGFR3S-transfected ECs. These results suggest that VEGFR3 signaling induces the expression of LYVE-1 and that the long and short forms of VEGFR3 do not confer significant differences in the induction of differentiation of vascular progenitor cells into ECs expressing LYVE-1.
VEGF-C(C152S) fails to induce the differentiation of VEGFR3-transfected VEGFR2+ cells into ECs
VEGF-C(C152S) is known as a selective agonist of VEGFR3.31 To investigate whether VEGFR3 signaling can compensate for that of VEGFR2 in the process of endothelial cell differentiation, we prepared a supernatant of HEK293 cells transfected with VEGF-C(C152S) cDNA [sVEGF-C(C152S)]. Tyrosine phosphorylation of VEGFR3 (L and S) by sVEGF-C(WT) and sVEGF-C(C152S) was similar (Figure 4A). Figure 4B shows that mock- and VEGFR3-transfected VEGFR2+ cells were induced to differentiate into ECs by treatment with sVEGF-C(WT), as observed in Figure 3C. Treatment with sVEGF-C(C152S), however, failed to induce the differentiation of either mock- or VEGFR3-transfected VEGFR2+ cells into ECs; instead, these cells differentiated into mural cells. This observation was confirmed by RT-PCR analysis that showed decreased expression of PECAM-1 and VE-cadherin (Figure 4C). Furthermore, the expression of LYVE-1 was restricted to the cells treated with sVEGF-C(WT), suggesting that LYVE-1 is expressed in ECs, but not in mural cells, in the presence of VEGFR3 signaling. The finding that sVEGF-C(C152S) failed to induce the differentiation of mock-transfected VEGFR2+ cells into ECs confirms that sVEGF-C(C152S) has lost the ability to stimulate VEGFR2. These results indicate that VEGFR3 signaling alone is not sufficient to induce the differentiation of vascular progenitor cells into ECs; instead, VEGFR2 signaling is essential for this process.
Effects of kinase-negative mutants of VEGFR3 on the differentiation of vascular progenitor cells into ECs
Kinase-negative mutants of VEGFR3 (G857R, R1041P) were originally identified in patients with hereditary lymphoedema, a disease that is characterized by swelling of the extremities.32 We examined the effects of VEGFR3 mutants on the endothelial differentiation of VEGFR2+ cells induced by VEGF-C. As shown in Figure 5A, VEGFR2+ cells expressing VEGFR3(G857R) or VEGFR3(R1041P) exhibited a reduced number of cells and small endothelial sheets upon treatment with sVEGF-C(WT) compared with mock- and VEGFR3(WT)–transfected cells. The total number of cells (ECs plus mural cells) was reduced by transfection of the VEGFR3 mutant cDNAs (Figure 5B). In contrast, the total number of cells treated with VEGF-A was only slightly reduced by transfection of VEGFR3(G857R) or VEGFR3(R1041P) (Figure 5C). VEGFR3 mutants thus inhibited the differentiation and survival of endothelial cells treated with VEGF-C but did not affect those treated with VEGF-A. These findings suggest that VEGFR2 signaling activated by VEGF-C can be blocked dominant negatively by the VEGFR3 mutants.
Kinase-negative mutants of VEGFR3 inhibited the differentiation of ES cell–derived VEGFR2+ cells into ECs induced by VEGF-C but not by VEGF-A. (A) VEGFR2+ cells transfected with mock and VEGFR3 (WT, G857R, R1041P) cDNAs were cultured in SFO3 serum-free medium in the presence of sVEGF-C(WT). After 2 days of culture, cells were observed by phase-contrast microscopy (top row) or doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies (bottom row). Scale bar, 100 μm. (B-C) The numbers of cells treated with sVEGF-C(WT) (B) and VEGF-A (C) were determined by Coulter counter.
Kinase-negative mutants of VEGFR3 inhibited the differentiation of ES cell–derived VEGFR2+ cells into ECs induced by VEGF-C but not by VEGF-A. (A) VEGFR2+ cells transfected with mock and VEGFR3 (WT, G857R, R1041P) cDNAs were cultured in SFO3 serum-free medium in the presence of sVEGF-C(WT). After 2 days of culture, cells were observed by phase-contrast microscopy (top row) or doubly stained with anti–PECAM-1 (purple) and anti-SMA (brown) antibodies (bottom row). Scale bar, 100 μm. (B-C) The numbers of cells treated with sVEGF-C(WT) (B) and VEGF-A (C) were determined by Coulter counter.
Discussion
In the present study, we have shown that the transfected VEGFR3 appears to heterodimerize with endogenous VEGFR2 in the ECs and that VEGF-C stimulates VEGFR2 signaling in the presence of VEGFR3. VEGF-C(C152S), which is able to activate VEGFR3 but not VEGFR2, failed to induce the differentiation of mock- and VEGFR3-transfected VEGFR2+ cells into ECs. Moreover, the VEGFR3 kinase-negative mutants inhibited the differentiation of vascular progenitor cells into ECs by VEGF-C, suggesting that the VEGFR3 mutants may affect the VEGFR2 signaling pathway through the formation of a heterodimer between VEGFR2 and the VEGFR3 mutants.
sVEGF-C(WT) induced the differentiation of mock- as well as VEGFR3-transfected VEGFR2+ cells into ECs. In contrast, recombinant VEGF-C (Figures 1, 2) and a low dose of sVEGF-C(WT) (Supplemental Figure S1) induced the endothelial differentiation of VEGFR3-transfected, but not mock-transfected, VEGFR2+ cells. In agreement with these observations, sVEGF-C(WT) induced phosphorylation of both VEGFR2 and VEGFR3, whereas recombinant VEGF-C phosphorylated VEGFR3 but not VEGFR2 (Figure 3A). We cannot rule out the possibility that other factors derived from the supernatants of HEK293 cells support the action of sVEGF-C(WT). However, sVEGF-C(C152S), which is able to act on VEGFR3 but not on VEGFR2, failed to induce the differentiation of the VEGFR3-overexpressing cells into ECs (Figure 4). These results suggest that VEGF-C can activate VEGFR2 only at high concentrations and that VEGFR3 signaling alone is not sufficient to induce the differentiation of vascular progenitor cells into ECs.
Matsumura et al33 reported that on the OP9 feeder layer, ECs formed packed clusters, while VEGF-A induced EC dispersion. In contrast, VEGF-C did not induce EC dispersion but induced morphologic changes of ECs, eg, an elongated spindle-like phenotype. OP9 cells produce various angiogenic growth factors, including VEGF-A and VEGF-C, and support the growth of EC cells. Similar to the observation by Matsumura et al,33 we found that addition of VEGF-C induced elongated morphology of ECs in the presence as well as in the absence of VEGF-A (Figure 2B). VEGFR2 and VEGFR3 may transduce different signaling pathways, and the pathways that induce elongated morphology of ECs may be specifically activated by VEGFR3. There are several papers regarding the morphology of LECs. Makinen et al10 reported that LECs show elongated morphology similar to our observation, whereas others did not see any morphologic differences between vascular ECs and LECs.34,35 These may be due to the differences in culture conditions. We have recently found that human umbilical vein endothelial cells (HUVECs) express high levels of VEGFR3 mRNA and show elongated morphology when they are transfected with Prox-1 cDNA (T.W., K. Miyazono, unpublished observations, July 12, 2004). These findings suggest that the morphologic alterations in HUVECs may also be induced by the VEGF-C–VEGFR3 signaling pathway.
In humans, but not in mice, VEGFR3 has 2 spliced variants.13,14 In the present study, we found that both forms of VEGFR3 stimulated EC differentiation and the up-regulation of mRNA of LYVE-1 but not of other LEC markers (Figure 3). These results suggest that the 2 spliced variants of VEGFR3 possess similar abilities to stimulate the differentiation of the vascular progenitor cells into ECs via VEGFR2. The long form of VEGFR3, but not the short form, has transforming ability in fibroblasts through the phosphorylation at tyrosine 1337 (Y1337), which is present only in the long form.36 Shc and Grb2 bind to the phosphorylated Y1337 and regulate the VEGFR3-mediated signal transduction.37,38 Therefore, signals induced by the long form of VEGFR3 may be important for oncogene-like activity such as cell proliferation and migration of differentiated LECs but not for endothelial differentiation.
LYVE-1 is the first lymph-specific HA receptor to be characterized and is involved in HA uptake and transport in lymphatic vessels.30 HA is an extracellular matrix glycosaminoglycan that plays an important role in maintaining tissue integrity, as well as facilitating the migration of cells during embryonic morphogenesis, inflammation, and wound repair. HA enters the afferent lymphatic vessels and is transported to the draining lymph nodes, where it is degraded before entering the circulation.39,40 Therefore, VEGFR3 signaling may determine the function of LECs through the expression of LYVE-1.
Overexpression of the homeobox transcription factor Prox-1 in the blood vascular ECs induced transcription of LEC-specific genes, including VEGFR3, and suppressed the expression of blood vascular EC-specific genes.41,42 These findings showed the diversity of endothelial cell phenotypes and suggested that Prox-1 determines the cell fate of ECs to LECs. In the present study, overexpression of VEGFR3 into ES cell–derived vascular progenitor cells stimulated the expression of LYVE-1 but not that of Prox-1 (Figure 3). Since Prox-1 has been reported to induce the expression of VEGFR3, the former might act as an upstream component of the latter and confer LEC-like phenotype on ECs during the process of lymphangiogenesis. However, LYVE-1 is expressed in all ECs of the anterior cardinal vein around embryonic day 9.5, whereas expression of Prox-1 is restricted to a subpopulation of ECs on one side of the vein at this stage,43 suggesting that expression of LYVE-1 may be regulated by signals other than Prox-1 or VEGF-C. Moreover, we found that podoplanin was not induced by VEGFR3, suggesting that VEGFR3 signaling alone is not sufficient to induce LECs. Further studies will be required to determine which signals are required for induction of LECs in cooperation with VEGF3 signaling.
Prepublished online as Blood First Edition Paper, November 23, 2004; DOI 10.1182/blood-2004-07-2547.
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Science, Sports and Technology of Japan.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank H. Niwa for providing the pCAGIP vector and MGZ-5 cells, and L. Claesson-Welsh for the VEGFR2 cDNA. We are grateful to T. Imamura and M. Saitoh for helpful discussions.