Abstract
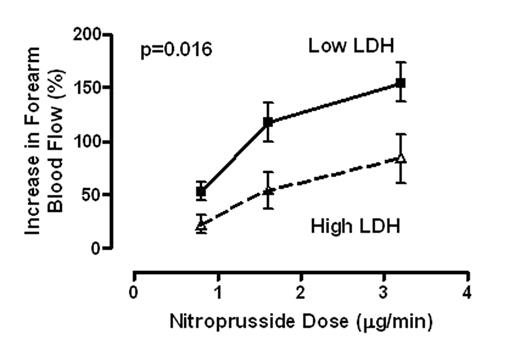
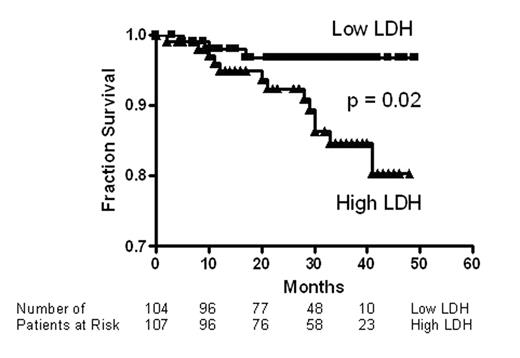
Pulmonary hypertension is prevalent in adult patients with sickle cell disease, and is strongly associated with markers of hemolysis, in particular serum lactate dehydrogenase (LDH), and early mortality. Intravascular hemolysis leads to a state of resistance to nitric oxide (NO), mediated by NO scavenging by plasma oxyhemoglobin and by arginine degradation by plasma arginase. We hypothesized that serum LDH may represent a convenient biomarker of intravascular hemolysis and NO bioavailability, characterizing a clinical subphenotype of hemolysis-associated vasculopathy. In a cohort of 213 patients with sickle cell disease, we found statistically significant associations of LDH with low levels of hemoglobin and haptoglobin and high levels of reticulocytes, bilirubin, plasma hemoglobin, aspartate aminotransferase, arginase and soluble adhesion molecules. LDH isoenzyme fractionation confirmed predominance of LD1 and LD2, the principal isoforms within erythrocytes. In a subgroup, LDH levels closely correlated with plasma cell free hemoglobin, accelerated nitric oxide consumption by plasma and impaired vasodilatory responses to an NO donor. Remarkably, this simple biomarker was statistically associated with a clinical subphenotype of pulmonary hypertension, leg ulceration, priapism, and risk of death in patients with sickle cell disease. We propose that LDH elevation identifies patients with a syndrome of hemolysis-associated NO-resistance, endothelial dysfunction and end-organ vasculopathy.
Author notes
Corresponding author