Comment on Trotta et al, page 3011
SHIP1, a hematopoietic cell–specific phosphatase, plays a critical role in down-regulating production of IFN-γ by human NK cells.
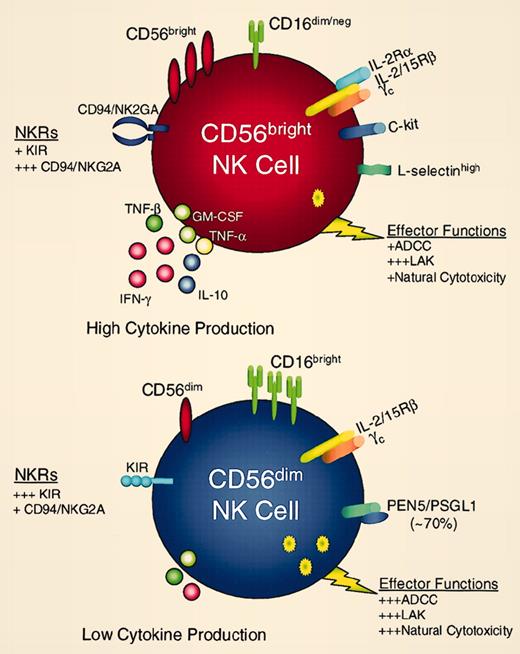
Natural killer (NK) cells are an important element of innate immunity and have both regulatory and effector functions. In humans, NK cells are best identified through expression of CD56, a cell surface protein also termed neural cell adhesion molecule (NCAM), and lack of expression of CD3 and T-cell receptors. Within this population of CD56+CD3– lymphocytes, which represents approximately 10% of lymphocytes in normal peripheral blood, previous studies have demonstrated the presence of at least 2 distinct subsets of NK cells based on different levels of expression of CD56.1 As summarized in the figure, CD56dim NK cells also express high levels of CD16. This subset represents approximately 90% of NK cells in normal peripheral blood and functions primarily as potent cytolytic effector cells. CD56dimCD16bright NK cells constitutively express intermediate affinity receptors for interleukin 2 (IL-2) and IL-15 and these cytokines markedly enhance the ability of these cells to kill antibody-coated targets or cells that lack expression of major histocompatibility complex (MHC) molecules. In contrast, CD56bright cells only represent a minor subset of NK cells in peripheral blood. CD56bright NK cells do not express CD16 and are not potent cytolytic effector cells. However, when activated, CD56bright NK cells secrete large amounts of various cytokines, including interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), TNF-β, and IL-10. In contrast to CD56dim cells, CD56bright NK cells constitutively express high-affinity receptors for IL-2. Thus, several previous studies in patients with cancer or HIV have demonstrated that prolonged treatment with low doses of recombinant IL-2 results in the marked expansion of CD56bright NK cells in vivo.2-4 With prolonged IL-2 therapy, CD56bright NK cells become the predominant circulating NK cell subset.
These distinct phenotypic characteristics suggest that CD56dim and CD56bright NK cells mediate different functions in vivo. Whereas CD56dim NK cells appear to be primarily cytolytic effector cells, CD56bright NK cells appear to have predominately regulatory functions. These regulatory functions are mediated through secretion of various cytokines in response to various stimuli. Indeed, CD56bright NK cells express L-selectin and CCR7 and are present in human lymph nodes where they appear to interact with both dendritic cells and T cells to enhance antigen presentation and generation of specific T cells.5 FIG1
CD56bright (red cell) and CD56dim (blue cell) NK cell subsets express different surface receptors and exhibit distinct innate immune functions. Reprinted from Cooper et al.1
CD56bright (red cell) and CD56dim (blue cell) NK cell subsets express different surface receptors and exhibit distinct innate immune functions. Reprinted from Cooper et al.1
Although CD56dim and CD56bright NK cells appear to have different functional roles, the cellular mechanisms responsible for these distinct functions have not previously been known. In this issue, Trotta and colleagues demonstrate that SHIP1 (Src homology 2 domain-containing inositol 5-phosphatase 1), a hematopoietic cell–specific phosphatase, plays an important role in suppressing cytokine secretion in CD56dim NK cells. CD56bright NK cells express low levels of SHIP1 but forced expression of this phosphatase markedly reduces the ability of these cells to secrete INF-γ following activation by monocytic cytokines such as IL-12, IL-15, and IL-18. Interestingly, these monokines also down-regulate the expression of SHIP1, leading to further enhancement of IFN-γ production by CD56bright NK cells. Having begun to define the signaling pathways that regulate cytokine secretion by CD56bright NK cells, further studies can now be directed to better define the relevant targets of SHIP1 that mediate these effects and to identify other molecules that regulate these important functions of human NK cells. ▪