Abstract
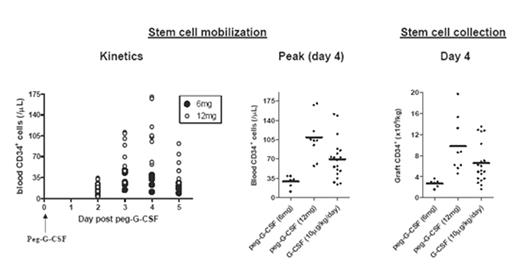
The mobilization of stem cells with pegylated-G-CSF (peg-G-CSF) modulates regulatory T cell and NKT cell function, separating graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) effects in animal models. We have initiated a phase I/II study to analyse the feasibility of mobilizing stem cells from sibling donors with peg-G-CSF and their ability to restore hematopoiesis in HLA matched transplant recipients who have received myeloablative conditioning. Results were compared to a cohort of donors mobilized with standard G-CSF at 10ug/kg/day (n=19). The administration of 6mg of peg-G-CSF (n=6) resulted in suboptimal stem cell mobilization with a peak peripheral blood CD34+ count of 29 ± 4/uL. Apheresis 4 days after peg-G-CSF administration yielded 2.7 ± 0.3 x106 CD34+ cells/kg recipient ideal body weight and all patients required a second collection on day 5 to yield a total of 4.0 ± 0.5 x106 CD34+ cells/kg recipient weight. Following escalation of the dose to 12mg (n=9), the peak CD34+ count was 109 ± 13/uL and all donors collected sufficient stem cells for transplantation in a single apheresis (9.8 ± 1.7 x106 CD34+ cells/kg recipient weight). The 6mg dose of peg-G-CSF was significantly inferior to standard G-CSF for stem cell mobilization (P<0.01) while the 12mg dose was at least equivalent (P=0.07).
Bone pain was similar between the 6mg and 12mg cohorts and to that seen with standard G-CSF. However, in addition to the expected rises in serum ALP and LDH, transient rises in hepatic transaminases were noted 5 to 12 days after peg-G-CSF administration in 7 of 9 donors receiving the 12mg dose. One donor developed NCI grade 3 hepatic toxicity and splenomegaly. After allogeneic transplantation of peg-G-CSF mobilized grafts (Cy/TBI conditioning in 13 of 14 recipients), median neutrophil and platelet engraftment occurred on days 18 and 14 respectively and was identical to that seen with grafts mobilized by standard G-CSF. With a median follow up of 165 days (range 55–532), the incidence of grade II-IV and grade III/IV acute GVHD is 50% and 21% respectively. No patients have relapsed to date and overall survival is 86%. The mobilization of stem cells with peg-G-CSF in normal donors is feasible and 12mg appears the optimal dose. Further data are required to more closely analyse the effect of peg-G-CSF on donor liver function and the ability of stem cell grafts to separate GVHD and GVL effects.
Author notes
Corresponding author