Abstract
Adenosine 5′-diphosphate (ADP) plays a central role in regulating platelet function by the activation of the G protein–coupled receptors P2Y1 and P2Y12. Although it is well established that aggregation responses of platelets to ADP desensitize, the underlying mechanisms involved remain unclear. In this study we demonstrate that P2Y1- and P2Y12-mediated platelet responses desensitize rapidly. Furthermore, we have established that these receptors desensitize by different kinase-dependent mechanisms. G protein–coupled receptor kinase (GRK) 2 and GRK6 are both endogenously expressed in platelets. Transient overexpression of dominant-negative mutants of these kinases or reductions in endogenous GRK expression by the use of specific siRNAs in 1321N1 cells showed that P2Y12, but not P2Y1, desensitization is mediated by GRKs. In contrast, desensitization of P2Y1, but not P2Y12, is largely dependent on protein kinase C activity. This study is the first to show that both P2Y1 and P2Y12 desensitize in human platelets, and it reveals ways in which their sensitivity to ADP may be differentially and independently altered.
Introduction
Platelets are activated by a variety of extracellular stimuli and are an essential component of the normal response to vascular injury. Central among these stimuli is adenosine 5′-diphosphate (ADP), which induces multiple platelet responses and potentiates platelet aggregation to other agonists (for reviews, see Gachet1 and Kunapuli et al2 ). Indeed, since it was recognized 40 years ago, ADP has been regarded as a central mediator of hemostasis and thrombosis by providing a positive feedback mechanism for the activation of platelets by multiple agonists. For example, both thrombin and collagen promote ADP release from platelet-dense granules; ADP subsequently acts on purinergic receptors to reinforce platelet aggregation responses1,2 and thrombus formation.
ADP acts on 2 G protein–coupled receptors (GPCRs), P2Y1 and P2Y12.1,2 The P2Y1 purinergic receptor was the first of the ADP receptors to be cloned.3-5 This receptor is widely expressed throughout the body and couples to Gq, leading to the activation of phospholipase Cβ, a subsequent increase in cytosolic calcium, and the activation of protein kinase C (PKC). The P2Y12 receptor was only recently identified6 and is the target of the clinically effective antithrombotic drugs clopidogrel and ticlopidine. P2Y12 couples through Gi to the inhibition of adenylyl cyclase and the activation of PI3-kinase. On the basis of pharmacologic and genetic studies, it is now accepted that P2Y1 is required for platelet activation by ADP, whereas P2Y12 is important in synergizing with P2Y1 or other Gq-coupled receptors to induce platelet activation by ADP and other agonists playing a major role in stabilizing platelet thrombi in vivo.
Given the established crucial role of ADP in platelet activation, it is likely that the responsiveness of platelets to ADP is tightly regulated, and knowledge of the mechanisms responsible for this regulation will be of considerable importance for the design of improved therapeutic strategies in the treatment of thrombotic disease. It has previously been demonstrated that human platelets can become refractory to activation after major surgery, possibly leading to an increased risk for postsurgical bleeding.7 Platelets have been shown to desensitize on continued exposure to ADP8-14 with targeted disruption of the CD39 gene, ablating the expression of endothelial ecto-apyrase in mice and causing a paradoxic platelet hyporesponsive phenotype; the authors explain this as the desensitization of platelet responses to ADP.15 This elegantly demonstrates that ADP sensitivity may vary in vivo and that desensitization mechanisms play a critical role in regulating platelet responsiveness to ADP. A more recent study suggested that this was attributed to desensitization of the P2Y1 receptor, with the P2Y12 receptor remaining functional, though the molecular mechanisms responsible for receptor desensitization were not investigated.9 In this study, we have reexamined in detail the desensitization of P2Y1 and P2Y12 receptor responses in platelets. To fully appreciate the molecular mechanisms responsible for receptor desensitization and to circumvent problems associated with examining these processes in human platelets, we also studied the regulation of heterologously expressed P2Y1 and P2Y12 receptors. In this investigation, we present evidence that the P2Y1 and P2Y12 purinergic receptors undergo desensitization in human platelets and that different families of protein kinases differentially regulate these 2 receptors.
Materials and methods
Materials
Dulbecco modified Eagle medium (DMEM), fetal bovine serum, and Lipofectamine 2000 transfection reagent were obtained from Invitrogen (Carlsbad, CA). Complete protease inhibitor tablets were from Roche. [3H]-cyclic adenosine monophosphate ([3H]-cAMP) and myo-[3H]-inositol (37 MBq mL-1) were from Amersham (Arlington Heights, IL). All other reagents were from Sigma (St Louis, MO).
Preparation of human platelets
Human blood was drawn from healthy, drug-free volunteers on the day of the experiment. Informed consent for blood taking was given by the Local Research Ethics Committee, United Bristol Healthcare Trust (project number D5736). Acid citrate dextrose (ACD; 120 mM sodium citrate, 110 mM glucose, 80 mM citric acid, used at 1:7 vol/vol) was used as anticoagulant. Platelet-rich plasma (PRP) was prepared by centrifugation at 200g for 17 minutes. Platelets were then isolated by centrifugation for 10 minutes at 1000g in the presence of 0.02 U/mL apyrase and prostaglandin E1 (PGE1; 140 nM) for all assays, other than measurements of intracellular cAMP, where PGE1 was omitted. The pellet was resuspended to a density of 4 × 108 platelets/mL in a modified Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (145 mM NaCl, 2.9 mM KCl, 10 mM HEPES, 1 mM MgCl2, 5 mM glucose; pH 7.3). To this platelet suspension, 10 μM indomethacin and 0.02 U/mL apyrase were added, and a 30-minute resting period was allowed before stimulation.
Measurement of platelet aggregation
Washed platelets were stimulated in the presence of 1 mg/mL fibrinogen in a Born aggregometer (Chrono-log, Havertown, PA) at 37°C under continuous stirring. Aggregation was monitored by optical aggregometry.
Receptor desensitization protocol for individual P2Y1 and P2Y12 responses
In all experiments to induce receptor desensitization, a desensitizing concentration of ADP was added for 10 to 300 seconds. Desensitizing ADP was then removed by the addition of 0.2 U/mL apyrase 3 minutes before the stimulation of cells. This time is calculated as 60 times that required for the complete breakdown of 10 μM ADP by apyrase under optimal conditions.16 In all experiments, controls were performed by following this protocol but not adding desensitizing ADP.
Measurement of cytosolic free calcium ([Ca2+]i) in platelets
Cytosolic calcium was measured as previously described.17 Briefly, 3 μM Fura-2AM (Molecular Probes, Eugene, OR) was added to platelet-rich plasma and was incubated at 37°C for 45 minutes in the presence of 10 μM indomethacin. Platelets were centrifuged and resuspended in modified Tyrode. ADP (10 μM)–induced calcium responses were subsequently measured at 37°C in desensitized and nondesensitized platelets using a Hitachi F-4500 spectrofluorometer (Hitachi, Tokyo, Japan) with fluorescence excitation made at 340 nm and 380 nm and emission at 510 nm.
Measurement of cAMP levels in platelets
After receptor desensitization and the addition of apyrase, as described above, EGTA (ethyleneglycotetraacetic acid) (1 mM) was added 1 minute before stimulatory ADP was added to inhibit platelet aggregation and to inhibit calcium-dependent apyrase activity,16 thereby preventing the breakdown of ADP added during the platelet-stimulation phase. This was important because prolonged stimulation with ADP is required for the significant measurable inhibition of cAMP accumulation. Platelets were stimulated in the presence of the phosphodiesterase inhibitor IBMX (3-isobutyl-1-methylxanthine) (100 μM) plus forskolin (10 μM), in the absence or presence of ADP, for 5 minutes at 37°C. Cyclic AMP accumulation was terminated by the addition of ice-cold 100% trichloroacetic acid (TCA), precipitates were removed by centrifugation at 4000g for 5 minutes, and supernatants were neutralized with 1 M NaOH and TE buffer (50 mM Tris-HCl, 4 mM EDTA; pH 7).4 Cyclic AMP levels were determined using a binding assay as previously described.18 Data are presented as a percentage of inhibition of forskolin-stimulated adenylyl cyclase or as cAMP accumulation (pmol cAMP/1 × 108 platelets).
Generation of P2Y1 and P2Y12 receptor constructs
Hemagglutinin-tagged P2Y1 and P2Y12 constructs were generated by polymerase chain reaction (PCR) using human P2Y1 and P2Y12 cDNA as templates. Primers were designed that introduced a Xho restriction site at the 5′ end, followed by a Kozak sequence, an ATG, and a 9-amino acid HA tag (YPYDVPDYA). The 3′ primer sequence contained either an XbaI site (P2Y1) or a SalI site (P2Y12) to facilitate directional cloning of the PCR product into the pCIneo vector (Promega, Madison, WI). The final construct was sequenced to ensure that no mutations were introduced during PCR.
Cell culture and transfection of 1321N1 astrocytoma cells
Human 1321N1 astrocytoma cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 U/mL-1 penicillin G, and 100 μg/mL-1 streptomycin sulfate at 37°C in a humidified atmosphere of 95% air and 5% CO2. For the generation of stable transfectants, pCIneo containing P2Y1, P2Y12, or vector alone was linearized, mixed with Lipofectamine 2000 (Invitrogen), and incubated with plated cells. After 48 hours, medium was supplemented with 400 μg/mL geneticin, and cells expressing HA-tagged receptor were isolated by immunofluorescence microscopy, as previously described.19 In transient transfections, cells were grown to 80% to 90% confluence and were transfected with 0.5 to 10 μg DNA using Lipofectamine 2000 (Invitrogen). Cells were analyzed 48 hours after transfection.
Transfection of GRK siRNAs
siRNA transfection of the G protein–coupled receptor kinase-2 (GRK2), GRK6, and scrambled (SC) RNA duplexes (Dharmacon, Lafayette, CO) was performed in 1321N1cells at approximately 90% confluence.20 Cells were transfected with siRNA duplexes (600 pmol siRNA in a 10-cm dish, final concentration of 40 nM) using Lipofectamine 2000 (Invitrogen). Cells were transfected with siRNA, split after 6 hours, transfected a second time after 24 hours, and analyzed for GRK expression and receptor activity 4 days after initial transfection. All siRNAs consisted of 21 nucleotide duplexes and had the following sequences: siRNA-GRK2, 5′-GAU CUU CGA CUC AUA CAU CdTdT-3′; siRNA-GRK6, 5′-GCC UCG AGC GUG ACU AUC AdTdT-3′; and siRNA-SC, 5′-GCG CGC UUU GUA GGA UUC GdTdT-3′.
Measurement of cytosolic free calcium ([Ca2+]i) in 1321N1 astrocytoma cells
Cytosolic free Ca2+ concentration ([Ca2+]i) was determined using Fura-2AM, as previously reported.17,21 Briefly, cells were grown on poly-l-lysine–coated glass coverslips and were used at approximately 60% confluence. Cells were washed twice and incubated with Fura-2AM (3 μM) at 37°C for 60 minutes. Coverslips were mounted into a quartz cuvette, and cells were continuously perfused at 37°C. Fluorescence was measured at 340 and 380 nm excitation and 510 nm emission, and [Ca2+]i was determined from radiometric data, as previously described.22
Inositol phosphate determination in 1321N1 astrocytoma cells
Inositol phosphate (IP) determination was undertaken as previously described.21 Briefly, cells stably transfected with P2Y1 were seeded into 24-well culture plates coated with 0.1 mg mL-1 poly-L-lysine. Cells were then labeled for 18 to 24 hours with myo-[3H]inositol (37 MBq mL-1) in DMEM (high glucose, without inositol), washed once, and incubated in DMEM containing ADP at 37°C. Apyrase (0.2 U/mL) was subsequently added for 1 minute to remove desensitizing ADP. Cells were then washed 3 times in ice-cold PBS and incubated with prewarmed media containing 20 mM LiCl and ADP. Reactions were terminated by the addition of 0.4 M perchloric acid, and samples were harvested into 0.4 mL of 0.72 M KOH, 0.6 M KHCO3. IP was separated on Dowex AG 1-X8 columns (Biorad, Hercules, CA) exactly as described previously,21 and total labeled IP was determined by scintillation counting. Data are presented as IP accumulation (fold over basal) or total [3H] IP/[3H] inositol.
Measurement of cAMP accumulation in 1321N1 astrocytoma cells
Cells were grown to 80% confluence and exposed to a desensitizing dose of ADP in the presence of the phosphodiesterase inhibitor Ro201724 (250 μM). Apyrase (0.2 U/mL) was then added directly to each well and was incubated for 1 minute at 37°C to remove the desensitizing ADP. Cells were then washed, forskolin (1 μM) was added in the absence or presence of ADP, and plates were incubated at 37°C for 10 minutes. Endogenous β2 adrenoceptor responses were also examined in 1321N1 cells by measuring isoproterenol (1 μM)–stimulated cAMP accumulation. Cyclic AMP accumulation was terminated by the addition of ice-cold 100% trichloroacetic acid, and supernatant was neutralized with 1 M NaOH and TE buffer. Cyclic AMP levels were determined as previously described.18 Data are expressed as cAMP production (pmol cAMP/well) or as a percentage of inhibition of forskolin-stimulated adenylyl cyclase.
Western blotting
Protein expression in 1321N1 cells and platelets was determined by Western blotting. Briefly, cells were lysed into ice-cold lysis buffer (20 mM HEPES, pH 7.4, 200 mM NaCl, 10 mM EDTA [ethylenediaminetetraacetic acid], 1% Triton X-100, supplemented with Complete protease inhibitors) and pelleted by centrifugation, and the pellet was lysed into sodium dodecyl sulfate loading buffer (63 mM Tris, pH 6.5, 100 mM dithiothreitol, 1% sodium dodecyl sulfate [SDS], 11.6% glycerol, and 0.02% bromophenol blue) and resolved by SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Gels were transferred to nitrocellulose membranes and blotted with GRK-specific antibodies: GRK2, mouse monoclonal antibody against residues 500 to 531 of bovine GRK223 or GRK6, and rabbit polyclonal antibody against residues 98 to 136 of human GRK6.23 Proteins were detected by enhanced chemiluminescence (ECL).
Experimental analysis and statistics
Data were analyzed using GraphPAD Prism (GraphPAD Software). Where appropriate, statistical significance of differences was assessed using the Mann-Whitney U test.
Results
Desensitization of P2Y1- and P2Y12-mediated responses in human platelets
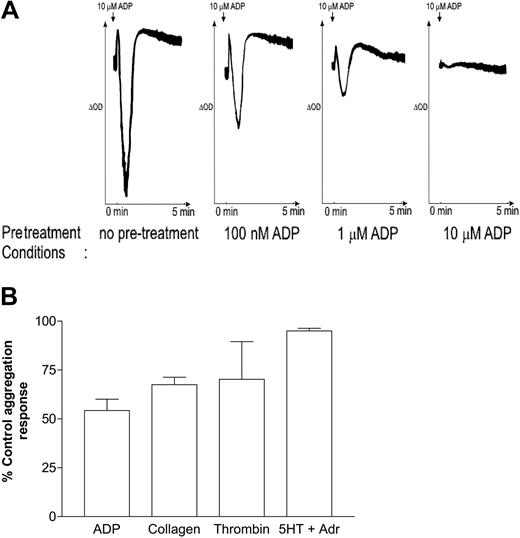
In agreement with previous studies,8-14 Figure 1A demonstrates that ADP-induced aggregation desensitized after prestimulation with various concentrations of ADP for 3 minutes. Pretreatment with ADP (1 μM; 3 minutes) also reduced aggregation induced by collagen (30 μg/mL) and thrombin (0.1 U/mL), whereas responses to combined stimulation with 5-hydroxytryptamine (5-HT) (20 μM) and adrenaline (20 μM) were largely unaffected (Figure 1B). Because the aggregation response to ADP is dependent on the stimulation of P2Y1 and P2Y12, we wanted next to determine whether desensitization was caused by decreased sensitivity of either or both of the platelet ADP receptors.
Desensitization of human platelet aggregation. (A) Washed platelets were preincubated under nonstirred conditions with vehicle (no pretreatment), 100 nM ADP, 1 μM ADP, or 10 μM ADP for 2 minutes, as indicated, and another minute with 1 mg/mL fibrinogen to allow aggregation. Platelets were then stimulated with 10 μM ADP, and the resultant aggregation response was monitored by optical aggregometry. Shown are traces representative of at least 3 experiments. (B) Washed platelets were preincubated under nonstirred conditions with vehicle or with 1 μM ADP for 2 minutes and for another minute with 1 mg/mL fibrinogen to allow aggregation. Platelets were then stimulated with ADP (10 μM), collagen (30 μg/mL), thrombin (0.1 U/mL), or 5-HT (20 μM) plus adrenaline (20 μM), and the resultant aggregation response was monitored by optical aggregometry. The maximal extent of aggregation 3 minutes after stimulation was measured, and data were expressed as a percentage of the control aggregation response (ie, in the absence of a desensitizing dose of ADP) and represent the mean ± SEM (n = 3).
Desensitization of human platelet aggregation. (A) Washed platelets were preincubated under nonstirred conditions with vehicle (no pretreatment), 100 nM ADP, 1 μM ADP, or 10 μM ADP for 2 minutes, as indicated, and another minute with 1 mg/mL fibrinogen to allow aggregation. Platelets were then stimulated with 10 μM ADP, and the resultant aggregation response was monitored by optical aggregometry. Shown are traces representative of at least 3 experiments. (B) Washed platelets were preincubated under nonstirred conditions with vehicle or with 1 μM ADP for 2 minutes and for another minute with 1 mg/mL fibrinogen to allow aggregation. Platelets were then stimulated with ADP (10 μM), collagen (30 μg/mL), thrombin (0.1 U/mL), or 5-HT (20 μM) plus adrenaline (20 μM), and the resultant aggregation response was monitored by optical aggregometry. The maximal extent of aggregation 3 minutes after stimulation was measured, and data were expressed as a percentage of the control aggregation response (ie, in the absence of a desensitizing dose of ADP) and represent the mean ± SEM (n = 3).
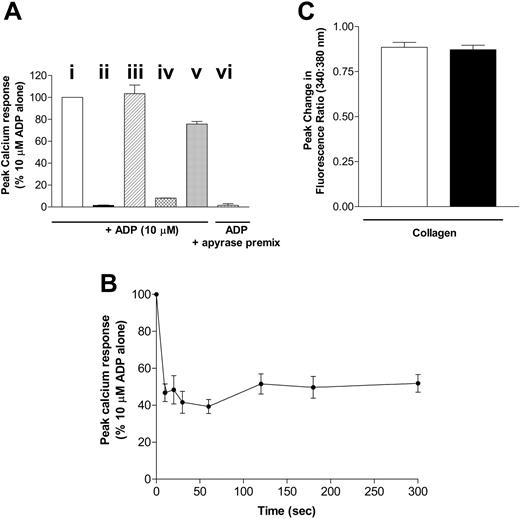
The ADP-induced calcium response depends on P2Y1 receptor activation; the inhibition of adenylate cyclase is downstream of P2Y12. We examined the desensitization of each of these responses using the following protocol. Platelets were pretreated with ADP for various times (0-300 seconds) to induce desensitization. The desensitizing ADP was then removed by treating the cells with apyrase (0.2 U/mL) for 3 minutes, and the signaling of each receptor was examined and compared with that in control non-ADP pretreated platelets in the presence of apyrase (0.2 U/mL). To validate this protocol, a number of control experiments were performed (Figure 2A).Although pretreatment causes a transient increase in cell calcium concentration, the concentration returns to basal levels by the time the second dose of agonist is added (data not shown). Pretreatment of platelets with the P2Y1 receptor antagonist A3P5P (1 mM; 5 minutes) abolished ADP (10 μM)–induced calcium responses (Figure 2Aii). Pretreatment of platelets with apyrase (0.2 U/mL; 3 minutes) did not significantly affect the calcium response to ADP (10 μM; Figure 2Aiii) when compared with control platelets in the absence of apyrase. Importantly, platelets pretreated with ADP (10 μM) and 30 seconds later with apyrase did show a significant reduction in calcium response to a subsequent addition of ADP (10 μM; Figure 2A(iv)) when compared with control cells (Figure 2Ai). Apyrase degrades ADP to AMP (with negligible generation of adenosine, as confirmed by high-performance liquid chromatography [HPLC] measurements; data not shown), and it was, therefore, important to rule out the possibility that AMP may mediate the apparent desensitization of responses seen. Figure 2A shows that, when mixed in vitro before addition to platelets, 0.2 U/mL apyrase is able to fully degrade 10 μM ADP within a 3-minute period to a level that is subsequently unable to induce platelet activation (Figure 2Avi). The maximal achievable concentration of AMP generated would be 10 μM.At this concentration, AMP, when added in combination with apyrase (0.2 U/mL), inhibited the calcium response of platelets to 10 μM ADP by only approximately 20% (Figure 2Av), substantially less than the reduction achieved by desensitization. All subsequent platelet experiments, however, used a 10-fold lower pretreatment concentration of 1 μM ADP, followed by apyrase (0.2 U/mL; 3 minutes), to minimize any role for AMP in the reduction in responsiveness.
P2Y1 purinergic receptor desensitization in human platelets. (A) Platelets were not pretreated (i; □) or were pretreated with (ii) P2Y1 receptor antagonist A3P5P 1 mM for 180 seconds (▪), (iii) apyrase alone 0.2 U/mL for 180 seconds (▨ ), (iv) ADP 10 μM for 30 seconds then apyrase 0.2 U/mL for 180 seconds (▩), or (v) AMP 10 μM for 30 seconds then apyrase 0.2 U/mL for 180 seconds (▦). ADP (10 μM)–induced calcium responses were subsequently measured, and peak responses were compared with those in nonpretreated control platelets. ADP (10 μM) premixed with apyrase for 3 minutes was also added to control platelets (vi; ▥), and subsequent calcium responses were measured. Data are expressed as peak calcium response (percentage of 10 μM ADP alone) and represent the mean ± SEM (n = 3). (B) Platelets were pretreated with ADP 1 μM for 0 to 300 seconds, and then apyrase 0.2 U/mL was added for 180 seconds. ADP (10 μM)–induced calcium responses were subsequently measured and compared with those of control platelets incubated with apyrase 0.2 U/mL only. Data are expressed as peak calcium response relative to control responses (percentage of 10 μM ADP alone) and represent the mean ± SEM (n = 6). (C) Platelets were pretreated with ADP 10 μM for 30 seconds, and then apyrase 0.2 U/mL was added for 180 seconds. Collagen (30 μg/mL)–induced calcium responses were subsequently measured, and peak responses (▪) were compared with those of nonpretreated control platelets (□). ADP/apyrase pretreatment did not affect peak calcium responses before collagen addition compared with nonpretreated controls (data not shown). Data are expressed as peak change in fluorescence ratio (340:380 nM) and represent the mean ± SEM (n = 3).
P2Y1 purinergic receptor desensitization in human platelets. (A) Platelets were not pretreated (i; □) or were pretreated with (ii) P2Y1 receptor antagonist A3P5P 1 mM for 180 seconds (▪), (iii) apyrase alone 0.2 U/mL for 180 seconds (▨ ), (iv) ADP 10 μM for 30 seconds then apyrase 0.2 U/mL for 180 seconds (▩), or (v) AMP 10 μM for 30 seconds then apyrase 0.2 U/mL for 180 seconds (▦). ADP (10 μM)–induced calcium responses were subsequently measured, and peak responses were compared with those in nonpretreated control platelets. ADP (10 μM) premixed with apyrase for 3 minutes was also added to control platelets (vi; ▥), and subsequent calcium responses were measured. Data are expressed as peak calcium response (percentage of 10 μM ADP alone) and represent the mean ± SEM (n = 3). (B) Platelets were pretreated with ADP 1 μM for 0 to 300 seconds, and then apyrase 0.2 U/mL was added for 180 seconds. ADP (10 μM)–induced calcium responses were subsequently measured and compared with those of control platelets incubated with apyrase 0.2 U/mL only. Data are expressed as peak calcium response relative to control responses (percentage of 10 μM ADP alone) and represent the mean ± SEM (n = 6). (C) Platelets were pretreated with ADP 10 μM for 30 seconds, and then apyrase 0.2 U/mL was added for 180 seconds. Collagen (30 μg/mL)–induced calcium responses were subsequently measured, and peak responses (▪) were compared with those of nonpretreated control platelets (□). ADP/apyrase pretreatment did not affect peak calcium responses before collagen addition compared with nonpretreated controls (data not shown). Data are expressed as peak change in fluorescence ratio (340:380 nM) and represent the mean ± SEM (n = 3).
Figure 2B shows the rapid desensitization of the calcium response to ADP (10 μM) after pretreatment with 1 μM ADP for 0 to 300 seconds. This P2Y1 receptor–dependent response was found to desensitize rapidly, reaching maximum desensitization by 30 seconds and remaining sustained thereafter. To confirm that the reduction in calcium responsiveness was not caused by a depletion of intracellular stores, we determined whether the calcium response to another agonist, collagen, was affected by the pretreatment of platelets with ADP. Figure 2C shows that the collagen-induced calcium response is not affected after desensitization of the ADP receptors, indicating that store emptying is not responsible for the apparent desensitization of the ADP-induced response and also indicating that no cross-desensitization occurs between these 2 agonists at the level of calcium signaling. Therefore, in agreement with previous studies,9 the P2Y1 purinergic receptor desensitizes rapidly for a sustained period in platelets.
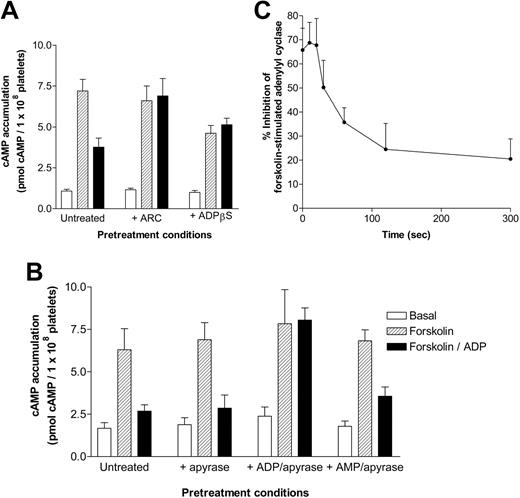
It was important to determine whether platelet P2Y12 receptor–mediated responses would also desensitize. Initially, we confirmed that ADP-induced inhibition of forskolin-stimulated adenylyl cyclase could be blocked by pretreatment with the P2Y12 purinergic receptor antagonist AR-C69931MX (1 μM; Figure 3A). Recently, Baurand et al9 used ADPβS to induce the desensitization of P2Y12 purinergic receptor responses. A major concern was that the desensitizing ADPβS—unlike ADP, which can subsequently be broken down by the addition of apyrase—could not be removed before platelet stimulation. Figure 3A shows that the ongoing presence of desensitizing ADPβS (1 mM) significantly decreases subsequent forskolin-stimulated adenylyl cyclase activity through the activation of P2Y12. Because ADPβS is still able to activate P2Y12, we concluded, in agreement with Baurand et al,9 that this agonist does not seem to induce full receptor desensitization. The continuing presence of ADPβS also diminished the ability of a subsequent dose of ADP to further decrease forskolin-stimulated adenylyl cyclase activity, though levels of cAMP accumulation were not significantly different from those in untreated platelets stimulated with forskolin and ADP, suggesting that maximal P2Y12 receptor activation had already been achieved. Thus, given that we were concerned that ADPβS pretreatment made interpretation of the data problematic, we used the ADP/apyrase protocol already outlined throughout the rest of this study.
P2Y12 purinergic receptor signaling and desensitization in human platelets. (A-B) cAMP accumulation (5 minutes) in the absence (basal; □) or presence of 10 μM forskolin (forskolin; ▨) ± ADP (10 μM; forskolin + ADP; ▪) was determined in (A) 3 different conditions; in the absence of pretreatment (untreated) or after pretreatment with either the P2Y12 receptor antagonist AR-C69931MX (1 μM; 5 minutes) or the P2Y receptor agonist ADPβS (1 mM; 5 minutes). (B) cAMP accumulation was determined in 4 different conditions: (1) in the absence of apyrase (untreated), (2) after pretreatment with apyrase (0.2 U/mL; 180 seconds; + apyrase), (3) after treatment with ADP (1 μM; 300 seconds) and subsequent treatment with apyrase (+ ADP/apyrase), and finally (4) after pretreatment with apyrase (0.2 U/mL; 180 seconds) and AMP (10 μM; 180 seconds) (+ AMP/apyrase). Data represent the mean ± SEM (n = 3) and are expressed as pmol cAMP per 1 × 108 platelets. (C) Platelets were desensitized to ADP, as described in “Materials and methods,” after ADP pretreatment for various time periods, and a percentage of inhibition of forskolin-stimulated cAMP accumulation was plotted as a function of pretreatment time. Data represent mean ± SEM (n = 3) and are expressed as a percentage of inhibition of forskolin-stimulated adenylyl cyclase.
P2Y12 purinergic receptor signaling and desensitization in human platelets. (A-B) cAMP accumulation (5 minutes) in the absence (basal; □) or presence of 10 μM forskolin (forskolin; ▨) ± ADP (10 μM; forskolin + ADP; ▪) was determined in (A) 3 different conditions; in the absence of pretreatment (untreated) or after pretreatment with either the P2Y12 receptor antagonist AR-C69931MX (1 μM; 5 minutes) or the P2Y receptor agonist ADPβS (1 mM; 5 minutes). (B) cAMP accumulation was determined in 4 different conditions: (1) in the absence of apyrase (untreated), (2) after pretreatment with apyrase (0.2 U/mL; 180 seconds; + apyrase), (3) after treatment with ADP (1 μM; 300 seconds) and subsequent treatment with apyrase (+ ADP/apyrase), and finally (4) after pretreatment with apyrase (0.2 U/mL; 180 seconds) and AMP (10 μM; 180 seconds) (+ AMP/apyrase). Data represent the mean ± SEM (n = 3) and are expressed as pmol cAMP per 1 × 108 platelets. (C) Platelets were desensitized to ADP, as described in “Materials and methods,” after ADP pretreatment for various time periods, and a percentage of inhibition of forskolin-stimulated cAMP accumulation was plotted as a function of pretreatment time. Data represent mean ± SEM (n = 3) and are expressed as a percentage of inhibition of forskolin-stimulated adenylyl cyclase.
As with the calcium studies, we confirmed that adding apyrase did not affect either the stimulation of adenylyl cyclase by forskolin or the ADP-induced inhibition of forskolin-stimulated adenylyl cyclase activity in human platelets (Figure 3B). Pretreatment with ADP (1 μM; 300 seconds) significantly reduced subsequent P2Y12 purinergic receptor stimulation (Figure 3B). This reduction in response was not secondary to AMP production because pretreatment with 10 μM AMP had no significant effect on the ability of ADP to inhibit forskolin-mediated cAMP response (Figure 3B). P2Y12 purinergic receptor desensitization was further characterized at different time points (0-300 seconds) and was found to desensitize rapidly over a 5-minute time course (Figure 3C). Therefore, in human platelets, P2Y1 and P2Y12 receptors undergo rapid desensitization after pretreatment with ADP.
Desensitization of P2Y1-mediated responses: the role of GRKs and PKC
To study the molecular mechanisms underlying desensitization, we expressed each receptor in a single cell type, 1321N1 human astrocytoma cells, which do not endogenously express purinergic receptors.24 We confirmed stable expression of each receptor by immunofluorescence microscopy (data not shown). We initially focused on the P2Y1 receptor and examined its ability to couple to Gq. As shown in Figure 4A, P2Y1 activation by ADP promoted IP accumulation in 1321N1 cells, whereas control cells transfected with vector alone showed no response. This response rapidly desensitized, as shown in Figure 4B, because pretreatment with ADP (1 μM; 5, 15, and 30 minutes) partially but significantly attenuated IP accumulation to a subsequent addition of ADP (1 μM). P2Y1 activation by ADP also promoted calcium mobilization in 1321N1 cells, whereas control cells transfected with vector alone showed no response. Interestingly, we were able to measure receptor desensitization with short periods of ADP exposure (2 minutes). Similar periods of agonist exposure failed to demonstrate significant and reproducible differences in IP accumulation (data not shown). Figure 4C shows an increasing rightward shift in the concentration-response relationship for this response, after pretreatment with a desensitizing addition of ADP (EC50 of 59.7 ± 15.3, 100.3 ± 11.3, 300 ± 4.9, and 384 ± 18.7 nM for ADP-induced calcium mobilization before and after 0.1, 1, and 10 μM ADP pretreatment, respectively).
Agonist-induced desensitization of calcium responses and inositol phosphate accumulation in cells stably expressing P2Y1 purinergic receptors. (A) IP accumulation was measured after the addition of ADP (1 nM-10 μM; 10 minutes) in cells stably expressing the P2Y1 purinergic receptor (⬡) or vector (pcNEO) alone (○). Data are the mean ± SEM of 5 independent experiments, and results are expressed as fold IP accumulation over basal. ADP stimulated IP accumulation with an EC50 of 14 ± 0.31 nM. (B) Cells either were not pretreated with ADP (control; ⬡) or were pretreated with ADP (1 μM; 5 [▿], 15 [○], or 30 minutes [▵]) and were incubated with apyrase (0.2 U/mL; 1 minute). Cells were then washed, and IP accumulation was measured after the addition of ADP (1 μM; 0-30 minutes). Data are mean ± SEM of 4 independent experiments, and results are expressed as fold IP accumulation over basal. (C) Cells stably transfected with P2Y1 purinergic receptor or vector alone (pcNEO; ▴) were either not pretreated (Control; ⬡) or were pretreated with agonist (ADP; 0.1 μM[▿], 1 μM[○], or 10 μM[▵] as indicated; 2 minutes). Peak calcium responses were measured on the addition of ADP (1 nM-100 μM). Values represent the mean ± SEM of 4 independent experiments. Results are expressed as the difference between basal resting and maximal response (peak rise) in cytosolic calcium concentration ([Ca2+]i nM), as assessed by Fura-2AM in intact cells. Prestimulation with ADP (0.1-1 μM) caused a shift in the dose-response curve for agonist-induced calcium mobilization (EC50 of 59.7 ± 15.3, 100.3 ± 11.3, 300 ± 4.9, and 384 ± 18.7 nM for ADP-induced calcium mobilization before and after 0.1, 1, and 10 μM ADP pretreatment, respectively).
Agonist-induced desensitization of calcium responses and inositol phosphate accumulation in cells stably expressing P2Y1 purinergic receptors. (A) IP accumulation was measured after the addition of ADP (1 nM-10 μM; 10 minutes) in cells stably expressing the P2Y1 purinergic receptor (⬡) or vector (pcNEO) alone (○). Data are the mean ± SEM of 5 independent experiments, and results are expressed as fold IP accumulation over basal. ADP stimulated IP accumulation with an EC50 of 14 ± 0.31 nM. (B) Cells either were not pretreated with ADP (control; ⬡) or were pretreated with ADP (1 μM; 5 [▿], 15 [○], or 30 minutes [▵]) and were incubated with apyrase (0.2 U/mL; 1 minute). Cells were then washed, and IP accumulation was measured after the addition of ADP (1 μM; 0-30 minutes). Data are mean ± SEM of 4 independent experiments, and results are expressed as fold IP accumulation over basal. (C) Cells stably transfected with P2Y1 purinergic receptor or vector alone (pcNEO; ▴) were either not pretreated (Control; ⬡) or were pretreated with agonist (ADP; 0.1 μM[▿], 1 μM[○], or 10 μM[▵] as indicated; 2 minutes). Peak calcium responses were measured on the addition of ADP (1 nM-100 μM). Values represent the mean ± SEM of 4 independent experiments. Results are expressed as the difference between basal resting and maximal response (peak rise) in cytosolic calcium concentration ([Ca2+]i nM), as assessed by Fura-2AM in intact cells. Prestimulation with ADP (0.1-1 μM) caused a shift in the dose-response curve for agonist-induced calcium mobilization (EC50 of 59.7 ± 15.3, 100.3 ± 11.3, 300 ± 4.9, and 384 ± 18.7 nM for ADP-induced calcium mobilization before and after 0.1, 1, and 10 μM ADP pretreatment, respectively).
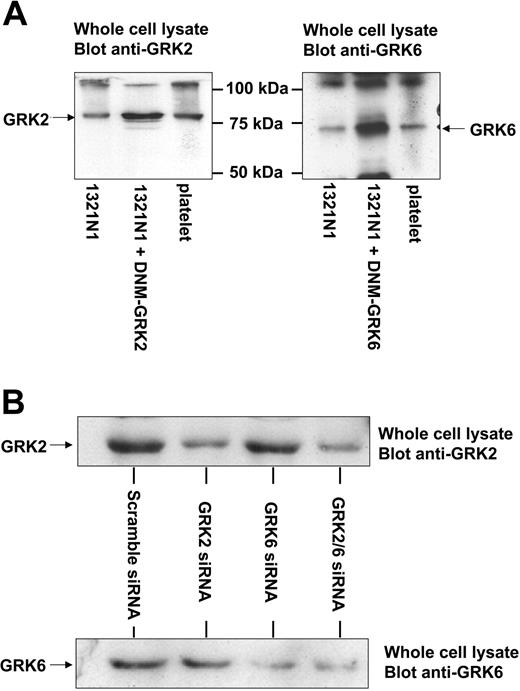
The molecular mechanisms responsible for P2Y1 receptor desensitization were then studied. Two major kinase-dependent mechanisms involved in the desensitization of GPCRs are mediated by the GRK and PKC families. Although investigation of the involvement of PKC isoforms can be addressed by pharmacologic means, this is not possible for the GRKs. Therefore, we chose to use 2 molecular approaches, overexpression of dominant-negative mutant (DNM) levels and reduction of endogenous levels by siRNA20 to selectively antagonize receptor kinases GRK2 and GRK6. Figure 5 shows overexpression of the DNM constructs (more than 5-fold over endogenous protein levels) and, importantly, shows that human platelets express GRK2 and GRK6, as shown by Western blotting. When overexpressed in 1321N1 cells, these DNMs block the phosphorylation and desensitization of a number of GPCRs.25,26 As a positive control, we show in Figure 6A that the expression of DNM-GRK2 and DNM-GRK6 significantly enhanced endogenous β2 adrenoceptor–dependent increases in cAMP production in 1321N1 cells, indicative of reduced receptor desensitization.18,27 As shown in Figure 5B, the transfection of GRK2 and GRK6 siRNAs selectively reduced endogenous GRK expression by approximately 70%. In this series of experiments, we chose to analyze the desensitization of the P2Y1 receptor–induced calcium response, which was a quicker and easier method to measure changes in receptor activity than IP accumulations. We chose to use 0.1 μM ADP as the stimulating concentration because, at this concentration, we had shown that desensitization with 1 or 10 μM ADP pretreatment produced the most significant change in peak calcium response (Figure 4C). Cells were pretreated with 1 μM rather than 10 μM ADP to reduce incidences of inadequate washout of desensitizing agonist. Expression of each DNM construct in 1321N1 cells did not block the desensitization of P2Y1 receptor–mediated responses (Figure 6B-C), though the expression of DNM-GRK2 did reduce the overall ADP-induced calcium signal (Figure 6B). Reduced GRK2 or GRK6 expression also did not affect the overall ADP-induced calcium signal or the desensitization of P2Y1 receptor–mediated responses. Because GRKs did not mediate the desensitization of P2Y1, we addressed the role PKC may play in receptor desensitization. Pretreatment with the PKC inhibitor GF109203X (2 μM), Ro317549 (1 μM), or Gö6976 (1 μM) significantly blocked the homologous desensitization of P2Y1 purinergic receptor responses (Figure 6B-C).
GRK2 and GRK6 expression in 1321N1 cells and platelets. (A) 1321N1 cells were transfected with 5 μg DNA containing empty vector or DNM-GRK constructs. Whole cell lysates from these cells or human platelets were subjected to SDS-PAGE, followed by immunoblotting with GRK-specific primary, as detailed in “Materials and methods.” Data shown are representative of 3 experiments. (B) Effect of siRNA treatment on endogenous GRK expression in 1321N1 cells. 1321N1 cells stably expressing P2Y12 purinergic receptors were transfected with scrambled, GRK2-, or GRK6-specific siRNAs twice in a 24-hour interval, and the cells were harvested after 4 days. GRK2 expression was analyzed by immunoblotting using an anti-GRK2 monoclonal antibody, whereas GRK6 expression was analyzed using an anti-GRK6 polyclonal antibody. These results are representative of at least 3 similar experiments. Similar results were obtained in 1321N1 cells stably expressing P2Y1 purinergic receptors (data not shown).
GRK2 and GRK6 expression in 1321N1 cells and platelets. (A) 1321N1 cells were transfected with 5 μg DNA containing empty vector or DNM-GRK constructs. Whole cell lysates from these cells or human platelets were subjected to SDS-PAGE, followed by immunoblotting with GRK-specific primary, as detailed in “Materials and methods.” Data shown are representative of 3 experiments. (B) Effect of siRNA treatment on endogenous GRK expression in 1321N1 cells. 1321N1 cells stably expressing P2Y12 purinergic receptors were transfected with scrambled, GRK2-, or GRK6-specific siRNAs twice in a 24-hour interval, and the cells were harvested after 4 days. GRK2 expression was analyzed by immunoblotting using an anti-GRK2 monoclonal antibody, whereas GRK6 expression was analyzed using an anti-GRK6 polyclonal antibody. These results are representative of at least 3 similar experiments. Similar results were obtained in 1321N1 cells stably expressing P2Y1 purinergic receptors (data not shown).
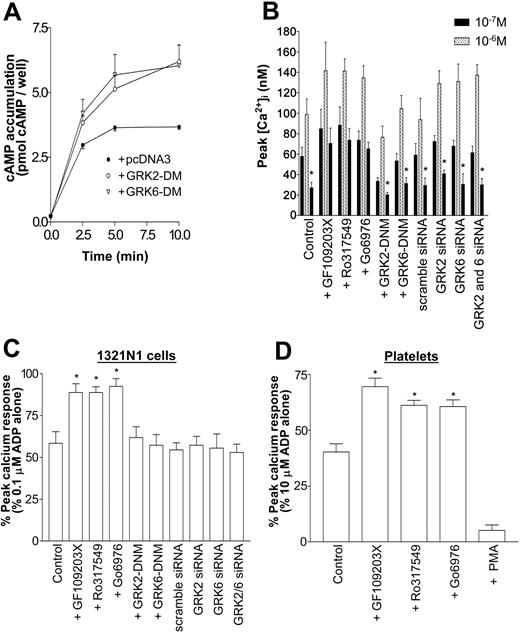
P2Y1 receptor desensitization is mediated through a PKC-dependent but GRK2- and GRK6-independent mechanism. Cells stably transfected with P2Y1 purinergic receptor construct were transiently transfected with pcDNA3-K220RGRK2 (GRK2-DNM), pcDNA3-K215RGRK6 (GRK6-DNM), vector alone (control), or scrambled GRK2- or GRK6-specific siRNA constructs. Cells were incubated in the absence or presence of the PKC inhibitors GF109203X (2 μM), Ro317549 (1 μM), or Gö6976 (1 μM) for 15 minutes before experiments. Desensitization was assessed by comparing calcium responses with those of ADP (0.1 μM) before or after a pretreatment addition of ADP (1 μM; 2 minutes), as detailed in “Materials and methods.” Values represent the mean ± SEM of 4 independent experiments. (A) Expression of GRK2-DNM (○) and GRK6-DNM (▿)in P2Y1-expressing 1321N1 cells enhances endogenous β2-adrenoceptor activity by decreasing the rate of desensitization of the receptor. Isoproterenol (1 μM)–induced cAMP accumulation (0-10 minutes) was assessed in 1321N1 cells. ⬡ indicates vector alone. Data represent mean ± SEM of 4 independent experiments and are expressed as pmol cAMP per well. (B) Fura-2AM–loaded cells were sequentially treated with ADP (0.1 μM) (left column of each triplet; ▪) to act as the control response, then with a desensitizing concentration of ADP (1 μM; middle column of each triplet; ▪), followed by a further test dose (0.1 μM ADP; right column in each triplet; ▪). Asterisks show responses where there is significant desensitization (right column compared with left column of each triplet; P < .05, Mann-Whitney U test). (C) Results are expressed as the response to 0.1 μM ADP after a desensitizing dose (1.0 μM ADP) as a percentage of the control response to 0.1 μM ADP. Asterisks indicate where responses are significantly desensitized relative to controls (P < .05, Mann-Whitney U test). (B-C) Data are mean ± SEM (n = 3). (D) P2Y1 receptor desensitization is mediated in part by PKC in human platelets. Platelets were pretreated with the PKC inhibitors GF109203X (2 μM), Ro317549 (1 μM), or Gö6976 (1 μM) or with the PKC activator PMA (100 nM; 15 minutes). Pretreatment of platelets with any of these agents induced no change in cytosolic calcium (data not shown). Platelets were treated with ADP (1 μM; 30 seconds) and then with 0.2 U/mL apyrase for another 180 seconds, and they were restimulated with 10 μM ADP. Receptor desensitization was assessed by comparing a percentage of desensitization of peak calcium responses to controls that had been treated identically except for the lack of pretreatment with desensitizing ADP. Values are mean ± SEM (n = 3), and results are expressed as the ADP response after a desensitizing dose as a percentage of the control response. Asterisks indicate statistical significance at P < .05 for data compared with ADP pretreatment without PKC activator or inhibitor (control; Mann-Whitney U test).
P2Y1 receptor desensitization is mediated through a PKC-dependent but GRK2- and GRK6-independent mechanism. Cells stably transfected with P2Y1 purinergic receptor construct were transiently transfected with pcDNA3-K220RGRK2 (GRK2-DNM), pcDNA3-K215RGRK6 (GRK6-DNM), vector alone (control), or scrambled GRK2- or GRK6-specific siRNA constructs. Cells were incubated in the absence or presence of the PKC inhibitors GF109203X (2 μM), Ro317549 (1 μM), or Gö6976 (1 μM) for 15 minutes before experiments. Desensitization was assessed by comparing calcium responses with those of ADP (0.1 μM) before or after a pretreatment addition of ADP (1 μM; 2 minutes), as detailed in “Materials and methods.” Values represent the mean ± SEM of 4 independent experiments. (A) Expression of GRK2-DNM (○) and GRK6-DNM (▿)in P2Y1-expressing 1321N1 cells enhances endogenous β2-adrenoceptor activity by decreasing the rate of desensitization of the receptor. Isoproterenol (1 μM)–induced cAMP accumulation (0-10 minutes) was assessed in 1321N1 cells. ⬡ indicates vector alone. Data represent mean ± SEM of 4 independent experiments and are expressed as pmol cAMP per well. (B) Fura-2AM–loaded cells were sequentially treated with ADP (0.1 μM) (left column of each triplet; ▪) to act as the control response, then with a desensitizing concentration of ADP (1 μM; middle column of each triplet; ▪), followed by a further test dose (0.1 μM ADP; right column in each triplet; ▪). Asterisks show responses where there is significant desensitization (right column compared with left column of each triplet; P < .05, Mann-Whitney U test). (C) Results are expressed as the response to 0.1 μM ADP after a desensitizing dose (1.0 μM ADP) as a percentage of the control response to 0.1 μM ADP. Asterisks indicate where responses are significantly desensitized relative to controls (P < .05, Mann-Whitney U test). (B-C) Data are mean ± SEM (n = 3). (D) P2Y1 receptor desensitization is mediated in part by PKC in human platelets. Platelets were pretreated with the PKC inhibitors GF109203X (2 μM), Ro317549 (1 μM), or Gö6976 (1 μM) or with the PKC activator PMA (100 nM; 15 minutes). Pretreatment of platelets with any of these agents induced no change in cytosolic calcium (data not shown). Platelets were treated with ADP (1 μM; 30 seconds) and then with 0.2 U/mL apyrase for another 180 seconds, and they were restimulated with 10 μM ADP. Receptor desensitization was assessed by comparing a percentage of desensitization of peak calcium responses to controls that had been treated identically except for the lack of pretreatment with desensitizing ADP. Values are mean ± SEM (n = 3), and results are expressed as the ADP response after a desensitizing dose as a percentage of the control response. Asterisks indicate statistical significance at P < .05 for data compared with ADP pretreatment without PKC activator or inhibitor (control; Mann-Whitney U test).
We subsequently examined the role of PKC in the desensitization of P2Y1 purinergic receptor–mediated calcium concentration increases in platelets (Figure 6D). Pretreatment of platelets with GF109203X (2 μM), Ro317549 (1 μM), or Gö6976 (1 μM) significantly, though not completely, attenuated ADP-induced P2Y1 purinergic receptor desensitization. Pretreatment of platelets with the PKC activator phorbol 12-myristate 13-acetate (PMA) (100 nM; 15 minutes) significantly enhanced desensitization, resulting in a substantial reduction in P2Y1-mediated calcium response. Therefore, PKC may regulate the desensitization of P2Y1 purinergic receptors in an expressed cell system and in platelets.
Mechanisms underlying desensitization of P2Y12-mediated responses: the role of GRKs and PKC
Expression of P2Y12 purinergic receptors in 1321N1 cells also produced a functionally active receptor. P2Y12 activation by ADP inhibited forskolin-stimulated adenylyl cyclase in a concentration-dependent manner (Figure 7A-B) and was blocked by pretreatment with the P2Y12 purinergic receptor antagonist AR-C69931MX (1 μM). As with platelets, we tested the ability of ADPβS to induce desensitization of P2Y12 purinergic receptor responses and found that the ongoing presence of desensitizing ADPβS significantly decreased subsequent forskolin-stimulated adenylyl cyclase activity. Therefore, pretreatment with ADPβS does not induce full receptor desensitization. Given that ADPβS pretreatment made interpretation of the data problematic, we subsequently used the ADP/apyrase protocol already outlined. Apyrase was shown not to affect basal, forskolin-activated, or ADP-induced inhibition of adenylyl cyclase stimulation (Figure 7A). Pretreatment with a maximal concentration of ADP (10 nM; 5 minutes), however, reduced subsequent agonist-induced P2Y12 receptor responses (Figure 7A-B). The time course of this homologous desensitization was examined by pretreating cells with ADP (10 nM; 0-15 minutes) and assessing the subsequent ability of agonist to inhibit forskolin-stimulated adenylyl cyclase. As with platelets, we found that P2Y12 underwent rapid homologous desensitization (Figure 7C).
Agonist-induced activation and desensitization of P2Y12 purinergic receptors in 1321N1 cells. (A) cAMP accumulation (10 minutes) in the absence (basal; □) or presence of 1 μM forskolin (forskolin; ▨) ± ADP (10 nM; forsk/ADP; ▪) was assessed under 5 different conditions: (1) in the absence of pretreatment (untreated), (2) after pretreatment with the P2Y12 receptor antagonist AR-C69931MX (1 μM; 5 minutes), (3) after pretreatment with the P2Y receptor agonist ADPβS (1 mM; 5 minutes), (4) after pretreatment with apyrase (0.2 U/mL; 180 seconds; + apyrase), and (5) after treatment with ADP (10 nM; 5 minutes) and subsequent treatment with apyrase (+ ADP/apyrase). Data represent mean ± SEM of 4 independent experiments and are expressed as pmol cAMP per well. (B) Concentration-dependent inhibition of forskolin (1 μM; 10 minutes)–stimulated adenylyl cyclase activity by P2Y12 purinergic receptor activation after pretreatment with ADP (10 nM; 5 minutes) (○) or vehicle alone (⬡). Values represent mean ± SEM of 4 independent experiments. Data are expressed as a percentage of reduction in maximal forskolin response. ADP pretreatment induced a significant (P < .05; 2-way analysis of variance [ANOVA]) reduction in P2Y12 purinergic receptor activity with a 30-fold shift in the dose-response curve (ADP-induced inhibition of forskolin-stimulated adenylyl cyclase had an EC50 of 2.5 ± 0.9 pM and 76.7 ± 4.2 pM before and after ADP pretreatment respectively). (C) Time dependence of desensitization of P2Y12 purinergic receptor activity. ADP pretreatment induced a rapid and significant reduction in P2Y12 purinergic receptor activity. Values represent mean ± SEM of 4 independent experiments. Data are expressed as a percentage of reduction in maximal forskolin response. (D) Cells stably transfected with P2Y12 purinergic receptor construct were transiently transfected with either pcDNA3-K220RGRK2 (GRK2-DNM), pcDNA3-K215RGRK6 (GRK6-DNM), vector alone (pcDNA3), or scrambled, GRK2-, or GRK6-specific siRNA constructs. Cells were incubated in the absence or presence of the PKC inhibitor GF109203X (GF; 2 μM) for 15 minutes before experimentation. Agonist (ADP; 10 nM)–dependent inhibition of forskolin (1 μM; 10 minutes)–stimulated adenylyl cyclase activity by P2Y12 purinergic receptor activation after pretreatment with ADP (10 nM; 5 minutes, ▪) or vehicle alone (□) was subsequently determined. Values represent the mean ± SEM of 4 independent experiments and are expressed as a percentage of inhibition of forskolin-stimulated adenylyl cyclase. *Statistical significance at P < .05 for data compared with respective nonpretreated agonist-induced inhibition of forskolin-stimulated controls (Mann-Whitney U test).
Agonist-induced activation and desensitization of P2Y12 purinergic receptors in 1321N1 cells. (A) cAMP accumulation (10 minutes) in the absence (basal; □) or presence of 1 μM forskolin (forskolin; ▨) ± ADP (10 nM; forsk/ADP; ▪) was assessed under 5 different conditions: (1) in the absence of pretreatment (untreated), (2) after pretreatment with the P2Y12 receptor antagonist AR-C69931MX (1 μM; 5 minutes), (3) after pretreatment with the P2Y receptor agonist ADPβS (1 mM; 5 minutes), (4) after pretreatment with apyrase (0.2 U/mL; 180 seconds; + apyrase), and (5) after treatment with ADP (10 nM; 5 minutes) and subsequent treatment with apyrase (+ ADP/apyrase). Data represent mean ± SEM of 4 independent experiments and are expressed as pmol cAMP per well. (B) Concentration-dependent inhibition of forskolin (1 μM; 10 minutes)–stimulated adenylyl cyclase activity by P2Y12 purinergic receptor activation after pretreatment with ADP (10 nM; 5 minutes) (○) or vehicle alone (⬡). Values represent mean ± SEM of 4 independent experiments. Data are expressed as a percentage of reduction in maximal forskolin response. ADP pretreatment induced a significant (P < .05; 2-way analysis of variance [ANOVA]) reduction in P2Y12 purinergic receptor activity with a 30-fold shift in the dose-response curve (ADP-induced inhibition of forskolin-stimulated adenylyl cyclase had an EC50 of 2.5 ± 0.9 pM and 76.7 ± 4.2 pM before and after ADP pretreatment respectively). (C) Time dependence of desensitization of P2Y12 purinergic receptor activity. ADP pretreatment induced a rapid and significant reduction in P2Y12 purinergic receptor activity. Values represent mean ± SEM of 4 independent experiments. Data are expressed as a percentage of reduction in maximal forskolin response. (D) Cells stably transfected with P2Y12 purinergic receptor construct were transiently transfected with either pcDNA3-K220RGRK2 (GRK2-DNM), pcDNA3-K215RGRK6 (GRK6-DNM), vector alone (pcDNA3), or scrambled, GRK2-, or GRK6-specific siRNA constructs. Cells were incubated in the absence or presence of the PKC inhibitor GF109203X (GF; 2 μM) for 15 minutes before experimentation. Agonist (ADP; 10 nM)–dependent inhibition of forskolin (1 μM; 10 minutes)–stimulated adenylyl cyclase activity by P2Y12 purinergic receptor activation after pretreatment with ADP (10 nM; 5 minutes, ▪) or vehicle alone (□) was subsequently determined. Values represent the mean ± SEM of 4 independent experiments and are expressed as a percentage of inhibition of forskolin-stimulated adenylyl cyclase. *Statistical significance at P < .05 for data compared with respective nonpretreated agonist-induced inhibition of forskolin-stimulated controls (Mann-Whitney U test).
We then studied the molecular mechanisms responsible for P2Y12 receptor desensitization. Again DNM-GRK2 and DNM-GRK6 were overexpressed, and expression of these proteins blocked P2Y12 receptor desensitization (Figure 7D), in contrast to data for P2Y1. Conversely, pretreatment with GF109203X did not significantly block desensitization, indicating that PKC does not play a role in the regulation of P2Y12 purinergic receptor responses. Reduced expression of either GRK2 or GRK6, by transfection with the relevant siRNA constructs, partially attenuated P2Y12 receptor desensitization. Interestingly, P2Y12 receptor desensitization was fully attenuated after reduction in the endogenous expression of both GRK isoforms. Hence, GRK2 and GRK6 seem to be the dominant mediators of P2Y12 purinergic receptor desensitization and may contribute equally to this desensitization.
Discussion
ADP is a central agonist regulating platelet activation during hemostasis and thrombosis, operating through the G protein–coupled receptors P2Y1 and P2Y12. Consequently, it is critical for normal platelet function that the sensitivity of these receptors to this agonist be continuously regulated to avoid inappropriate thrombosis or excessive bleeding. In this study, we demonstrate for the first time that P2Y1 and P2Y12 receptor responses undergo rapid homologous desensitization in human platelets. We show that the 2 receptors are differentially regulated: P2Y1 is regulated in a PKC-dependent, GRK-independent manner, whereas the reverse is true for P2Y12, which undergoes GRK-dependent but PKC-independent desensitization.
To date, studies have shown that platelets pretreated with ADP become unresponsive to subsequent ADP-induced stimulation.8-14 We also observed reduced ADP-induced aggregation after pretreatment with this agonist (Figure 1A-B). Moreover, ADP pretreatment reduced subsequent collagen-induced platelet aggregation (Figure 1B). It is well established that collagen induces the release of ADP from dense granules; therefore, it is consistent to find that prior desensitization of ADP receptors leads to reduced collagen responsiveness. Interestingly, ADP pretreatment did not reduce subsequent collagen-induced calcium mobilization (Figure 2C), probably because collagen-induced calcium release is an early signaling event and is less dependent on released ADP. Downstream events, such as aggregation, are partly dependent on secreted ADP and are, therefore, sensitive to purinergic receptor desensitization. Thrombin-induced platelet aggregation responses were also slightly reduced by ADP pretreatment, consistent with a role for ADP in thrombin-induced aggregation, possibly suggesting some crosstalk between PAR1/PAR4 and purinergic receptors in human platelets.
A recent study demonstrated that the P2Y1 purinergic receptor is desensitized in platelets, though the molecular basis of this phenomenon was not examined.9 In the same study, it was suggested that P2Y12 purinergic receptors remain largely refractory to receptor desensitization. A significant number of methodological differences are apparent between this study and our own. In our study we used ADP, a full agonist of P2Y12, to promote receptor desensitization, whereas Baurand et al9 used ADPβS as the desensitizing agonist. Studies have shown that ADPβS is less potent at P2Y12 than P2Y128 ; ADP has also recently been shown to be more efficacious at P2Y12 than ADPβS.29 ADPβS is reported to be a partial agonist at P2Y12,30 and partial agonists are often unable to promote full desensitization of receptor responses.31 In addition, the lack of breakdown of ADPβS allows its continued presence, inhibiting forskolin responsiveness and leading to a reduction in the window through which subsequent ADP-induced inhibition of adenylyl cyclase could be seen, possibly explaining the apparent lack of desensitization of P2Y12 observed by these authors. For these reasons, we decided to use the endogenous ligand ADP as our desensitizing agonist and developed a protocol to allow use of the agonist for this purpose.
In our investigation, desensitizing ADP was removed by the addition of apyrase before restimulation by agonist. At 0.2 U/mL, apyrase removes 10 μM ADP (the maximum concentration used to desensitize in this study) in 3 seconds, under ideal conditions.16 Therefore, under our experimental conditions, all desensitizing ADP was consumed before restimulation. Our control experiments validated this approach by showing that premixing of ADP (10 μM) with apyrase (0.2 U/mL) ablates P2Y1-dependent increases in cytosolic calcium (Figure 2A(vi)), clearly demonstrating that through this approach, all desensitizing ADP was removed before restimulation. Our experiments examining P2Y12 were confirmation and showed, in addition, that apyrase treatment effectively removed desensitizing ADP because forskolin stimulation in Figure 3B (hatched bars) was comparable throughout all pretreatment conditions. If desensitizing ADP had not been effectively removed by apyrase, subsequent forskolin stimulation would have been significantly attenuated, but this was not the case.
Apyrase itself did not affect the ADP-induced rapid calcium response or cAMP responses (Figures 2A, 3B). This might have been because the apyrase was unable to significantly degrade stimulating ADP within the short time the peak calcium response was generated. According to our measurements of cAMP accumulation, apyrase was inhibited by EGTA, added immediately before restimulation with ADP. Indeed, we confirmed that EGTA inhibits the breakdown of ADP by apyrase by HPLC measurements (data not shown).
AMP weakly binds to ADP receptors and is formed after the breakdown of ADP by apyrase. It was thought that this AMP would block ADP receptor activation in the subsequent restimulation step. We ruled out this possibility given that even in the presence of 10 μM AMP, a concentration 10 × higher than that achievable with full degradation of the 1 μM desensitizing dose of ADP, there was a negligible effect on ADP-induced cAMP accumulation and only a small reduction in ADP-induced calcium response when compared with pretreatment with 10 μM ADP. Finally, we confirmed that no cross-desensitization of the collagen-induced calcium response occurred by pretreatment of platelets with ADP. This suggested that the reduction in response caused by the pretreatment of platelets with ADP did not result from emptying of intracellular calcium stores.
We showed that the P2Y1 and P2Y12 purinergic receptors undergo desensitization in human platelets. Consequently, we sought to determine the molecular regulators of this process. Accordingly, in this investigation, we expressed each GPCR in a single cell type, 1321N1 human astrocytoma cells. This cell line does not express endogenous purinergic receptors, unlike CHO, COS-7, and HEK293 cells,24 thus allowing a clean background for analysis of results. Like platelets, these cells have the machinery required to support agonist-induced receptor desensitization, including GRKs and arrestins.32 Indeed 1321N1 cells have already been used for heterologous expression of P2Y receptors, including P2Y1.33 As such, 1321N1 cells provide an ideal environment for the study of purinergic receptor regulation. P2Y1 and P2Y12 receptors were expressed in 1321N1 cells at similar levels (Bmax, 14.1 ± 5.8 pmol/mg; protein, 13.1 ± 2.3 pmol/mg) that are higher than estimates for their endogenous expression in human platelets.34 The similarities in levels allows comparison between the 2 receptors in this system. Although care should be taken when interpreting and relating data between overexpressed and endogenous GPCR populations, it should be noted that the molecular regulation of other receptors of importance in the cardiovascular system, such as the β AR,35 thrombin,36 and platelet-activating receptors,37 has been determined by heterologous overexpression of epitope-tagged receptors in noncardiovascular cell types.
We initially confirmed that, as in platelets, P2Y1 and P2Y12 receptors became desensitized. P2Y1 receptor desensitization seemed to be less complete in 1321N1 cells than in platelets. For example, peak calcium responses in 1321N1 cells were subject to limited desensitization, though a clear rightward shift in EC50 for ADP was evident after acute desensitization. A decrease in agonist potency without a reduction in maximal response is a common phenomenon when desensitization is examined in cell systems heterologously overexpressing GPCRs.38 Indeed, even in platelets, the P2Y1 receptor population does not completely desensitize. This may be a consequence of desensitized receptors recycling back to the cell surface after agonist-induced internalization and resensitization. A number of studies have examined desensitization of P2Y1 purinergic receptors in a variety of settings, including neuronal and endothelial cells.39,40 In agreement with our investigation, they also concluded that this receptor undergoes rapid desensitization, consistent with regulation by kinases.
Two families of protein kinases are predominantly involved in the regulation of GPCRs: GRKs, which phosphorylate agonist-occupied GPCRs to mediate homologous receptor desensitization,41 and second messenger-activated kinases, such as PKC, which phosphorylate ligand-bound and inactive GPCRs in a heterologous manner.42,43 In this study we decided to overexpress catalytically dead dominant-negative GRK constructs to block endogenous GRK activity41 and to reduce endogenous GRK expression by the use of specific siRNAs.20 It should be noted that although GRK2 and GRK6 have similar basic structures, each belongs to 2 distinct subfamilies of GRKs because they have distinct structural elements that differentially affect their regulation and membrane association.44 Expression of these DNM constructs or reduced GRK expression did not attenuate P2Y1 purinergic receptor desensitization; however, P2Y12 receptor desensitization was inhibited, suggesting a selective role for GRKs in the regulation of P2Y12. Studies examining endogenous β2 adrenoceptor function in P2Y1-expressing 1321N1 cells confirmed that both constructs were operating as true dominant negatives in this cell system. Because both GRKs are expressed in platelets (Figure 5), it is plausible that these kinases may regulate P2Y12 function in platelets. The P2Y12 receptor has a number of putative phosphorylation sites in its C-terminus, and studies are ongoing to investigate which regions of this GPCR may be regulated by GRKs. Interestingly, the overexpression of DNM-GRK2 reduced P2Y1 purinergic receptor–induced calcium mobilization, likely because of the binding of Gq to the RGS homology (RH) domain of GRK2,45 which thereby attenuated PLC-β activity. Interestingly as well, DNM-GRK2 or DNM-GRK6 almost completely ablated the desensitization of P2Y12 responses, whereas desensitization of this receptor was abolished only by combined knockdown of GRK2 and GRK6 using the siRNA approach. This suggests that GRK2 and GRK6 contribute equally to the desensitization of P2Y12 and that the DNM of each GRK may cause the inhibition of both.
Because the activation of P2Y1 receptors activates PKC, we decided to examine whether this kinase family regulates this GPCR. A number of Gq-coupled GPCRs are regulated by PKC phosphorylation of PLC or receptor, leading to subsequent decreases in receptor activity.42,46-48 Pretreatment with 3 structurally unrelated PKC inhibitors (GF109203X, Ro317549, Gö6976) increased ADP-induced Ca2+ mobilization, as has been seen for a number of Gq-coupled receptors. Moreover, the desensitization of P2Y1 receptors was attenuated by the blockade of PKC activity, indicating that PKC is likely to regulate this receptor. Further studies in platelets confirmed our findings in 1321N1 cells, with GF109203X, Ro317549, or Gö6976 attenuating agonist-induced desensitization of P2Y1 and pretreatment with the PKC activator PMA substantially inhibiting subsequent ADP-induced calcium responses. Therefore, it seems likely that PKC plays a role in the regulation of P2Y1 in platelets. A recent study examining the regulation of P2Y1 receptors in neuronal cells also identified the importance of PKC in the regulation of this GPCR.39 A specific residue, Thr339, in the C-terminus of this receptor was phosphorylated by PKC and thereby promoted P2Y1 desensitization. Studies in this laboratory, therefore, are under way to elucidate the role of Thr339 and its phosphorylation in P2Y1 receptor regulation in platelets. We also plan to ascertain whether this mechanism is of importance in heterologous regulation of P2Y1 by other receptors given that PKC is known to play a role in this type of receptor regulation.
In conclusion, given that a delicate balance must be maintained between rest and activation of platelets in the circulation and because ADP performs a pivotal role in the formation of stable platelet aggregates, ADP receptor desensitization may represent a key mechanism by which this balance is maintained. Our study demonstrates for the first time that the platelet-expressed P2Y1 and P2Y12 purinergic receptors undergo rapid agonist-induced desensitization by different molecular mechanisms involving PKC and GRK, respectively. Regulation of receptor activities in this way may allow platelets to fine-tune their responses to ADP by differential regulation of each of the 2 receptors for this agonist.
Prepublished online as Blood First Edition Paper, January 21, 2005; DOI 10.1182/blood-2004-07-2893.
Supported by an A.J. Clark Studentship from the British Pharmacological Society (A.R.H.), by project grants from the Wellcome Trust (nos. 064785 and 069572) (A.W.P.), and by a grant from the British Heart Foundation (no. PG/2000087). S.J.M. is a British Heart Foundation Research Fellow (grant no. FS/03/002/15102).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Suzanne Delaney (Portola Pharmaceuticals) for valuable discussion of results in the preparation of this manuscript.

![Figure 4. Agonist-induced desensitization of calcium responses and inositol phosphate accumulation in cells stably expressing P2Y1 purinergic receptors. (A) IP accumulation was measured after the addition of ADP (1 nM-10 μM; 10 minutes) in cells stably expressing the P2Y1 purinergic receptor (⬡) or vector (pcNEO) alone (○). Data are the mean ± SEM of 5 independent experiments, and results are expressed as fold IP accumulation over basal. ADP stimulated IP accumulation with an EC50 of 14 ± 0.31 nM. (B) Cells either were not pretreated with ADP (control; ⬡) or were pretreated with ADP (1 μM; 5 [▿], 15 [○], or 30 minutes [▵]) and were incubated with apyrase (0.2 U/mL; 1 minute). Cells were then washed, and IP accumulation was measured after the addition of ADP (1 μM; 0-30 minutes). Data are mean ± SEM of 4 independent experiments, and results are expressed as fold IP accumulation over basal. (C) Cells stably transfected with P2Y1 purinergic receptor or vector alone (pcNEO; ▴) were either not pretreated (Control; ⬡) or were pretreated with agonist (ADP; 0.1 μM[▿], 1 μM[○], or 10 μM[▵] as indicated; 2 minutes). Peak calcium responses were measured on the addition of ADP (1 nM-100 μM). Values represent the mean ± SEM of 4 independent experiments. Results are expressed as the difference between basal resting and maximal response (peak rise) in cytosolic calcium concentration ([Ca2+]i nM), as assessed by Fura-2AM in intact cells. Prestimulation with ADP (0.1-1 μM) caused a shift in the dose-response curve for agonist-induced calcium mobilization (EC50 of 59.7 ± 15.3, 100.3 ± 11.3, 300 ± 4.9, and 384 ± 18.7 nM for ADP-induced calcium mobilization before and after 0.1, 1, and 10 μM ADP pretreatment, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-07-2893/6/m_zh80090577700004.jpeg?Expires=1764960727&Signature=tYtdXaDuOA2lmF9trbiRiNMEikK1KEqutReD8ZpTyLpfeVeetPNYNz2Lo5-LmvMFTM0rdt5lVQ7qUvcexe49L-IhsVM-NcMVjPLKhg34Jkzn7R6qabp9TEC2XjhqCp-6WO-I7z-EgdgQQzuQICQEbWHWBNs8HlbO8tlnSHktaKTrpX6AKw-MdgVUisuWg66TCGCpSSDAKZ8~mUYZy8C2O3oDK8086KtKVcqn3nhVBXiy-2cMFIDnTeYNlD7uaaV0~t7WnsJ0-EtoFJGCb75ssFjNA12v1X8xV0HZnzF9e6fXH7w68jqsUU3FwrjaQDwDpjumDs42zrVaa4imIPJ6Xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Agonist-induced activation and desensitization of P2Y12 purinergic receptors in 1321N1 cells. (A) cAMP accumulation (10 minutes) in the absence (basal; □) or presence of 1 μM forskolin (forskolin; ▨) ± ADP (10 nM; forsk/ADP; ▪) was assessed under 5 different conditions: (1) in the absence of pretreatment (untreated), (2) after pretreatment with the P2Y12 receptor antagonist AR-C69931MX (1 μM; 5 minutes), (3) after pretreatment with the P2Y receptor agonist ADPβS (1 mM; 5 minutes), (4) after pretreatment with apyrase (0.2 U/mL; 180 seconds; + apyrase), and (5) after treatment with ADP (10 nM; 5 minutes) and subsequent treatment with apyrase (+ ADP/apyrase). Data represent mean ± SEM of 4 independent experiments and are expressed as pmol cAMP per well. (B) Concentration-dependent inhibition of forskolin (1 μM; 10 minutes)–stimulated adenylyl cyclase activity by P2Y12 purinergic receptor activation after pretreatment with ADP (10 nM; 5 minutes) (○) or vehicle alone (⬡). Values represent mean ± SEM of 4 independent experiments. Data are expressed as a percentage of reduction in maximal forskolin response. ADP pretreatment induced a significant (P < .05; 2-way analysis of variance [ANOVA]) reduction in P2Y12 purinergic receptor activity with a 30-fold shift in the dose-response curve (ADP-induced inhibition of forskolin-stimulated adenylyl cyclase had an EC50 of 2.5 ± 0.9 pM and 76.7 ± 4.2 pM before and after ADP pretreatment respectively). (C) Time dependence of desensitization of P2Y12 purinergic receptor activity. ADP pretreatment induced a rapid and significant reduction in P2Y12 purinergic receptor activity. Values represent mean ± SEM of 4 independent experiments. Data are expressed as a percentage of reduction in maximal forskolin response. (D) Cells stably transfected with P2Y12 purinergic receptor construct were transiently transfected with either pcDNA3-K220RGRK2 (GRK2-DNM), pcDNA3-K215RGRK6 (GRK6-DNM), vector alone (pcDNA3), or scrambled, GRK2-, or GRK6-specific siRNA constructs. Cells were incubated in the absence or presence of the PKC inhibitor GF109203X (GF; 2 μM) for 15 minutes before experimentation. Agonist (ADP; 10 nM)–dependent inhibition of forskolin (1 μM; 10 minutes)–stimulated adenylyl cyclase activity by P2Y12 purinergic receptor activation after pretreatment with ADP (10 nM; 5 minutes, ▪) or vehicle alone (□) was subsequently determined. Values represent the mean ± SEM of 4 independent experiments and are expressed as a percentage of inhibition of forskolin-stimulated adenylyl cyclase. *Statistical significance at P < .05 for data compared with respective nonpretreated agonist-induced inhibition of forskolin-stimulated controls (Mann-Whitney U test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-07-2893/6/m_zh80090577700007.jpeg?Expires=1764960727&Signature=Pe7Ib1~UY8CpIGbenRCK~rdlFUpk9J9ssWuqLDMxxlvVsFbJqe2SJv8zx7tONSldLu8OSTZR0U5gPlavMDZc8eU1OowW9jk2Y0ezdoGQb2fyA2NAU8SGiZKo7OXU0ocSRXEJzP1m9x6MPLBRYuQhdh01VG9L0qmYQXaZ2lQ-L9RWLtZO6v2Mm2JL6LWZxhJNqKDdSO--~o9e-Tqg0R6iqgPq~mPobT~a-hWZRdrz73cDeXFFJjk1GozvA4sl4MNrftZCAjX3Me8FWGlBuLr8W9XAcg6d9QF0Ros35wWImqZ78Zd8smwQK2mdN6wEy8p918qbv0k4Oloxxd8qnuA0LQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
