Abstract
It remains controversial whether alloreactive donor-derived natural killer (NK) cells display graft-versus-leukemia reactions after unmodified allogeneic hematopoietic stem cell transplantation (HSCT). The present study evaluated the role of inhibitory killer immunoglobulin–like receptor (KIR) ligand incompatibility using a well-defined and uniform setting of unmodified allogeneic HSCT in 374 patients with myeloid leukemias. The most striking finding was a significant heterogeneity in the 5-year estimates of hematologic leukemic relapse after human leukocyte antigen (HLA)–identical (n = 237; 22%), HLA class I–disparate (n = 89; 18%), and KIR ligand–incompatible transplantations (n = 48; 5%) (P < .04). Multivariate analysis confirmed that the relative relapse risk (RR) was influenced by HLA class I disparity alone (RR 0.49), but was lowest after HLA class I–disparate, KIR ligand–incompatible transplantations (RR 0.24) (P < .008). The primary graft failure rates, however, increased from 0.4% after HLA class I–identical to 2.3% after HLA class I–disparate, and to 6.3% after KIR ligand–incompatible transplantations, respectively (P < .02). Unlike some other reports, no beneficial effect of KIR ligand incompatibility on other major endpoints of allogeneic HSCT (transplantation-related mortality, and overall and event-free survival) was detectable in the present study. In conclusion, unmodified allogeneic HSCT from KIR ligand–incompatible donors provides a superior long-term antileukemic efficacy in patients with myeloid malignancies.
Introduction
It is well established that the curative effect of allogeneic hematopoietic stem cell transplantation (HSCT) largely depends on allospecific donor immune cells, which can react against a broad array of polymorphic antigens expressed on residual malignant recipient cells. In transplantations between major histocompatibility complex (MHC) matched related or unrelated individuals, these graft-versus-leukemia (GvL) reactions are predominantly attributable to donor T lymphocytes as demonstrated by compelling laboratory and clinical evidence.1-6 Anovel antileukemic mechanism, which relies on allospecific interactions between donor natural killer (NK) cells and recipient leukemic cells, has been recently demonstrated in the clinical setting of CD34+ cell–enriched, and thereby T-cell–depleted HSCT from MHC haplotype–disparate related donors.7,8 The pivotal structural elements underlying this mechanism are inhibitory killer immunoglobulin–like receptors (KIRs), which are clonally expressed on the surface of NK cells or certain T-cell subsets, and membrane-bound MHC class I molecules as their natural ligands on target cells.9-11 If inhibitory KIRs recognize and bind to self–MHC class I epitopes on target cells, a transmembrane signal cascade leads to inhibition of the cytolytic NK cell repertoire. Inhibitory KIRs predominantly bind to HLA-C molecules, and the specificity of this receptor-ligand interaction is based upon a genetic dimorphism in exon 2 of the human leukocyte antigen (HLA)–C α-domain gene.12 Depending on the inhibitory KIR binding motifs of the corresponding amino acid residues at positions 77 and 80, 2 distinct groups of HLA-C molecules can be distinguished. Group 1 HLA-C molecules (Ser77Asn80) are the natural ligands for allelic KIR2DL2 and KIR2DL3 receptors, whereas group 2 HLA-C molecules (Asn77Lys80) recognize the KIR2DL1 receptor.9 In addition, HLA-A and HLA-B molecules are involved in the allorecognition process of inhibitory KIRs. While the HLA-A alleles A*03 and A*11 represent natural ligands for the inhibitory receptor KIR3DL2, other HLA-A alleles have no role in the interactions with KIRs.13 Similarly, the HLA-B alleles with the Bw4 public epitope are recognized by KIR3DL1, while no KIRs for the Bw6 motif have so far been identified.11
In contrast to the receptor-ligand interactions of inhibitory KIRs, the natural ligands of most MHC class I–specific activating KIRs are currently not well defined. The activating KIR2DS1 and KIR2DS2/3 receptors have identical extracellular domains and recognize the same binding motifs of HLA-C molecules as their inhibitory counterparts.14 Several features of inhibitory KIRs have been described which potentially explain their dominant influence on NK cell function over their activating counterparts. Among others, inhibitory KIRs can actively prevent the transfer of lipid rafts, which contain activating accessory molecules to the immune synapse.15-17 A higher affinity of inhibitory compared with activating KIRs to their common HLA recognition sites may also contribute to the dominance of inhibition. It has further been suggested that inhibiting and activating receptors with specificity for the same HLA allele may respond differentially depending on the peptides bound by their HLA ligands.18 Due to the predominance of inhibitory KIRs on NK cell function, NK cells will only become activated if inhibition is not present and activating KIRs are at the same time stimulated by their as-yet-undefined high-affinity natural ligands. Candidate ligands include non-MHC molecules, such as foreign or microbial antigens expressed on infected cells, normal cell-surface proteins that are aberrantly expressed, stress-induced proteins, or other pathogen-derived peptides bound to MHC class I molecules.
As a consequence of these receptor-ligand interactions, only those donor NK cell clones will become operative against residual recipient leukemic cells after allogeneic HSCT, whose inhibitory KIRs remain unstimulated by their group-specific MHC class I alleles.8,19 It has been unequivocally demonstrated that HLA-C molecules are the predominant MHC class I allotypes involved in the inhibitory and activating regulation of NK cells, since alloreactive donor NK cell clones with lytic activity against recipient cells can be regularly detected if a donor group–specific HLA-C allele is not shared by the recipient.7,8 In contrast, other KIR ligand incompatibilities between donor and recipient are generally associated with varying frequencies of detectable antihost NK cell clones. As strong experimental support for the clinical significance of the antileukemic activity of alloreactive NK cells, it has recently been demonstrated that the transfer of KIR ligand–incompatible NK cell clones induces durable remissions in a human myeloid leukemia nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model.8
While the superior antileukemic efficacy of inhibitory KIR ligand–incompatible CD34+ cell–enriched HSCT from MHC haplotype–disparate related donors in patients with myeloid leukemias has been convincingly demonstrated, it is currently controversial whether unmodified transplants from MHC class I partially matched related or unrelated donors (URDs) can also contribute to the curative potential of allogeneic HSCT, if a KIR ligand incompatibility in graft-versus-host direction is present. One recent retrospective analysis in patients who underwent URD marrow transplantation indeed supports that KIR ligand incompatibility not only reduces the risk of relapse in patients with myeloid leukemias, but additionaly exerts a dramatic beneficial effect on overall and disease-free survival.20 In contradiction, a previous study in URD marrow transplantation patients revealed no differences in the major transplantation endpoints between KIR ligand–compatible and –incompatible donor-recipient pairs.21 Since the unresolved question of an enhanced antileukemic efficacy associated with inhibitory KIR ligand–incompatible transplantations might have important implications for the donor selection process, we exploited our comprehensive experience with allogeneic HSCT using partially matched related or unrelated donors to further evaluate the clinical outcome of KIR ligand incompatibilities as predicted by MHC class I genotyping in a large single-center cohort of patients with myeloid leukemias.22 Focusing on hematologic leukemic relapse as well as on other major clinical endpoints of allogeneic HSCT, particular consideration was given to the effects exerted by exclusive MHC class I disparities compared with those additionally encompassing KIR ligand incompatibilities.
Patients, materials, and methods
Patient population
The present study is based on patients who underwent allogeneic HSCT at our institution between January 1997 and December 2002. The initial inclusion timepoint was chosen to ensure that for all analyzed donor-recipient pairs DNA-based HLA class I and class II genotyping had been prospectively performed according to the previously published methods.22 In order to reduce or exclude confounding factors related to the transplantation, the following eligibility criteria were applied: (1) first transplantation (ie, no prior allo- or autotransplantations); (2) T-cell–replete grafts; (3) institutional standard myeloablative regimen (2.5 Gy cobalt 60 total body irradiation per day administered on 4 consecutive days followed by 120 mg/kg body weight [BW] cyclophosphamide); (5) institutional standard acute graft-versus-host disease (GVHD) prophylaxis using short-course methotrexate and cyclosporine.23 Since a reduced relapse risk of KIR ligand–incompatible transplantations has been predominantly described in patients with myeloid malignancies as the underlying disease at present, the analysis was further restricted to patients who underwent allogeneic HSCT for acute myeloid leukemia (AML), chronic myeloid leukemia (CML), or myelodysplastic syndrome (MDS) after leukemic transformation.8,20 The study was approved by the institutional review board of the Department of Bone Marrow Transplantation at the University Hospital of Essen. Written informed consent was obtained for all aspects of the transplantation procedure in accordance with the Declaration of Helsinki.
Histocompatibility testing and assignment of KIR ligand incompatibility
Pretransplantantion histocompatibility testing of donors and recipients generally consisted of low- and intermediate-resolution HLA-A, -B, -C, and high-resolution HLA-DRB1 and -DQB1 DNA-based typing as previously published.22 If low-resolution typing revealed the antigens HLA-Cw3, Cw4, Cw7, Cw12, Cw14, Cw15, and Cw16, HLA-C sequence–specific typing was additionally performed for the following reasons: the presence or absence of the natural ligands for the inhibitory receptors KIR2DL1, KIR2DL2, KIR2DL3, KIR3DL1, and KIR3DL2 was predicted according to HLA-typing results as previously proposed by others.7,8,19 However, in case of the above HLA-C antigens, a definite assignment of their KIR ligand group specificity can only be assured by HLA-C sequencing, because their individual allelic gene products act as ligands for different group-specific KIR receptors. As an example, most Cw7 alleles such as *0701, *0702, *0703, and so forth, belong to the Ser77Asn80 (group 2) amino acid sequence group and are therefore ligands of inhibitory KIR2DL2 and KIR2DL3 receptors. In contrast, the alleles *0707 and *0709 have the Asn77Lys80 dimorphism, and thus bind to the inhibitory KIR2DL1 receptor. No sequencing was performed for the HLA-C antigens Cw17 and Cw18 because all currently defined Cw17 and Cw18 alleles are ligands for KIR2DL1. Genotypic KIR ligand incompatibility in graft-versus-host direction was only assumed if donor HLA-C alleles with an identical group specificity for inhibitory KIR receptors were not present in the recipient. Patient and donor demographic characteristics categorized to subsets of HLA class I–identical transplantations (group 1), HLA class I–disparate and KIR ligand–compatible transplantations (group 2), and HLA class I–disparate, but KIR ligand–incompatible transplantations (group 3) are summarized in Table 1.
Clinical study endpoints
Diagnosis of leukemic relapse after transplantation was based on cytomorphologic and flow-cytometry studies together with cytogenetic or molecular analysis of disease-specific markers whenever applicable. Thus, isolated cytogenetic or molecular findings of disease persistence or recurrence without progression to hematologic relapse were not considered as relapsed leukemic disease. For the purpose of the analysis on transplantation outcome, patients were stratified into 2 categories according to the risk of leukemic relapse after transplantation. An early disease stage was assumed if patients were treated in the first remission of acute leukemia or in the first chronic phase of CML. This category further included all patients with MDS whose pretransplantation marrow blast content was below 5% at the time of transplantation. Accordingly, all other disease stages were categorized as advanced disease. Primary graft failure (GF) was assumed if sustained blood cell recovery was not detectable until day 30 after transplantation (with day 0 designating the day of transplantation), studies of chimerism revealed no evidence for donor cell hematopoiesis, and day-30 marrow biopsies demonstrated severe reduction of hematopoietic tissue. The diagnosis and grading of acute and chronic GVHD followed the commonly accepted definitions.24,25 Patients with GF were censored for the endpoints of relapse and acute and chronic GVHD at day 30 after transplantation. Transplantation-related mortality (TRM) included lethal events from any cause other than hematologic relapse after transplantation. Overall survival (OS) was defined as survival from transplantation without lethal event from any cause. Event-free survival (EFS) was defined as survival from transplantation in hematologic remission without lethal events from any cause.
Statistical analysis
All patient/donor demographic and transplantation outcome data were prospectively collected and entered into the institutional HSCT database. Comparisons between classified covariates were performed by the 2-tailed Fisher exact test unless otherwise stated. Continuous covariates were compared by the Wilcoxon rank-sum or Kruskal-Wallis statistics. Time-to-event estimates were calculated by the product-limit method with right censoring of subjects at the last timepoint at which they were at risk for a given event. Time-to-event distribution functions between strata were compared by the log-rank test. To evaluate the influence of different explanatory covariates on the times to achieve the major endpoints of transplantation outcome, forward and backward stepwise proportional hazard general linear model (PHGLM) analysis was performed. Explanatory covariates were allowed to enter model-building with an error significance level below 5% after adjustment for covariates in the model. Acute (grades 0-I vs II-IV) and chronic GVHD (no vs yes) were used as time-dependent covariates during model-building. To account for the multiple-test situation, only those explanatory covariates with an adjusted error significance level below 1% after forward and backward PHGLM selection were regarded significant. Relative risk (RR) estimates and their 95% confidence limits (95% CL) were derived from the PHGLM analysis after adjustment for all significant covariates in the models. The median follow-up time of survivors in the 3 patient subsets is 41 months (range, 14-86 months) in group 1; 56 months (15-86 months) in group 2; and 59 months (range, 15-86 months) in group 3. Date of the final data analysis was September 30, 2004.
Results
Leukemic relapse
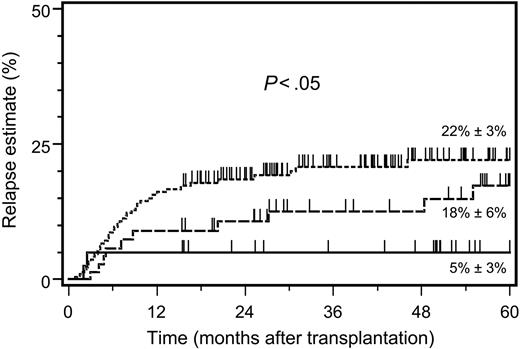
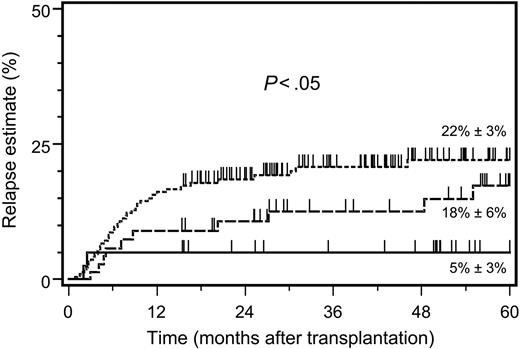
The 5-year relapse estimate was clearly lower in patients with KIR ligand–incompatible donors compared with HLA-identical and HLA class I–disparate, KIR ligand–compatible donors (Figure 1). Notably, the 2 relapse events after KIR ligand–incompatible transplantations occurred exclusively within the first 3 months after transplantation, while in the 2 other patient subsets, relapse events emerged continuously throughout the 5-year follow-up period (Figure 1). The striking influence of KIR ligand incompatibility on the 5-year relapse estimate was detectable in early as well as in advanced disease stages and surpassed substantially the influence exerted by HLA class I disparity alone (Table 2). Multivariate analysis confirmed that patient assignment to the 3 study subsets remained an independent predictor of the relapse risk after adjustment for the predominant effect of the disease stage (Table 3).
Five-year relapse estimates after allogeneic hematopoietic stem cell transplantation. Estimates are shown after HSCT from HLA-identical donors (dotted line; n = 237), HLA class I–disparate, inhibitory KIR ligand–compatible donors (dashed line; n = 89), and HLA class I–disparate, inhibitory KIR ligand–incompatible donors (solid line; n = 48). Significance for the heterogeneity between the 3 study groups tested by log-rank statistics.
Five-year relapse estimates after allogeneic hematopoietic stem cell transplantation. Estimates are shown after HSCT from HLA-identical donors (dotted line; n = 237), HLA class I–disparate, inhibitory KIR ligand–compatible donors (dashed line; n = 89), and HLA class I–disparate, inhibitory KIR ligand–incompatible donors (solid line; n = 48). Significance for the heterogeneity between the 3 study groups tested by log-rank statistics.
Primary graft failure
There was significant heterogeneity between the 3 study groups regarding GF as the primary endpoint, and the highest GF risk was associated with KIR ligand–incompatible transplantations (P < .02; Table 2). Although the rareness of GF events precluded further multidimensional statistical analysis on this endpoint, the present analysis suggests that the increased GF risk after HLA class I–disparate transplantations is even higher if KIR ligand incompatibility is additionally involved. This can be explained by the fact that in KIR ligand–incompatible donor-recipient pairs, HLA class I disparity in host-versus-graft direction is inevitably involved, and thereby further increases the risk of GF. In contrast, an isolated HLA class I disparity in graft-versus-host direction (ie, an HLA class I antigen–homozygous donor for an HLA class I antigen–heterozygous recipient sharing 1 HLA antigen) entails KIR ligand compatibility and, consequently does not affect the risk of GF.
Acute and chronic graft-versus-host disease
Grades II-IV acute GVHD estimates were nearly identical for HLA class I–disparate, KIR ligand–compatible transplantations (58% ± 5%) and for KIR ligand–incompatible transplantations (55% ± 8%), whereas HLA-identical transplantations were associated with a significantly lower risk of grades II-IV acute GVHD (39% ± 3%) (P < .001). Multivariate analysis confirmed that HLA class I disparity was the only independent predictor of acute GVHD and was associated with a 1.8 (95% CL: 1.3-2.5) times higher relative risk for this condition as opposed to HLA-identical transplantations (P < .001). Estimates of chronic GVHD increased from 43% ± 4% (group 1) to 65% ± 6% (group 2), and to 78% ± 9% (group 3), respectively (P < .002). Two independent factors for the development of chronic GVHD were identified by multivariate analysis: preceding grades II-IV acute GVHD (RR 1.9 [95% CL: 1.5-2.7], P < .001) and patient assignment to study group (HLA class I–disparate, KIR ligand–compatible transplantations: RR 1.4 [95 CL: 1.1-1.6] and HLA class I–disparate, KIR ligand–incompatible transplantations: RR 1.9 [95 CL: 1.6-2.4], P < .008).
Transplantation-related mortality, overall survival, and event-free survival
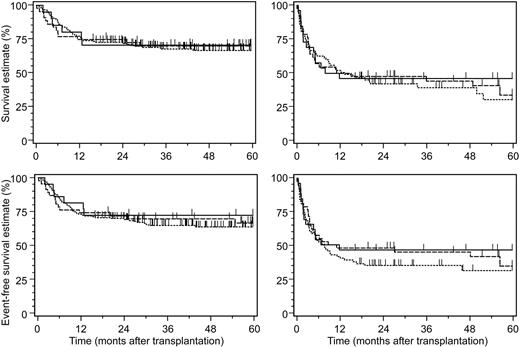
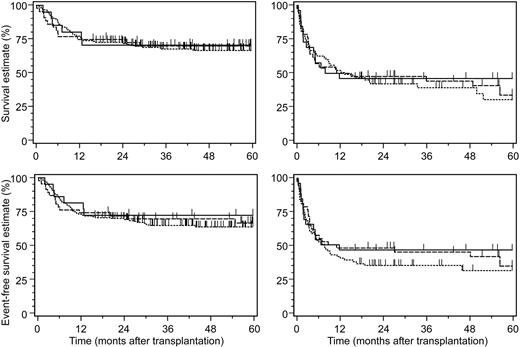
The 3 patient subsets did not differ significantly in their estimates on TRM, OS, and EFS (Table 2). These clinical endpoints were predominantly determined by the pretransplantation disease stage and patient age, and furthermore by the development of grades II-IV acute GVHD after transplantation (Table 3). Despite the increased rates of primary GF and of GVHD after KIR ligand–incompatible transplantation, the complete lack of leukemic relapses beyond 3 months after transplantation apparently compensated for these adverse factors on OS and EFS when compared to KIR ligand–compatible transplantations (Figure 2).
Five-year survival estimates after allogeneic hematopoietic stem cell transplantation. Shown are survival estimates (top row) and 5-year event-free survival estimates (bottom row) according to disease stage (left panels indicate early disease stages; right panels, advanced disease stages) after allogeneic hematopoietic stem cell transplantation from HLA-identical donors (dotted line), HLA class I–disparate, inhibitory KIR ligand–compatible donors (dashed line), and HLA class I–disparate, inhibitory KIR ligand–incompatible donors (solid line). All differences between the 3 study groups were not significant
Five-year survival estimates after allogeneic hematopoietic stem cell transplantation. Shown are survival estimates (top row) and 5-year event-free survival estimates (bottom row) according to disease stage (left panels indicate early disease stages; right panels, advanced disease stages) after allogeneic hematopoietic stem cell transplantation from HLA-identical donors (dotted line), HLA class I–disparate, inhibitory KIR ligand–compatible donors (dashed line), and HLA class I–disparate, inhibitory KIR ligand–incompatible donors (solid line). All differences between the 3 study groups were not significant
Discussion
The present study investigated the role of genotypic inhibitory KIR ligand incompatibility between recipient and donor on the major clinical endpoints of allogeneic HSCT in a well-defined and uniform transplantation setting. We have chosen this approach to avoid procedural heterogeneities, which may interfere with the potential impact of KIR ligand incompatibility on these endpoints. This appears particularly important because previous reports led to conflicting results with regard to the influence of KIR ligand incompatibility in conventional transplantation settings, which can partly be attributed to differences between the investigated patient populations and to varying transplantation procedures.20,21 In addition, the number of patients with KIR ligand–incompatible donors was relatively small in previous reports as a consequence of the current practice of HLA matching. Hence, potential biologic effects exerted by KIR ligand incompatibility in conventional transplantation settings are difficult to detect, because they pertain only to a very limited number of patients.
The most striking finding in the present study was a substantially decreased risk of leukemic relapse among patients with KIR ligand–incompatible donors. In particular, the 2 relapse events after KIR ligand–incompatible transplantations exclusively emerged within the immediate posttransplantation course, whereas the relapse rate continued to increase during the entire follow-up period in the other 2 patient subsets. This finding is similar to the results of 1 recent study in a conventional URD marrow transplantation setting, in which KIR ligand incompatibility was associated with a complete elimination of leukemic relapse in patients with myeloid leukemias.20 Further support that KIR ligand incompatibility reduces the posttransplantation relapse risk comes from a recent retrospective study of the Japan Marrow Donor Program, in which no relapse events occurred after KIR ligand–incompatible URD marrow transplantation in patients with AML, while the relapse rate of comparable patients with HLA-C allele–disparate, but KIR ligand–compatible donors was 25%.26 In contrast, no beneficial effect of KIR ligand incompatibility on the relapse risk was demonstrable in 2 recent reports, which evaluated transplantation settings with several heterogeneities in terms of patient populations, underlying diseases, preparative regimens, graft manipulation techniques, and posttransplantation immunosuppressive regimens.21,27 Consequently, these differing results are in accordance with the assumption that the impact of KIR ligand incompatibility on the risk of posttransplantation relapse cannot only be influenced, but might even be superimposed by varying patient populations and transplantation techniques. The only 2 heterogeneities in our study were the inclusion of related and unrelated donors, as well as of marrow and blood stem cell grafts, but neither donor source nor graft type affected the risk of relapse. Compared with other reports on this issue, the nucleated cell doses in the present study appear relatively high, but were homogeneously distributed between the 3 patient subsets.20,21,27 This was clearly a consequence of the inclusion of peripheral blood stem cell transplantations, which on the average contain substantial higher numbers of donor hematopoietic progenitor cells, T cells, and, in particular, NK cells.28 The type of graft had no detectable impact on the risk of leukemic relapse in this study, and antileukemic effects after unmodified KIR-incompatible URD transplantations have been recently demonstrated with lower bone marrow cell doses.20 Taking these findings together, it appears reasonable to assume that the major determinant of the observed antileukemic effect is the degree of histoincompatibility between recipient and donor, which is most pronounced in KIR-incompatible combinations.
Acute and chronic GVHD were more common after transplantations from HLA class I–disparate donors in the present study. This, however, cannot explain the observed differences in the relapse risk, because both conditions did not influence this endpoint after adjustment for the dominant influcene of disease stage and study group. Stratification of patients according to the presence of HLA class I disparity in graft-versus-host direction alone or in combination with KIR ligand incompatibility allowed a superior discrimination of distinct patient risk sets for leukemic relapse than stratification exclusively based on KIR ligand incompatibility. This may point to a role of donor T-cell alloimmunity directed against HLA class I–disparate alleles expressed on leukemia cells as one mechanism mediating GvL reactions after unmodified HLA class I–disparate HSCT, which can be augmented by the antileukemic effects of donor-derived NK cell alloimmunity, if KIR ligand incompatibility is additionally present. HLA class I (in particular, HLA-C) disparity in graft-versus-host direction has been shown to reduce the risk of leukemic relapse in one comprehensive study, but this has so far not been confirmed by others.29,30 Whether donor-derived NK cells actually contribute to the antileukemic effects of KIR ligand–incompatible unmodified HSCT, as previously demonstrated for KIR ligand–incompatible CD34+ cell-enriched HSCT from haploidentical related donors, remains currently undefined.7,8 Alternatively, another candidate effector cell population of GvL reactions in this setting are donor-derived cytotoxic T-cell receptor-γδ lymphocytes (TCRγδ–cytotoxic T-lymphocytes [CTLs]), which, like NK cells, constitutively express inhibitory KIR receptors.31 TCRγδ-CTLs are capable of lysing leukemia cells very efficiently, if their KIR receptors are not engaged with their natural inhibitory HLA-C ligands on target cells.32 Consequently, the cytolytic repertoire of TCRγδ-CTLs will become activated by either a weak or missing expression of inhibitory KIR ligands on residual leukemia cells. As for NK cells, the clinical relevance of donor-derived TCRγδ-CTLs in mediating GvL reactions after unmodified HLA class I–disparate HSCT still needs to be defined. Since donor-derived alloreactive NK cell clones could only be isolated from the patient's blood within the first 4 months after transplantation, a durable NK cell–mediated immune surveillance of residual leukemia cells appears unlikely.7 The relatively short-lived antileukemic NK cell effect may nevertheless allow for sustained disease control if residual leukemic cells have been permanently eradicated. Alternatively or supplementary to antileukemic NK cell effects, the superior long-term antileukemic efficacy observed in the present study may have been mediated by alloimmune donor-derived T-cell clones.
The second important finding in the present study is an increased risk of primary GF after KIR ligand–incompatible transplantations, which was more than 2.5-fold higher compared with HLA class I–disparate, but KIR ligand–compatible donor-recipient pairs. This can be ascribed to the fact that KIR ligand incompatibility inevitably includes HLA class I antigen disparity in host-versus-graft direction, which has been identified as a particular risk factor for primary GF if HLA class I disparity relies on serologically detectable mismatches or if the recipient is homozygous at the mismatched HLA class I locus.30 Since about 30% of HLA class I–disparate, but KIR ligand–compatible donor-recipient pairs in the present study were not exposed to an increased risk according to this risk profile, the higher GF rate observed after KIR ligand–incompatible transplantations appears sufficiently explained. A similar adverse influence of KIR ligand incompatibility on primary GF has been recently demonstrated by Morishima et al26 in a Japanese study, in which the risk of GF increased from 2.8% after HLA class I–disparate, KIR ligand–compatible transplantations to 12.7% after KIR ligand–incompatible transplantations.26 Contrarily, no adverse influence of KIR ligand incompatibility on engraftment kinetics and the risk of GF was detectable in another recent study of URD marrow transplantation patients.20 In this study, pretransplantation antithymocyte globulin (ATG) was applied in combination with posttransplantation short-course methotrexate and cyclosporine as prophylaxis of acute GVHD, and it was therefore hypothesized that ATG may have facilitated engraftment due to its potent immunosuppressive effects on host cells capable of mediating graft rejection. This assumption, however, needs to be further substantiated in well-defined risk sets of HLA class I disparity between donor and recipient, because primary GF constitutes a rare event after HLA class I antigen–disparate transplantations, and the use of ATG has no validated beneficial effect in URD marrow transplantation for hematologic malignancies.22,30,33 Effective suppression of graft rejection by alloreactive donor-derived NK cells with cytotoxic host cell reactivity has been demonstrated in CD34+ cell-enriched HSCT from KIR ligand–incompatible haploidentical related donors and is further strongly supported by experimental animal data.7,8 Whether donor-derived NK cells can be similarly operative against GF after conventional HLA class I–disparate transplantations remains to be determined. The current clinical evidence speaks for a predominant influence of T-cell alloreactivity on the occurrence of GF after conventional allogeneic HSCT, because this risk clearly increases with the number of HLA class I–disparate antigens in host-versus-graft direction.30,34 This is further supported by one recent study in pediatric recipients of minimally T-cell–depleted marrow transplants from HLA partially matched donors in which the adverse influence of T-cell alloreactivity surmounted NK cell alloreactivity in terms of the occurrence of GF.27 Thus, in a conventional transplantation setting donor selection on the basis of KIR incompatibility is expected to result in a somewhat increased GF rate compared to completely HLA class I antigen–matched donors.
Finally, the superior antileukemic efficacy after KIR ligand–incompatible HSCT did not translate into improved overall and event-free survival in the present study. However, the reduced relapse risk completely compensated for the adverse effects on these endpoints, which resulted from the increased risk of acute GVHD and primary GF after HLA class I–disparate, and in particular, KIR ligand–incompatible transplantations. Thus, the present study does not confirm the dramatic beneficial effects on the major transplantation endpoints observed in 1 single study after conventional KIR ligand–incompatible URD marrow transplantation.20 Nevertheless, our results support a broader application of KIR ligand–incompatible transplantations for patients with myeloid leukemias, who have a substantially increased risk of posttransplantation leukemic relapse. In consequence, genotyping of donor and recipient HLA-C alleles should generally be performed in clinical situations in which KIR ligand incompatibility can substantially contribute to the antileukemic efficacy of allogeneic HSCT. Future strategies investigating KIR ligand–incompatible donor transplantations should implement preparative regimens with more potent immunosuppressive properties in order to diminish the increased risk of primary GF associated with this approach. Additional studies are required to precisely define a “perfect” HLA-C allele disparity in graft-versus-host direction that provides the most favorable ratio between the benefits and risks of unmodified allogeneic HSCT.
Prepublished online as Blood First Edition Paper, November 9, 2004; DOI 10.1182/blood-2004-04-1441.
Supported in part by Aktion “Kampf dem Krebs” of the German Cancer Society (D.W.B.), by Deutsche Krebshilfe (A.H.E.), and by the German José Carreras Foundation (S.F., H.O. and H.G.-W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.