Abstract
Regulatory T cells (TREGs) control the key aspects of tolerance and play a role in the lack of antitumor immune responses. Cyclophosphamide (CY) is a chemotherapeutic agent with a dose-dependent, bimodal effect on the immune system. Although a previous study demonstrated that CY reduces the number of TREGs, the mechanism involved in this process has yet to be defined. In this report, it is established that low-dose CY not only decreases cell number but leads to decreased functionality of TREGs. CY treatment enhances apoptosis and decreases homeostatic proliferation of these cells. Expression of GITR and FoxP3, which are involved in the suppressive activity of TREGs, is down-regulated after CY administration, though the level of expression varies depending on the time studied. This is the first report demonstrating that CY, in addition to decreasing cell number, inhibits the suppressive capability of TREGs. The relevance of the loss of suppressor functionality and the changes in gene expression are further discussed.
Introduction
Regulatory T cells (TREGs) account for 5% to 10% of the CD4+ T cell population and constitutively express CD25.1,2 TREGs control key aspects of tolerance to self-antigens, rendering them crucial in the prevention of autoimmune responses.3-5 Because tumor-associated antigens are derived from self-antigens, TREGs may be partially responsible for the lack of antitumor immune responses.6 It has been shown that increasing tumor burden is associated with a drastic increase in the proportion of TREGs.7,8 In animal models it has also been shown that the removal of CD4+25+ TREGs enhances antitumor immune responses.6,9 The presence of the TREGs may also hinder the development of antitumor immune responses after the delivery of an immunotherapeutic agent. For this reason, methods of abrogating the activity of TREGs may be critical for the successful immunotherapeutic treatment of cancer.
In several studies examining the feasibility of enhancing antitumor responses through the inhibition of regulatory cell activity, antibodies against the CD25 cell surface marker have been used. Although depletion of TREGs by this method does lead to enhanced immunity in tumor models,3-5 CD25 is an insufficiently specific marker for TREGs. Removing cells expressing CD25 may lead to the removal of activated CD4+ and CD8+ cells, which also express CD25. Depleting TREGs using other cell markers, such as CTLA-4, may not be adequate given that TREGs from CTLA-4 knockout mice are still capable of suppressing the immune response.10 Therefore, there would be considerable therapeutic potential for a treatment modality that could temporarily suppress the activation of TREGs during immunotherapeutic treatment without detrimental effects to the CD8+ and CD4+25- tumor-specific cells.
Cyclophosphamide (CY) is a chemotherapeutic agent used to treat various types of cancer. The high doses of the drug that are required for effective chemotherapy lead to immunosuppression, a property that is the basis for its other clinical uses, such as preventing graft-versus-host disease in bone marrow transplantation11,12 and minimizing chances of organ rejection in organ transplantation.13,14 Although CY can cause immunosuppression, low doses of the drug can lead to enhanced immune responses against a variety of antigens.15-18 During the 1980s many hypotheses for this effect were postulated, including an inhibitory action on an unspecified putative immunosuppressive cell type.15 However, since TREGs have been identified only recently, this hypothesis to explain the effect of CY was never sufficiently tested. Recently, it has been demonstrated that CY decreases the number of CD4+25+ TREGs in tumor-bearing rats, but neither the mechanism of action of CY nor the functionality of the surviving TREGs was investigated.19 In this investigation, we examined the effects of low-dose CY treatment on mouse TREGs; we demonstrated for the first time that, in addition to decreasing cell number, CY directly inhibits the suppressive capability of CD4+25+ TREGs. In addition, real-time polymerase chain reaction (PCR) was used to address the role of FoxP3 and GITR in the function of the TREG population and the mechanism of action of CY.
Materials and methods
Mice and reagents
Female C57BL/6 mice, 8 weeks of age (mean body weight, 20 g) were used in all experiments. Antibodies to CD4, CD8, CD19, CTLA-4, CD45RB, CD62L, and CD25 were purchased from BD Biosciences PharMingen (San Diego, CA).
Kinetics of cell-population changes after CY administration
Female C57BL/6 mice, 8 weeks of age, were injected intraperitoneally with 2 mg CY (Sigma, St Louis, MO) on day 0. Control mice received no CY. At various time points after injection, mice were killed, and spleen and lymph node (LN) cells were collected and stained for cell surface markers.
Lymphocyte separation by flow cytometry
CD4+ cells were positively selected from splenocytes using anti-CD4 beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. CD4+ cells were stained for CD4 and CD25α and then sorted (FACSVantage; Becton Dickinson, San Jose, CA) into 2 populations: CD4+25- and CD4+25+. The identity of the CD4+25+ cells as TREGs was confirmed by staining for CTLA-4, GITR (DTA-1 hybridoma, a generous gift of Dr Shimone Sakaguchi, Laboratory of Immunopathology, Research Center for Allergy and Immunology, Institute for Physical and Chemical Research, Yokohama, Japan), CD45RB, and CD62L.
Lymphocyte separation by magnetic beads
CD4+25+, CD4+25-, and CD8+ cells were isolated from splenocytes using the Regulatory T Cell Isolation kit and anti-CD8 beads from Miltenyi Biotec according to the manufacturer's protocol.
Cell culture
CD8+ cells (5 × 104) from control mice were incubated with 1 × 105 antigen-presenting cells (APCs) (spleen cells depleted of CD4+ and CD8+ cells from untreated control mice irradiated with 30 Gy) and 1 μg/mL anti-CD3 (BD Biosciences PharMingen) in the presence or absence of 5 × 104 TREGs from either control mice or mice that had received 2 mg CY. Cells were incubated for 72 hours (3H-thymidine was added to the culture for the last 12 hours) and harvested. Control wells containing TREGs, APCs, and anti-CD3 were used to determine background levels of proliferation in culture. The lack of proliferation of the CD4+25+ cells further confirmed their identification as TREGs.
Apoptosis
Tdt-mediated dUTP nick-end labeling (TUNEL) (APO-DIRECT kit; BD Biosciences PharMingen) and Annexin V (TACS Annexin V-FITC kit; R&D Systems, Minneapolis, MN) staining on single-cell suspensions of spleen and LN cells was carried out according to the manufacturer's instructions.
In vivo proliferation of cells
C57BL/6 mice were placed on bromodeoxyuridine (BrdU) water (0.8 mg/mL H2O). Control mice received no CY, whereas experimental mice received 2 mg CY intraperitoneally. After 24 and 48 hours, mice were killed and spleen and LN cells were collected and stained for CD4, CD25, and BrdU incorporation. Gates were placed around either the CD4+25+ cells or the CD4+25- cells, and the amount of BrdU incorporation was determined for each cell type.
Real-time PCR
For reverse transcription, 3 μg RNA was used to synthesize single-strand cDNA (SuperScript First-Strand Synthesis System; Invitrogen, Carlsbad, CA) according to the manufacturer's guidelines. Real-time PCR was performed using the FastStart DNA Master SYBR Green 1 kit (Roche Diagnostics, Indianapolis, IN) and a Roche LightCycler, as described elsewhere.20 Reaction information is summarized in Table 1.
Statistical analysis
Flow cytometry data for apoptosis was statistically evaluated using the Kolmogorov-Smirnov test in CellQuest software (Becton Dickinson).21
Results
Effect of cyclophosphamide on T and B lymphocytes
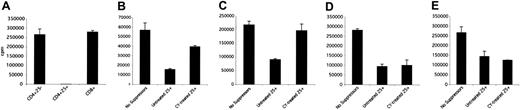
To investigate the effect of low-dose CY on T and B lymphocytes in the spleens and LNs, mice were treated with 2 mg CY intraperitoneally. As depicted in Figure 1A, treating mice with a low dose of CY led to a decrease (14%) in the number of spleen cells within 24 hours of treatment. The decrease in cell number peaked at a 50% drop on the fourth day after CY administration. After day 4, the total spleen cell number began to increase (Figure 1A); by 10 days after CY administration, the spleen cell number was back to pretreatment levels (data not shown). Analyzing spleens and LNs of mice treated with CY demonstrated that, in addition to the general cell loss, the proportions of the lymphocytes were altered in spleens and LNs. Relative numbers of CD4+ and CD8+ T cells increased in spleens and LNs after the administration of CY, whereas the relative number of CD19+ cells decreased sharply (Figure 1B-C). By day 10, the spleen phenotype was returning to pretreatment status (Figure 1B) whereas the LNs remained affected (Figure 1C).
Effect of CY on the absolute and relative numbers of lymphocytes in spleens and LNs. (A) The effect of CY on spleen cell number. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, total splenocytes were counted using a hemocytometer after lysis of red blood cells with ammonium chloride (ACK) buffer. Results are representative of 2 experiments. Results are presented as the mean ± SD of results from 3 mice in each of 2 experiments. (B) Effect of CY on spleen cell phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleen cells were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+( ), CD8+(▪), and CD19+ cells (▴) in the spleens were determined by flow cytometry. (C) Effect of CY on LN phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, LNs were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+, CD8+, and CD19+cells in the LNs were determined by flow cytometry. Results are presented as mean ± SD of results from 3 mice. (D) Effect of CY on the percentage of CD4+cells coexpressing CD25. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleens (
), CD8+(▪), and CD19+ cells (▴) in the spleens were determined by flow cytometry. (C) Effect of CY on LN phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, LNs were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+, CD8+, and CD19+cells in the LNs were determined by flow cytometry. Results are presented as mean ± SD of results from 3 mice. (D) Effect of CY on the percentage of CD4+cells coexpressing CD25. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleens ( ) and LNs (▪) were stained with fluorescence-labeled antibodies against TREG markers. The percentage of CD4+ cells that express CD25 was measured using dual-color flow cytometry. Results are the mean ± SD average of triplicate stains for 2 different mice. (E) Effect of CY on the number of CD4+25+ T cells in the spleens. The number of CD4+25+ cells in the spleens at various times after CY administration was calculated [(spleen cell count) × (% CD4+) × (% CD25+ of CD4+)].
) and LNs (▪) were stained with fluorescence-labeled antibodies against TREG markers. The percentage of CD4+ cells that express CD25 was measured using dual-color flow cytometry. Results are the mean ± SD average of triplicate stains for 2 different mice. (E) Effect of CY on the number of CD4+25+ T cells in the spleens. The number of CD4+25+ cells in the spleens at various times after CY administration was calculated [(spleen cell count) × (% CD4+) × (% CD25+ of CD4+)].
Effect of CY on the absolute and relative numbers of lymphocytes in spleens and LNs. (A) The effect of CY on spleen cell number. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, total splenocytes were counted using a hemocytometer after lysis of red blood cells with ammonium chloride (ACK) buffer. Results are representative of 2 experiments. Results are presented as the mean ± SD of results from 3 mice in each of 2 experiments. (B) Effect of CY on spleen cell phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleen cells were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+( ), CD8+(▪), and CD19+ cells (▴) in the spleens were determined by flow cytometry. (C) Effect of CY on LN phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, LNs were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+, CD8+, and CD19+cells in the LNs were determined by flow cytometry. Results are presented as mean ± SD of results from 3 mice. (D) Effect of CY on the percentage of CD4+cells coexpressing CD25. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleens (
), CD8+(▪), and CD19+ cells (▴) in the spleens were determined by flow cytometry. (C) Effect of CY on LN phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, LNs were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+, CD8+, and CD19+cells in the LNs were determined by flow cytometry. Results are presented as mean ± SD of results from 3 mice. (D) Effect of CY on the percentage of CD4+cells coexpressing CD25. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleens ( ) and LNs (▪) were stained with fluorescence-labeled antibodies against TREG markers. The percentage of CD4+ cells that express CD25 was measured using dual-color flow cytometry. Results are the mean ± SD average of triplicate stains for 2 different mice. (E) Effect of CY on the number of CD4+25+ T cells in the spleens. The number of CD4+25+ cells in the spleens at various times after CY administration was calculated [(spleen cell count) × (% CD4+) × (% CD25+ of CD4+)].
) and LNs (▪) were stained with fluorescence-labeled antibodies against TREG markers. The percentage of CD4+ cells that express CD25 was measured using dual-color flow cytometry. Results are the mean ± SD average of triplicate stains for 2 different mice. (E) Effect of CY on the number of CD4+25+ T cells in the spleens. The number of CD4+25+ cells in the spleens at various times after CY administration was calculated [(spleen cell count) × (% CD4+) × (% CD25+ of CD4+)].
Although the relative number of CD4+ T cells increased, the amount of TREGs as a percentage of the CD4+ population decreased by day 4 to almost half the level seen in control cells (Figure 1D). By day 10, the number of CD4+25+ cells in the LNs was back to pretreatment levels, whereas the numbers in the spleen remained depressed (Figure 1D). Although the changes were minor, the same result was obtained in 4 separate experiments. In addition to the changes seen in the relative numbers of CD4+25+ cells, the absolute number of these cells in the spleen dropped sharply after CY treatment (Figure 1E). On day 4 after treatment, the number of CD4+25+ cells was less than 40% of the cell number in control mice. At day 10, when the total spleen cell count returned to pretreatment levels, the absolute number of CD4+25+ cells was also normal (Figure 1E).
Effect of CY on the functionality of TREGs
To examine the effects of CY on the functionality of TREGs, CD4+25+ cells from healthy and CY-treated mice were sorted by bead separation for in vitro experiments. The separated population consisted of 94% to 97% pure CD4+25+ cells. Because the mice were healthy and unimmunized, we expected the CD4+25+ population to consist of TREGs and not activated CD4+ cells that have up-regulated CD25 on their cell surfaces. To further identify these cells, staining for GITR and CTLA-4 was performed after separation. CD4+25+ cells expressed high levels of GITR and low levels of CTLA-4 (see the Supplemental Figure link at the top of the online article on the Blood website), which identified them as TREGs. As a further confirmation that the CD4+25+ cells were TREGs, these cells were incubated with soluble anti-CD3 and APCs. This incubation did not lead to the proliferation of the cells (Figure 2A), confirming that the CD4+25+ cells are TREGs.
Proliferation of CD4+ and CD8+ T lymphocytes in response to anti-CD3. (A) Proliferation of CD4+25+cells compared with CD4+25-and CD8+ cells. T cells (5 × 104/well) were incubated with 1 × 105 irradiated APCs and 1 μg/mL anti-CD3. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. (B) Effect of CD4+25+ TREGs on the proliferation of CD8+ cells in response to anti-CD3. Two days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD8+ cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no T, 5 × 104, 5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments. (C) Effect of CD4+25+ TREGs on the proliferation of CD4+25- cells in response to anti-CD3. Two days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD4+25- cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no TREGs, 5 × 104 ,5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments. (D) Effect of CD4+25+ TREGs on the proliferation of CD8+ cells in response to anti-CD3. Ten days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD8+ cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no TREGs 5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments. (E) Effect of CD4+25+ TREGs on the proliferation of CD4+25- cells in response to anti-CD3. Two days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD4+25- cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no TREGs 5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments.
Proliferation of CD4+ and CD8+ T lymphocytes in response to anti-CD3. (A) Proliferation of CD4+25+cells compared with CD4+25-and CD8+ cells. T cells (5 × 104/well) were incubated with 1 × 105 irradiated APCs and 1 μg/mL anti-CD3. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. (B) Effect of CD4+25+ TREGs on the proliferation of CD8+ cells in response to anti-CD3. Two days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD8+ cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no T, 5 × 104, 5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments. (C) Effect of CD4+25+ TREGs on the proliferation of CD4+25- cells in response to anti-CD3. Two days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD4+25- cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no TREGs, 5 × 104 ,5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments. (D) Effect of CD4+25+ TREGs on the proliferation of CD8+ cells in response to anti-CD3. Ten days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD8+ cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no TREGs 5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments. (E) Effect of CD4+25+ TREGs on the proliferation of CD4+25- cells in response to anti-CD3. Two days after the intraperitoneal administration of 2 mg CY, CD4+25+ TREGs were separated from spleens of treated and control mice. CD4+25- cells (5 × 104) from healthy mice were incubated with 1 × 105 irradiated APCs from healthy mice and 1 μg/mL anti-CD3 along with either no TREGs 5 × 104 TREGs from untreated mice, or 5 × 104 TREGs from CY-treated mice. Proliferation was measured by 3H-thymidine incorporation during the last 12 hours of a 72-hour culture. Results are the mean ± SD of triplicate wells. Data are representative of 3 experiments.
Because CD4+25+ cells have been shown to suppress the proliferation of other T lymphocytes in cultures with APCs and soluble anti-CD3,22 we designed a similar experiment to measure the effect of CY on the suppressor function of TREGs. We cultured CD8+ cells or CD4+25- cells and APCs from healthy, untreated mice, along with 1 μg/mL anti-CD3, in the presence or absence of TREGs from control or CY-treated mice (2 days after treatment) for 72 hours (Figure 2B-C). The proliferation of CD8+ and CD4+25- cells in the presence of TREGs from untreated mice was significantly decreased (50%-70%) compared with the proliferation of CD8+ and CD4+25- cells cultured with CY-treated TREGs, indicating that CY treatment inhibits the suppressor function of TREGs.
We performed an identical experiment 10 days after CY administration. At this time, the proliferation of CD8+ and CD4+25- T cells was the same in the presence of TREGs from control and CY-treated mice (Figure 2D-E). These data suggest that by day 10 after CY treatment, TREGs have regained full suppressive function.
Apoptosis of TREGs after CY administration
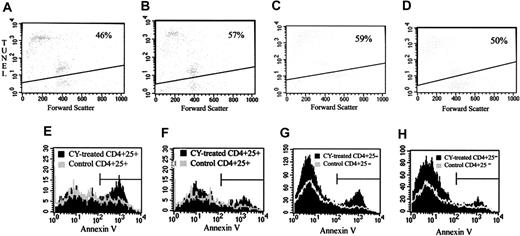
One possible explanation for the loss of suppressor function and for the decrease in the number of CD4+25+ T cells after CY administration is that CY may cause apoptosis in the TREG compartment of the immune system. To examine this prospect, we cultured TREGs from untreated control mice or from CY-treated mice (after 2 days) with immobilized 10 μg/mL anti-CD3 for 24 hours and analyzed the cells using the TUNEL assay. CD4+25+ spleen cells from CY-treated mice had an increased sensitivity to apoptosis in vitro compared with cells from control mice (57% apoptosis vs 46%) (P < .001 by Kolmogorov-Smirnov analysis; see Materials and Methods) (Figure 3A-B). In contrast, CD4+25- spleen cells from CY-treated mice were less sensitive to apoptosis induction in vitro than CD4+25- cells from control mice (50% apoptosis vs 59%) (P < .001 by Kolmogorov-Smirnov analysis) (Figure 3C-D). These experiments were replicated 3 times with similar results.
Effect of CY on apoptosis in CD4+25+TREGs and CD4+25- cells. (A-B) TUNEL staining of CD4+25+ cells. Two days after the intraperitoneal administration of 2 mg CY, spleen cells were removed, and CD4+25+ cells were separated by magnetic bead separation and fluorescence-activated cell sorting (FACS). CD4+25+ cells from treated or control mice were incubated with bound anti-CD3, and TUNEL staining was performed after 24 hours in culture. (A) CD4+25+ cells from untreated control mice. (B) CD4+25+ cells from CY-treated mice. Data are representative of 2 separate experiments. (C-D) TUNEL staining of CD4+25- cells. Two days after the intraperitoneal administration of 2 mg CY, spleen cells were removed, and CD4+25- cells were separated by magnetic bead separation and FACS. CD4+25- cells from treated or control mice were incubated with bound anti-CD3 and after 24 hours in culture, TUNEL staining was performed. (C) CD4+25- cells from untreated control mice. (D) CD4+25- cells from CY-treated mice. Data are representative of 2 separate experiments. Percentages in panels A-D indicate the percent of apoptotic cells. (E-F) Annexin V staining of CD4+25+ cells. Two days after the intraperitoneal administration of 2 mg CY, spleen and LN cells were removed. Freshly isolated cells were stained for cell surface markers, and Annexin V staining was performed on the samples. (E) Annexin V profile of CD4+25+ LN cells from CY-treated mice (black solid histogram) compared with CD4+25+ LN cells from control mice (gray line histogram). (F) Annexin V profile of CD4+25+ spleen cells from CY-treated mice (black solid histogram) compared with CD4+25+ spleen cells from control mice (gray line histogram). (G-H) Annexin V staining of CD4+25- cells. Two days after the intraperitoneal administration of 2 mg CY, spleen and LN cells were removed. Freshly isolated cells were stained for cell surface markers, and Annexin V staining was performed on the samples. (G) Annexin V profile of CD4+25- LN cells from CY-treated mice (black solid histogram) compared with CD4+25- LN cells from control mice (gray line histogram). (H) Annexin V profile of CD4+25- spleen cells from CY-treated mice (black solid histogram) compared with CD4+25- spleen cells from control mice (gray line histogram). Horizontal bars in panels E and H are gates indicating what percent of cells are positive for apoptotic cells.
Effect of CY on apoptosis in CD4+25+TREGs and CD4+25- cells. (A-B) TUNEL staining of CD4+25+ cells. Two days after the intraperitoneal administration of 2 mg CY, spleen cells were removed, and CD4+25+ cells were separated by magnetic bead separation and fluorescence-activated cell sorting (FACS). CD4+25+ cells from treated or control mice were incubated with bound anti-CD3, and TUNEL staining was performed after 24 hours in culture. (A) CD4+25+ cells from untreated control mice. (B) CD4+25+ cells from CY-treated mice. Data are representative of 2 separate experiments. (C-D) TUNEL staining of CD4+25- cells. Two days after the intraperitoneal administration of 2 mg CY, spleen cells were removed, and CD4+25- cells were separated by magnetic bead separation and FACS. CD4+25- cells from treated or control mice were incubated with bound anti-CD3 and after 24 hours in culture, TUNEL staining was performed. (C) CD4+25- cells from untreated control mice. (D) CD4+25- cells from CY-treated mice. Data are representative of 2 separate experiments. Percentages in panels A-D indicate the percent of apoptotic cells. (E-F) Annexin V staining of CD4+25+ cells. Two days after the intraperitoneal administration of 2 mg CY, spleen and LN cells were removed. Freshly isolated cells were stained for cell surface markers, and Annexin V staining was performed on the samples. (E) Annexin V profile of CD4+25+ LN cells from CY-treated mice (black solid histogram) compared with CD4+25+ LN cells from control mice (gray line histogram). (F) Annexin V profile of CD4+25+ spleen cells from CY-treated mice (black solid histogram) compared with CD4+25+ spleen cells from control mice (gray line histogram). (G-H) Annexin V staining of CD4+25- cells. Two days after the intraperitoneal administration of 2 mg CY, spleen and LN cells were removed. Freshly isolated cells were stained for cell surface markers, and Annexin V staining was performed on the samples. (G) Annexin V profile of CD4+25- LN cells from CY-treated mice (black solid histogram) compared with CD4+25- LN cells from control mice (gray line histogram). (H) Annexin V profile of CD4+25- spleen cells from CY-treated mice (black solid histogram) compared with CD4+25- spleen cells from control mice (gray line histogram). Horizontal bars in panels E and H are gates indicating what percent of cells are positive for apoptotic cells.
To determine whether a similar phenomenon occurred in vivo, mice were injected with low-dose (2 mg) CY intraperitoneally. Control mice remained untreated. 2 days later, spleens and LNs were removed, and cell surface marker and Annexin V staining were performed on freshly isolated cells. CD4+25+ cells from LNs showed increased apoptosis after CY administration (46% vs 28% Annexin V+)(P < .001 by Kolmogorov-Smirnov analysis) (Figure 3E). CD4+25+ cells from the spleens also showed a slight increase in the number of apoptotic cells (21% vs 14% Annexin V+) (P < .001 by Kolmogorov-Smirnov analysis) (Figure 3F). CD4+25- cells from lymph nodes showed a slight increase in apoptosis after CY administration (21% vs 14% Annexin V+) (P < .001 by Kolmogorov-Smirnov analysis) (Figure 3G). CD4+25- cells from the spleens showed little or no change in the number of apoptotic cells (11% vs 12% Annexin V+) (Figure 3H).
In vivo proliferation of TREGs after CY administration
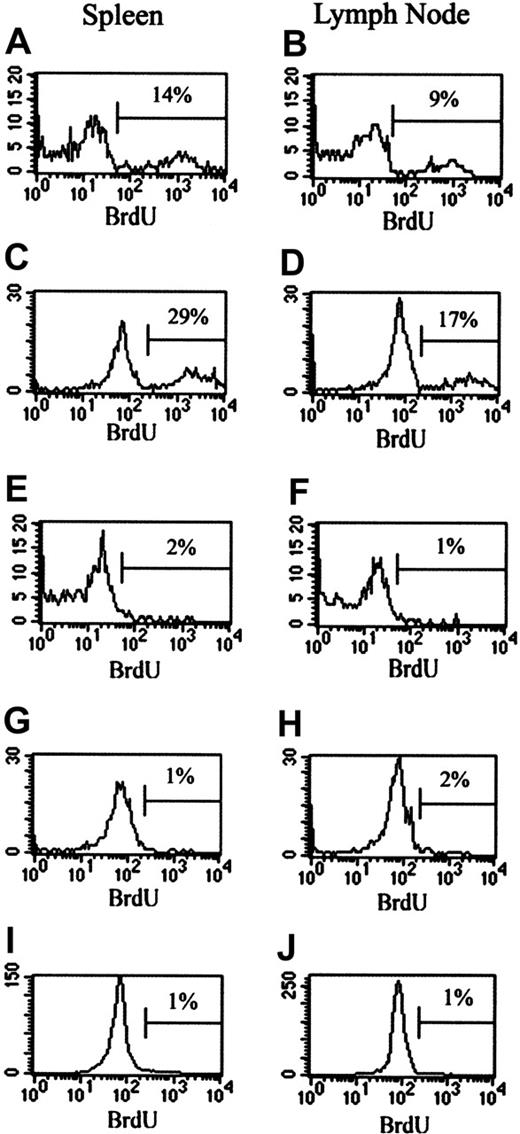
To investigate the effect of CY on the in vivo proliferation of TREGs, BrdU studies were performed. Mice were injected with CY (2 mg intraperitoneally) and given BrdU water. Control mice received no CY but were given BrdU water. Cells from the LNs and spleens were stained for CD4, CD25, and BrdU. Gates were placed around either the CD4+25- population or the CD4+25+ population to determine BrdU incorporation by each cell type. After 24 hours of BrdU treatment in vivo, 9% to 14% of TREGs were proliferating in the spleens and LNs of control mice (Figure 4A-B). This number increased to 17% to 28% of the TREGs in 48 hours (Figure 4C-D). This proliferating population represents homeostatic maintenance of the TREG population. The administration of CY completely obliterated this homeostatic proliferation at 24 hours in spleens and LNs (Figure 4E-F). At 48 hours after CY administration, homeostatic proliferation was still absent in spleens and LNs (Figure 4G-H). In comparison, no BrdU incorporation was measured after 24 hours (data not shown) or 48 hours in the CD4+25- cells from untreated mice (Figure 4I-J). CY treatment did not affect the lack of BrdU incorporation in these cells (data not shown). Therefore, it seems that CY directly and specifically interferes with the maintenance and homeostasis of TREGs. The elimination of homeostatic proliferation after CY administration is another likely contributor to the loss of TREG suppressor function.
Effect of CY on in vivo proliferation of CD4+25+ and CD4+25- cells. Mice were injected intraperitoneally with 2 mg CY. Control mice received no CY. Treated and control mice were given BrdU water beginning at the time of injection. Mice were killed at either 24 or 48 hours, cell surface marker staining was performed on spleen and LN cells, and cells were stained for BrdU incorporation. (A-H) BrdU incorporation by CD4+25+ cells. (A) Spleen cells from control mice at 24 hours after CY administration. (B) LN cells from control mice at 24 hours after CY administration. (C) Spleen cells from control mice at 48 hours after CY administration. (D) LN cells from control mice at 48 hours after CY administration. (E) Spleen cells from CY-treated mice at 24 hours after CY administration. (F) LN cells from CY-treated mice at 24 hours after CY administration. (G) Spleen cells from CY-treated mice at 48 hours after CY administration. (H) LN cells from CY-treated mice at 48 hours after CY administration. (I-J) BrdU incorporation by CD4+25- cells. (I) LN cells from control mice after 48 hours. (J) Spleen cells from control mice after 48 hours. Percentages indicate the percent of BrdU+ cells. Horizontal bars are gates indicating what percent of cells are positive for BrdU+ cells.
Effect of CY on in vivo proliferation of CD4+25+ and CD4+25- cells. Mice were injected intraperitoneally with 2 mg CY. Control mice received no CY. Treated and control mice were given BrdU water beginning at the time of injection. Mice were killed at either 24 or 48 hours, cell surface marker staining was performed on spleen and LN cells, and cells were stained for BrdU incorporation. (A-H) BrdU incorporation by CD4+25+ cells. (A) Spleen cells from control mice at 24 hours after CY administration. (B) LN cells from control mice at 24 hours after CY administration. (C) Spleen cells from control mice at 48 hours after CY administration. (D) LN cells from control mice at 48 hours after CY administration. (E) Spleen cells from CY-treated mice at 24 hours after CY administration. (F) LN cells from CY-treated mice at 24 hours after CY administration. (G) Spleen cells from CY-treated mice at 48 hours after CY administration. (H) LN cells from CY-treated mice at 48 hours after CY administration. (I-J) BrdU incorporation by CD4+25- cells. (I) LN cells from control mice after 48 hours. (J) Spleen cells from control mice after 48 hours. Percentages indicate the percent of BrdU+ cells. Horizontal bars are gates indicating what percent of cells are positive for BrdU+ cells.
Analysis of FoxP3 and GITR gene expression in TREGs after CY administration
Real-time PCR was used to examine the expression of GITR and FoxP3 genes, each of which is believed to play a role in regulatory T cell function. Gene expression was examined at multiple time points to investigate the kinetics of CY effects (Table 2). At 14 hours after CY administration, GITR was down-regulated 1.7-fold in CD4+25+ cells from CY-treated mice compared with CD4+25+ cells from untreated mice. At 24 hours, GITR was down-regulated 2.1-fold; by 48 hours, the down-regulation reached 2.5-fold. FoxP3 was down-regulated 1.5-fold in CD4+25+ cells 14 hours after CY administration, 2.2-fold 24 hours after, and 1.5-fold 48 hours after compared with untreated CD4+25+ cells. Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as the housekeeping gene, and the results for the housekeeping gene were confirmed by the use of ribosomal protein S8.
Real-time PCR results can also be used to address the question of the identity of the CD4+25+ cells. Control CD4+25- cells showed 3.8-fold less expression of GITR than CD4+25+ cells from untreated mice (Table 2). FoxP3 expression was 8.5-fold lower in control CD4+25- cells than in CD4+25+ cells. These gene expression results identified the CD4+25+ cells as regulatory T cells, not activated CD4+ cells, confirming the cell surface marker expression results.
Effect of CY on cell surface expression of proteins
To determine whether the alteration in gene expression levels translates into differential expression of proteins, we used fluorescent antibodies to examine the expression of GITR on the surfaces of CD4+25+ spleen cells. Of 5 mice studied, 2 showed measurable decreases in GITR expression at day 1, and 1 showed a decrease in GITR expression at day 2, whereas 2 of the mice showed no change in GITR expression compared with untreated mice (data not shown).
Discussion
In animals and humans, several studies have demonstrated that the combination of CY with a vaccine leads to enhanced immune and clinical responses.23-25 Although it has been speculated15 that these results may be attributed to the action of CY on a regulatory T cell population, the hypothesis was never validated. Recently, it has been demonstrated that a single dose of CY led to a significant decline in the number of CD4+25+ TREGs in rats with established tumors19 ; however, this study did not address the functionality of the surviving TREGs or the mechanism of action of CY. Our work addresses these crucial questions.
It has never before been demonstrated that CY has a direct inhibitory action on the TREG population in healthy mice, affecting not only cell number but also the suppressive functionality of the surviving cells. In addition, our results, including functional studies of apoptosis and in vivo proliferation, address the mechanism of the decrease in cell number after CY administration. Our data allow the delineation of some of the mechanisms of action of CY on TREGs.
Our results conclusively demonstrate that TREGs from mice that had been treated with CY are significantly less capable of inhibiting CD8+ and CD4+25- proliferative responses than TREGs from untreated mice. Because the CD8+ cells, CD4+25- cells, and APCs in the culture came from untreated mice, we can state that the enhanced response resulted from an inhibitory effect of CY on the TREGs rather than an amplifying effect on some other aspect of the immune system.
Interestingly, 10 days after CY treatment, the absolute numbers of CD4+25+ cells returned to normal and the cells regained full suppressive function, indicating that the effects of CY on TREGs are short-lived and resolve within 10 days. The transient nature of the effect of CY allows for the inhibition of TREGs without a permanent or prolonged reduction of tolerance in the body. Administration of immunotherapeutic modalities, such as vaccines, during this limited period of regulatory T cell inhibition would theoretically allow for enhanced immune responses with a decreased likelihood of autoimmunity.
Functional assays indicate that TREGs are more susceptible to apoptosis after CY treatment, both in vitro and in vivo, whereas CD4+25- cells do not appear to be as susceptible to apoptosis after CY treatment. Furthermore, our results demonstrate, for the first time, that the homeostatic level of the proliferation of TREGs is obliterated by CY treatment. It has been previously documented that low-dose CY can lead to enhanced immune responses; however, the mechanism behind this has not been elucidated.23-25 Homeostatic proliferation of CD4+25+ cells is required to maintain self-tolerance, and the temporary destruction of it by CY may account for some of these reports of enhanced immune responses. In addition, it has been shown that homeostatic proliferation of TREGs augments their suppressive function.26 Clearly, then, the loss of this proliferation after CY administration is a significant finding.
Altered expression of certain molecules directly implicated in the immune response and TREG function may be involved in the inhibition of TREG function by CY. It has been postulated that GITR and FoxP3 are important in suppressor function.27-30 Although the precise function and mechanism of these genes remain unknown, there is a great deal of evidence that they play significant roles in the suppressor function of TREGs. This report marks the first time that the effect of CY on these molecules has been studied.
Experiments using agonist antibody against GITR indicate that the GITR ligand may play a role in modifying the action of TREGs.27 Antibody against GITR reverses the ability of CD4+25+ cells to suppress antigen-specific CD4+25- and CD8+ responses in vitro. Further experiments with recombinant GITR ligand indicate that GITR may function as a costimulatory molecule for T cell receptor (TCR)-stimulated T cells (TREGs and effector CD4+ and CD8+ cells).31 Moreover, in TREGs, increased expression of GITR mRNA is protective against TCR-mediated cell death.32-34 The down-regulation of GITR in TREGs after the administration of CY may be associated with the increased susceptibility of these cells to apoptosis. The death of cells that lose their GITR expression may explain why the difference in GITR expression on the cell surface is difficult to demonstrate consistently; perhaps the cells that experience the loss of GITR are being killed and lost from the spleens. Finally, if GITR is involved in the activation of TREGs, the loss of this molecule could inhibit the suppressive effects of the TREGs through the prevention of their activation.
FoxP3 is a transcriptional repressor that, in mice, is exclusively expressed in CD4+25+ TREGs; unlike CD25 and other molecules, it is not induced on activation in CD4+25- cells.29,30,35 Multiple experiments have shown that FoxP3 is necessary and sufficient for the development and function of CD4+25+ TREGs.29,30,35 FoxP3 was identified when mice with a lymphoproliferative disorder were found to be lacking the protein scurfin, a product of the FoxP3 gene.36 Sakaguchi et al35 demonstrated that FoxP3 was produced in TREGs regardless of activation state and that retroviral transfer of FoxP3 into naive T cells could convert them to a regulatory cell phenotype functionally similar to that of native CD4+25+ TREGs. Rudensky et al30 created a FoxP3-/- mouse strain and demonstrated that these mice lacked TREGs but developed a lethal lymphoproliferative disorder that could be resolved through the transfer of CD4+25+ TREGs from healthy mice. CD4+25+ TREGs from FoxP3 transgenic mice were shown by Ramsdell29 to produce greater amounts of FoxP3 than wild-type mice. This increased expression of FoxP3 was correlated with increased suppressive functions of these cells. It is evident that FoxP3 plays a role in the development and function of TREGs. Our results show a slight down-regulation (1.5- to 2.2-fold decrease) of FoxP3 expression in CD4+25+ TREGs after low-dose CY treatment, peaking at 24 hours after treatment. Our results indicate that the loss of suppressive activity by these cells was caused by multiple effects of the drug on TREGs and that the effects of CY on the expression of FoxP3 might be attributed slightly to this loss.
We postulate that the mechanism of action of CY on TREGs is multifactorial. At the most basic level, cell death and lack of proliferation of the TREG population in vivo play a role in the inhibition of suppression by CY, but the situation is more complex. This loss of suppressive action can also be attributed to the loss of molecules that may be necessary for signaling (GITR) or the development of regulatory function (FoxP3). The loss of the ability to respond to activation by these mechanisms likely has a significant impact on the function of TREGs in vivo.
The studies reported here demonstrate for the first time that CY has a direct inhibitory action on CD4+25+ TREGs, impairing the functionality of these cells in addition to decreasing cell number. In addition, our data allow us to begin discussing possible mechanisms of action of the drug on these cells. Gaining insight into the mechanism of action of CY on TREGs will allow for the development of additional strategies to inhibit TREGs to enhance immune responses. In addition, knowing the mechanism of action of CY on TREGs may uncover crucial information that may elucidate the normal function of TREGs. This information can be used to design treatments for conditions that would benefit from an increased TREG component, such as autoimmune disease or transplantation, and for diseases that may be ameliorated by inhibition of the TREG population.
Prepublished online as Blood First Edition Paper, December 9, 2004; DOI 10.1182/blood-2004-06-2410.
This study was conducted as part of the intramural research program of the National Cancer Institute, National Institutes of Health, Bethesda, MD.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Judith DiPietro for technical assistance throughout the experiments, Marjorie Duberstein for assistance with the real-time PCR, and Veena Kapoor and William Telford from the Experimental Transplantation and Immunology Branch at NCI (FACS core facility) for assistance with cell separation. We also thank Debra Weingarten for her assistance with manuscript preparation.

![Figure 1. Effect of CY on the absolute and relative numbers of lymphocytes in spleens and LNs. (A) The effect of CY on spleen cell number. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, total splenocytes were counted using a hemocytometer after lysis of red blood cells with ammonium chloride (ACK) buffer. Results are representative of 2 experiments. Results are presented as the mean ± SD of results from 3 mice in each of 2 experiments. (B) Effect of CY on spleen cell phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleen cells were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+(), CD8+(▪), and CD19+ cells (▴) in the spleens were determined by flow cytometry. (C) Effect of CY on LN phenotype. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, LNs were stained with fluorescence-labeled antibodies against lymphocyte markers. Relative numbers of CD4+, CD8+, and CD19+cells in the LNs were determined by flow cytometry. Results are presented as mean ± SD of results from 3 mice. (D) Effect of CY on the percentage of CD4+cells coexpressing CD25. At various time points after the administration of low-dose (2 mg/mouse) CY intraperitoneally, spleens () and LNs (▪) were stained with fluorescence-labeled antibodies against TREG markers. The percentage of CD4+ cells that express CD25 was measured using dual-color flow cytometry. Results are the mean ± SD average of triplicate stains for 2 different mice. (E) Effect of CY on the number of CD4+25+ T cells in the spleens. The number of CD4+25+ cells in the spleens at various times after CY administration was calculated [(spleen cell count) × (% CD4+) × (% CD25+ of CD4+)].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-06-2410/6/m_zh80070576670001.jpeg?Expires=1769201423&Signature=FFsG~0haC3AgaJu5uz0Pkq07uJIEDuE1TBJ5yW0gu5huB0-aT9sbMgk9GUsOz7P-DK4Gfzlp2e0GU5q3ST~OCv9OR6X8SAfrETJsr8K1zMh9ILPB3MQFNyyHSpAAthqXHjzLlanTujDQViLGGRMXvOcmwdH4VJDOlIhTd6ImMI5FCfvtI5iTSnVbNJTz9l3vrtcAtA-Eqr~d5hwNAgtwLTHCFbJ0zsfH2xgD3NLlLZVvlPPOE2NHjxOj8uBzeEQPfQe79wuEGGznusZiUtPFCePp5p0e-4201mKEP76Fnb1dBkPgQpRUQ7uXcUCnZHN~IZUExOXec9QXop5Myysi7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)