Abstract
Thymic function is critical for immune reconstitution after hematopoietic stem cell transplantation (HSCT). We evaluated recipient thymic function before HSCT by quantifying T-cell receptor excision circles (TRECs) in pretransplantation peripheral blood lymphocytes from 102 patients who received HSCs from an HLA-identical sibling for malignant (n = 87) or nonmalignant diseases (n = 15). Median TREC value before transplantation was 257 TRECs per 150 000 CD3+ cells (range, 0-42 746). We assessed 172 TRECs per 150 000 CD3+ cells as the most discriminating TREC value for survival in a first cohort of patients (n = 62). This cut-off was validated in a second independent prospective group of 40 patients. In the 102 patients, a TREC value greater than or equal to 172 was associated with a better survival (P < .000 01), a decreased incidence of grade II-IV acute graft-versus-host disease (GVHD; P = .017), chronic GVHD (P = .023), and bacterial (P = .003) and cytomegalovirus (CMV) infection (P = .024). In a multivariate analysis, low pretransplantation TREC values were associated with a higher incidence of CMV infection (hazard ratio [HR] = 2.0, P = .06) and severe bacterial infections (HR = 2.8, P = .036). Finally, high TREC values (HR = 6.6, P = .002) and ABO compatibility (HR = 2.7, P = .02) were associated with a better survival. Therefore, recipient host thymic function assessment could be helpful in predicting HSCT outcome and identifying patients who require a close immunologic monitoring.
Introduction
Impaired T-cell immune reconstitution after allogeneic hematopoietic stem cell transplantation (HSCT) is associated with the occurrence of opportunistic infections, graft-versus-host disease, and relapse and is, therefore, a major cause of morbidity and mortality.1,2 T-cell recovery after HSCT proceeds normally along 2 different pathways. The thymic-independent pathway includes transfer of graft-derived mature donor T cells followed by antigen or cytokine-driven expansions and has limited T-cell receptor (TCR) diversity. The thymic-dependent pathway that involves generation of new naive T cells from donor-derived precursor cells accounts for the more durable reconstitution of the T-cell compartment and generates a more diverse TCR repertoire.3 Thymic function and production of recent thymic emigrants (RTEs) may be directly evaluated through the quantification, by real-time polymerase chain reaction (PCR), of the T-cell receptor excision circles (TRECs), which result from the deletion of the TCRδ region during TCRα locus rearrangement.4,5 After transplantation, TREC production has been clearly associated with the generation of naive T cells6,7 and with major parameters in transplantation outcome, including acute and chronic graft-versus-host disease (GVHD),1,6 opportunistic infections, and the overall morbidity and mortality.8 Assessing factors that could impact the quality of thymus-dependent T-cell immune reconstitution would therefore be useful in predicting patient outcome. Thymic function before transplantation has been recently associated with the rate of T-cell reconstitution in children.9 However, a direct correlation of pretransplantation thymic function with clinical HSCT outcome is still elusive and the evidence that recipient TREC quantification could be of predictive value in clinical practice is still lacking. This may be due to the existing correlation between thymic function and age, one of the strongest predictors of HSCT outcome.10 The gradual decrease in thymic function that occurs with age, especially after 25 years,4 could explain differences between adults and children in immune reconstitution timing and HSCT outcome.11,12 Although age and TRECs are linked, TREC values are highly variable among individuals within the same age range, especially in adults.4,9,13 Here, we studied a group of 102 patients who underwent HLA-identical sibling HSCT to determine whether recipient TREC values before transplantation as a marker of thymic function could, in addition to age, be predictive of HSCT outcome.
Patients, materials, and methods
Patient characteristics
Patient characteristics, treatments, and posttransplantation events are summarized in Table 1. The study population consisted of 102 transplant recipients who received an HSCT from an HLA-identical sibling at the Bone Marrow Transplantation Unit, Hôpital Saint-Louis (Paris, France). Patients who received a reduced-intensity conditioning regimen were excluded and none received a T-cell–depleted graft. Bone marrow and mobilized peripheral blood stem cells were used in 83 and 20 patients, respectively (1 patient received both). The study included 2 separate cohorts: an initial cohort (n = 62) of patients treated between March 1994 and August 2000, from whom blood samples were obtained and frozen before the conditioning regimen; and a second cohort of 40 consecutive patients who underwent transplantation between March 2001 and December 2002 in the same center (the second cohort was used for confirmatory analysis). For the latter patients, samples were collected prospectively at 3, 6, 12, and 24 months after transplantation, analyzed in flow cytometry, and stored for TREC analysis. Patient characteristics, including conditioning regimen and GVHD prophylaxis, were not statistically different between the 2 series. Both cohorts were combined for statistical analysis on patient outcome. For TREC quantification, a control group was composed of 20 healthy volunteer blood donors and 21 HSCT donors. The investigation was approved by the Committee on Medical Ethics of the Hôpital Saint-Louis. Informed consent was provided according to the Declaration of Helsinki.
TREC quantification
Peripheral blood mononuclear cells (PBMCs) were separated on lymphocyte separation medium (Eurobio, les Ulis, France) and stored in liquid nitrogen. They were thawed, stained with CD3–fluorescein isothiocyanate (FITC) (Becton Dickinson, Le Pont de Claix, France), and genomic DNA was extracted using TriReagent (Molecular Research Center, Cincinnati, OH). Quantification of thymic signal joint TRECs was done by real-time quantitative PCR (ABI PRISM7700; Applied Biosystems, Foster City, CA) as described.7 Values were normalized for the genomic copy number using albumin gene quantification,14 and corrected for the percentage of CD3+ cells. In the second cohort, we could also directly calculate the number of TRECs per milliliter of blood.
Definitions of endpoints
Acute and chronic GVHD were diagnosed and graded according to published criteria.15 All patients were considered at risk for acute GVHD (aGVHD) as of day +1 after transplantation. Occurrence of chronic GVHD (cGVHD) was evaluated among patients who survived with sustained engraftment from day +100 after transplantation. Survival was calculated from transplantation to death from any cause. First episodes of severe bacterial, viral, and invasive fungal infections were analyzed up to 180 days after transplantation according to the criteria described in Rocha et al.16 Cytomegalovirus (CMV) infection was defined by a positive antigenemia (presence of 2 or more positive nuclei per 200 000 leukocytes). CMV disease was diagnosed according to previously published criteria.17
Flow cytometry analysis
Lymphocyte immunophenotyping was performed on fresh whole blood samples on EDTA (ethylenediaminetetraacetic acid), by direct 2- or 4-color immunofluorescence. Measurements of forward and side scatter were combined with CD45 and CD14 determinations to identify lymphocytes and exclude monocytes. Lymphocyte gate purity was equal to at least 98%. The following monoclonal antibodies and combinations were used: CD45-FITC/CD14–phycoerythrin (PE), CD3-FITC/CD16-PE/CD56-PE, CD62L-FITC/CD45RA-PE/CD4–peridinin chlorophyll protein (PerCP)/CD8–allophycocyanin (APC; Becton Dickinson), and CD62L (Beckman Coulter, Villepinte, France). Absolute counts of total, CD4+, and CD8+ lymphocytes were determined with the Multitest CD3-FITC, CD8-PE, CD45-PerCP, CD4-APC (Becton Dickinson). Appropriate isotype-matched controls were carried out on each sample. Gated lymphocytes (5000) were analyzed with a FACScalibur analyzer (Becton Dickinson).
Statistical analysis
In order to find the most discriminating cut-off TREC value, we categorized patients into 5 groups of equal numbers of cases (Figure 2A). The defined cut-off (172 TRECs/150 000 CD3+ cells) split the groups into those patients with “low” and those with “high” TRECs. Differences in categorical variables between the 2 groups were evaluated by chi-square analysis. Clinical variables were the recipient's age (< or ≥ 25 years, according to reported data, showing that thymic function decreases more sharply after this age4 ), recipient and donor genders (female donor to a male recipient versus others), recipient and donor CMV serology (donor negative/recipient positive versus other combinations), ABO incompatibility (major versus minor or none), diagnosis (malignant versus nonmalignant), status of disease (low, medium, or high risk), conditioning regimen (use of a radiation or busulfan-based conditioning), and nucleated cell and CD34+ marrow cell doses (median).
Association between pretransplantation TREC values and survival. Kaplan-Meier representatives of patient survival. (A) Patients (n = 62) were categorized in 5 groups of equal number according to pretransplantation TREC level (ranges are TRECs per 150 000 CD3+ cells: 0-18, solid gray line; 19-83, dashed gray line; 84-171, dotted gray line; 172-927, solid black line; and 928-7000, dashed black line). A first cohort (n = 62) and a confirmatory independent cohort (n = 40) of genoidentical HSCT patients were further split into “high” (≥ 172 TRECs/150 000 CD3+ cells; black lines) and “low” (< 172 TRECs/150 000 CD3+ cells; gray lines) groups, and analyzed separately (B and C, respectively) and together (D).
Association between pretransplantation TREC values and survival. Kaplan-Meier representatives of patient survival. (A) Patients (n = 62) were categorized in 5 groups of equal number according to pretransplantation TREC level (ranges are TRECs per 150 000 CD3+ cells: 0-18, solid gray line; 19-83, dashed gray line; 84-171, dotted gray line; 172-927, solid black line; and 928-7000, dashed black line). A first cohort (n = 62) and a confirmatory independent cohort (n = 40) of genoidentical HSCT patients were further split into “high” (≥ 172 TRECs/150 000 CD3+ cells; black lines) and “low” (< 172 TRECs/150 000 CD3+ cells; gray lines) groups, and analyzed separately (B and C, respectively) and together (D).
Continuous variables were compared between these groups by 2-tailed unpaired t tests. Correlations and variance inflating factors were calculated between variables in order to avoid colinearity in multifactorial analysis. Univariate Kaplan-Meier analysis was used to describe risk factors of death. Univariate analysis using the competing risk method as described by Fine and Gray18 was used for assessment of prognosis factors of GVHD and infections with death as a competing event. Cox proportional hazards model analysis was used to identify independent risk factors for death, infections, and acute or chronic GVHD. All tests were 2 sided and the type 1 error rate was fixed at 0.05. The Statistical Package for Social Scientists (SPSS 11.5; SPSS, Chicago, IL) was used for data management and analysis by Kaplan Meier and Cox methods. The R Package “cmprsk” developed by Gray was used for competing risk analysis.
Results
TREC values and pretransplantation patient parameters
The number of TREC copies per 150 000 CD3+ cells was measured by real-time PCR in preconditioning PBMC samples in 102 patients who underwent HSCT using an HLA-identical sibling donor and in a group of 41 healthy donors (Figure 1). We observed, as did others,4,9,13 that, despite a high variability in TREC values within the same age range, age correlated with TREC values in both patient and control group (Figure 1, P = .001, r = -0.34, which reflects a shared variance of about 11%, Pearson correlation). The median TREC value for the 102 patients was 257 TRECs per 150 000 CD3+ cells (range 0-42 746). It was lower but not significantly different (P = .10) than the median TREC value of 480 TRECs per 150 000 CD3+ cells (range 18-4338) in healthy donors of similar age. TREC values were significantly lower in patients affected by a malignant versus a nonmalignant disease (median, 150 TRECs per 150 000 CD3+ cells, range 0-19 390; and median, 1533, range, 86-42 746, respectively; P < .0001) but median age was also significantly lower in the former group (median age, 17.5 years, range, 3.4-43.3 years; and median, 35.9 years, range 7.2-55.8 years, respectively; P < .005). Age and disease risk were included in the subsequent multivariate analysis. Other pretransplantation parameters (gender, CD34+ cell dose infused, total body irradiation, CMV status, ABO compatibility) were not associated with recipient TREC differences. Median TRECs were not statistically different in the initial group (median, 141; range, 0-7000) and in the second group of patients (median, 490; range 0-42 746) (P = .10). In this last group, we could also calculate the number of TRECs per milliliter of blood (range, 0-137 973 copies/mL). Comparison with values obtained per 150 000 CD3+ cells resulted in a strong correlation (r = 0.957, P < .0001) between these 2 modes of calculation.
Association between pretransplantation TREC values and age. TREC copies/150 000 CD3+ cells (y axis) were plotted against patient age (x axis). ○ represents first cohort patients;, second cohort patients; *, healthy controls. Solid line represents linear regression for all patients; dashed line, for controls.
Association between pretransplantation TREC values and age. TREC copies/150 000 CD3+ cells (y axis) were plotted against patient age (x axis). ○ represents first cohort patients;, second cohort patients; *, healthy controls. Solid line represents linear regression for all patients; dashed line, for controls.
TRECs and survival
We first attempted to define a threshold value for pretransplantation TRECs by categorization of the 62 patients from the initial cohort in 5 series of the same number of cases analyzed for survival (Figure 2A). We observed a striking difference between the groups in which the TREC values were lower than the 60th percentile of the TREC value distribution (survival < 60%) compared with those for which TREC values were higher than the 60th percentile (survival > 80%). This cut-off TREC value split the group into “high TREC” patients who have 172 or more TRECs per 150 000 CD3+ cells before conditioning and “low TREC” patients with less than 172 TRECs per 150 000 CD3+ cells. Patients with a high TREC number had a significantly better survival rate (P = .003, Figure 2B).
In order to validate this threshold, an independent cohort of 40 additional patients was analyzed prospectively. The threshold value of TRECs determined initially still holds true and was even stronger when considering this second cohort (P = .0006, Figure 2C). These 2 groups of patients did not differ in terms of disease and transplantation procedures (Table 1) and were therefore combined for subsequent statistical analysis. Impact of pregraft TREC value on survival was very strong in the whole group of patients (P < .000 01, Figure 2D). To evaluate the impact of pretransplantation TREC values on survival compared with other parameters, a Cox multifactorial analysis was designed, which included recipient age of 25 years, donor/recipient CMV serology, ABO incompatibility, sex mismatch, and disease risk (Table 2). ABO incompatibility was found to have a significant impact on survival. Notably, patients with low pretransplantation TREC values had a statistically significant lower survival rate independently of age. The next issue was to dissect the correlations of pretransplantation TREC levels on the major posttransplantation clinical outcome events. As the number of relapses was low in these patients (n = 9), we focused the analysis on GVHD and the occurrence of severe opportunistic infections.
TRECs and GVHD
Occurrence of acute and chronic GVHD has a major impact on overall survival.19,20 Among the 102 patients, 41% had aGVHD of grade II or higher. Using death as a competing factor, there was a significant association between low pretransplantation TREC counts and a higher incidence of grade II-IV aGVHD (P = .017, Figure 3A).
Association between pretransplantation TREC values and GVHD. Cumulative incidence of grades II to IV acute GVHD (A) and chronic GVHD (B) using death as a competing risk for the group of 102 patients who underwent HLA-identical sibling donor HSCT. Gray lines indicate low TREC levels; black lines, high TREC levels.
Association between pretransplantation TREC values and GVHD. Cumulative incidence of grades II to IV acute GVHD (A) and chronic GVHD (B) using death as a competing risk for the group of 102 patients who underwent HLA-identical sibling donor HSCT. Gray lines indicate low TREC levels; black lines, high TREC levels.
Since aGVHD is the most important risk factor for cGVHD,19,20 we next analyzed the relationship between TRECs and cGVHD. Thirty-three percent of the patients had limited or extensive cGVHD. Using death as competing risk, cumulative incidence of cGVHD was also significantly lower for patients with a high TREC value (P = .023, Figure 3B).
Using the same parameters (age of 25 years, donor/recipient CMV serology, ABO incompatibility, sex mismatch, and disease risk) as for survival, we evaluated the effect of a low TREC value before graft on acute and chronic GVHD in a Cox multifactorial analysis. Only disease risk was found to have an impact on aGVHD, with malignant low-risk or malignant high- and intermediate-risk patients having an increased risk to develop aGVHD than nonmalignant patients (hazard ratio [HR] = 3.8 and 4.9, P = .08 and .05 respectively). ABO incompatibility (HR = 1.9, P = .04) and a lower pretransplantation TREC value (HR = 2.2, P = .011), were statistically associated with a higher incidence of cGVHD.
TRECs and infections
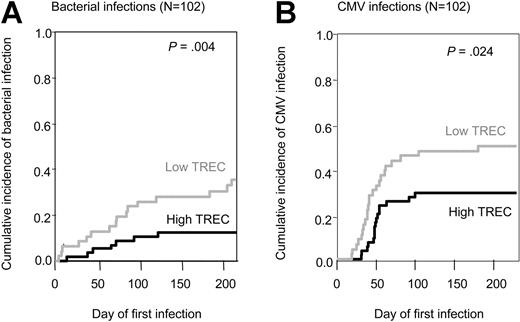
Infections are also a major cause of morbidity and mortality after HSCT. We recorded 24 severe bacterial infections (14 septicemia, 7 pneumonia, and 3 septic shock syndromes), 15 fungal infections, and 40 CMV infections among the total group of 102 patients. Fungal infections were not significantly associated with recipients' pretransplantation TREC values (P = .80, data not shown). However, there was a higher incidence of severe bacterial infection in patients with a low TREC value, as compared with those with a high TREC value (P = .0034, Figure 4A). There was also a significant association between low TREC value and higher CMV infection incidence (P = .024, Figure 4B), taking into account death as a competing event.
Association between pretransplantation TREC values and infections. Cumulative incidence of bacterial infections (A) and CMV infections (B) using death as a competing risk. Gray lines indicate low TREC levels; black lines, high TREC levels.
Association between pretransplantation TREC values and infections. Cumulative incidence of bacterial infections (A) and CMV infections (B) using death as a competing risk. Gray lines indicate low TREC levels; black lines, high TREC levels.
By multivariate analysis, a low pretransplantation TREC value was clearly associated to a higher incidence of bacterial (HR = 2.8, P = .036) and CMV infection (HR = 2.0, P = .06). As expected, donor and recipient CMV serology was also found to be associated with CMV infections (Table 2).
TREC values and T-cell reconstitution
T-cell reconstitution was prospectively monitored by immunophenotyping in the second cohort of patients (n = 40). Absolute counts of different subsets of lymphocytes were calculated using CD3, CD4, CD8, CD16, and CD56 surface markers. Naive T-cell populations were characterized by expression of CD45RA and CD62L. Before transplantation, absolute counts of CD3, CD4, CD8, and natural killer (NK) cells were in the normal range for both groups of patients (Figure 5). A higher naive absolute cell count before transplantation in both CD4 and CD8 subpopulations correlated with a high TREC number (P = .04 and P = .009, respectively). There was no statistical difference in lymphocyte counts between the 2 groups during follow-up, although patients with a high pretransplantation TREC value appeared to have higher CD3 and CD8 counts at 6 months and 1 year after transplantation (Figure 5). However, we should be aware that there is an intrinsic bias in such a prospective analysis due to the higher mortality rate in patients with low TREC values before transplantation, accounted for by the low number of patients alive in this group (47% and 96% survival at 12 months in “low” and “high” TREC groups, respectively).
Kinetics of T-cell reconstitution in the second cohort of patients (n = 40). Graphs show the mean numbers of cells/mm3 of blood plus or minus the standard error of the mean (SEM) for the patients with high TREC values ( ) and the patients with low TREC values (□) in CD3+ cells, NK cells, global and naive CD4+ and CD8+ T-cell subsets before and at 3, 6, 12, and 24 months after transplantation. *P < .05 between patients with high TREC levels and those with low TREC levels. Shaded areas indicate values in healthy controls.
) and the patients with low TREC values (□) in CD3+ cells, NK cells, global and naive CD4+ and CD8+ T-cell subsets before and at 3, 6, 12, and 24 months after transplantation. *P < .05 between patients with high TREC levels and those with low TREC levels. Shaded areas indicate values in healthy controls.
Kinetics of T-cell reconstitution in the second cohort of patients (n = 40). Graphs show the mean numbers of cells/mm3 of blood plus or minus the standard error of the mean (SEM) for the patients with high TREC values ( ) and the patients with low TREC values (□) in CD3+ cells, NK cells, global and naive CD4+ and CD8+ T-cell subsets before and at 3, 6, 12, and 24 months after transplantation. *P < .05 between patients with high TREC levels and those with low TREC levels. Shaded areas indicate values in healthy controls.
) and the patients with low TREC values (□) in CD3+ cells, NK cells, global and naive CD4+ and CD8+ T-cell subsets before and at 3, 6, 12, and 24 months after transplantation. *P < .05 between patients with high TREC levels and those with low TREC levels. Shaded areas indicate values in healthy controls.
Discussion
In this study, we found that recipient thymic function as assessed by TREC quantification may be predictive for outcome in HLA-identical HSCT from sibling donors. TREC quantification is the most direct way to approach thymic function ex vivo but, as with other biologic markers, it may be influenced by many factors. This explains the large variability observed even among healthy individuals, especially in adults. In this respect, our data are consistent with those from other studies.4,9,13 Age alone does not explain solely this variability, which could be due to genetic but also to acquired factors. TREC levels were lower in the whole group of patients than in the control group although values overlapped. Among patients, disease and prior chemotherapy could clearly impair thymic function. Consistently, we observed lower TREC levels in patients affected by a malignant versus a nonmalignant disease, as previously reported.9,21 However, the association between TRECs and a given chemotherapy could not be shown in these studies. In our hands, age and malignancy (which takes into account prior treatment) were the only 2 factors associated with TREC values and were therefore included in the Cox multivariate analysis (Table 2).
Using categorization on a first cohort of 62 patients, we could define a pretransplantation cut-off TREC value (172/150 000 CD3+ cells) that has a remarkable impact on HSCT outcome, with 86% and 47% of survival at 3 years for patients with high and low TREC values, respectively. This value was also validated in a second, and independent, cohort of 40 patients. However, due to the lack of TREC standardization at the present time, it could be difficult to transpose this value in other laboratories to achieve biologic risk assessment before transplantation. But, whereas absolute TREC counts may vary from one laboratory to another, the cut-off defined here identified as high-risk patients those with TREC values below the 60th percentile, an estimate that could be considered in other centers as well. Notably, the pretransplantation recipient TREC value cut-off predicting impaired T-cell reconstitution after HSCT in the report by Chen et al9 corresponds also to patients with TREC values before transplantation of lower than the 60th percentile (16/26 patients). Thus, in these 2 independent studies, the definition of “low” and “high” recipient TREC values were fully consistent.
In this group of 102 genoidentical sibling HSCT patients, the impact of TREC value on survival was strong and clearly independent of age and disease risk. In this cohort, only 2 factors were associated with a better survival: ABO compatibility22 and high TREC value in the transplant recipient. Notably, when age and TREC value were included in the multivariate analysis, the effect of TREC value overcame age as a predictive factor for survival (HR = 6.6, P = .002). High TREC value in the recipient was associated with decreased incidence of acute and chronic GVHD. In the multivariate analysis for aGVHD, TREC value was not selected as an independent factor, disease risk (malignant versus nonmalignant patients) being the most important factor. However, in cGVHD, the impact of TREC values became clearly apparent (HR = 2.2, P = .011). Interestingly, the strongest effect of recipient thymic function on HSCT outcome events was on the incidence of bacterial and CMV infections (Table 2). In multivariate analysis, recipient TREC value and the recipient and donor CMV serologic status were the only 2 parameters to be associated with CMV infection incidence. The only other transplant situation in which recipient thymic function has been evaluated is autologous HSCT in patients with myeloma.23 In this setting also, low recipient TREC levels before transplantation were associated with a higher incidence of infectious complications and a lower overall survival.
The simplest mechanistic explanation is that the residual recipient thymic function after conditioning and transplantation is dependent on the pretransplantation thymic activity itself, which is heterogeneous and not solely reflected by age. Residual thymic activity could be a major factor for donor lymphoid progenitors engraftment and differentiation after transplantation. Supporting our data, Chen et al9 recently showed that the rate of T-cell reconstitution after allogeneic HSCT is dependent on the competence of thymic function in the recipient before transplantation. However, in this study, no correlation with clinical posttransplantation events was reported. Considering the time range of infection monitoring in our study (day 180), recipient thymic-dependent T-cell immune reconstitution could clearly play a role. In this regard, Chen et al9 found that patients with the highest pretransplantation TREC values were also those with the earliest thymic function recovery, beginning 2 months after transplantation. This may suggest that classical immune monitoring by lymphocyte immunophenotypes may underestimate the kinetics and the quality of T-cell immune reconstitution. De novo thymic-dependent T-cell reconstitution could therefore take over the role of donor-derived, memory-specific T cells in the control of infections. Another point to be mentioned is the relationship between thymic function before and after transplantation and GVHD. Several studies6,7 agree that GVHD impairs thymic function and TREC production. This is not in contradiction with our data. Our working hypothesis is that, in case of a low thymic function prior to transplantation (due to age, treatment, etc), the attack of GVHD on the thymus will be even more critical, resulting in a delay in immune reconstitution. Besides, another mechanism relevant to thymic function could implicate CD4+CD25high regulatory T cells. These cells being selected in the thymus,24 a potent residual thymic function could help maintaining regulatory T cells, which could be associated with a favorable transplantation outcome. This assumption has been recently evidenced in humans by the positive correlation between FOXP3 gene expression, the occurrence of recent thymic emigrants and a lower severity of GVHD.25
In conclusion, we provide here data supporting that recipient thymic function analysis could be a marker of clinical importance in allogeneic HSCT to identify patients requiring a close immunologic monitoring. We therefore propose that TREC quantification could be added to a transplant recipient's biologic risk assessment prior to transplantation.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-04-1667.
Supported by research grants from the Fondation pour la Recherche Médicale (ARS 2000), AP-HP (PHRC AOM 97093), Cancéropôle Ile-de-France, EUROCORD III (QLRT-2001-01918), EUROBANK (QLRI-CT-2000-00010), and FP6 ALLOSTEM (no. 503319).
V.R., K.T., and M.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.