Abstract
The role of all-trans retinoic acid (ATRA) in pediatric acute promyelocytic leukemia (APL) is the topic of several ongoing studies. The results of the Italian pediatric experience with the multicentric Gruppo Italiano per le Malattie Ematologiche dell'Adulto (GIMEMA)–Italian Pediatric Hematology and Oncology Group (AIEOP) “AIDA” (ATRA and idarubicin) trial are presented. Of the 983 patients with APL enrolled in this protocol between January 1993 and June 2000, 124 (13%) had younger than 18 years. Treatment consisted of ATRA and idarubicin induction followed by 3 polychemotherapy consolidation courses. Molecular response by reverse transcriptase–polymerase chain reaction (RT-PCR) was assessed after consolidation and patients who were PCR– were randomized for different maintenances. One hundred and seven children were eligible and evaluable for induction: 103 (96%) achieved a hematologically complete remission. Overt ATRA syndrome was observed in 2 patients and pseudotumor cerebri was observed in 10 patients. Ninety-four patients were evaluable for RT-PCR analysis at the end of consolidation: 91 (97%) proved PCR+ and 3 PCR–. The overall survival and event-free survival (EFS) are 89% (95% confidence interval [c.i.]: 83%-95%) and 76% (c.i.: 65%-85%), respectively, at more than 10 years. A white blood cell (WBC) count at diagnosis of greater than 10 × 109/L had a significant impact on EFS (59% vs 83% at 10 years). These results highlight the efficacy and feasibility of the AIDA protocol in the pediatric APL population.
Introduction
Since the successful introduction of all-trans retinoic acid (ATRA) in the treatment of acute promyelocytic leukemia (APL), several clinical trials have been performed with various combinations of ATRA and chemotherapy in an attempt to obtain more durable remissions and reduced ATRA-related toxicity. As demonstrated by the European APL 91 Group randomized trial, these combinations clearly improve the disease-free survival (DFS) of APL patients compared with the results achieved with chemotherapy alone.1 Moreover, the results of the Italian multicenter GIMEMA (Gruppo Italiano per le Malattie Ematologiche dell'Adulto) studies in adult APL initiated in 1983 have demonstrated that anthracyclines alone are equally effective in inducing complete remissions (CRs) as polychemotherapy combinations; in particular, induction monochemotherapy with a single course of idarubicin (Ida) was associated with a high CR rate in newly diagnosed APL.2 For this reason, in 1993 a protocol that combined ATRA and Ida (AIDA protocol) was designed by the GIMEMA, first as a pilot study and, thereafter, in association with the Italian Pediatric Hematology and Oncology Group (AIEOP), as a large multicentric study for the treatment of APL in both adults and children.
We hereby report the long-term results obtained in the pediatric population (aged younger than 18 years) enrolled in this trial.
Patients, materials, and methods
Eligibility criteria
Criteria for inclusion were as follows: (1) Age older than 12 months. (2) Confirmed genetic diagnosis based on the presence of the promyelocytic leukemia retinoic acid receptor (PML-RAR) alpha transcript or the t(15;17) translocation. Molecular evidence of the fusion gene was generally required; however, patients with cytogenetically proven APL and failure of the polymerase chain reaction (PCR) test were also considered eligible. (3) No cardiac contraindications to anthracycline chemotherapy. (4) Serum creatinine level less than 3 times the normal upper limit. (5) Serum alkaline phosphatase level less than 3 times the normal upper limit. (6) Serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) levels less than 3 times the upper normal limit. (7) WHO performance status less than 4. (8) Written informed consent by parents or legal guardian.
Protocol design
Induction therapy consisted of oral ATRA 25 mg/m2/d, divided into 2 doses administered every 12 hours, up to hematological remission and for a maximum of 90 days, and 4 brief intravenous infusions of Ida 12 mg/m2 on days 2, 4, 6, and 8. Patients in hematological complete remission (HCR) received 3 monthly consolidation courses consisting of cytosine arabinoside (ARA-C) 1 000 mg/m2/d by intravenous infusion over 6 hours on days 1, 2, 3, and 4 and Ida 5 mg/m2/d by brief intravenous infusion on days 1, 2, 3, and 4, 3 hours after the end of the ARA-C infusion (course 1); mitoxantrone (MTZ) 10 mg/m2/d by brief intravenous infusion on days 1, 2, 3, 4, and 5 and etoposide (VP-16) 100 mg/m2/d by intravenous infusion lasting 45-60 minutes on days 1, 2, 3, 4, and 5 (12 hours after the start of MTZ) (course 2); Ida 12 mg/m2 intravenous infusion on day 1, ARA-C 150 mg/m2 every 8 hours given subcutaneously on days 1, 2, 3, 4, and 5, and 6-thioguanine (6-TG) 70 mg/m2 every 8 hours on days 1, 2, 3, 4, and 5 (course 3). Each consolidation course was administered at recovery from the previous cycle, when polymorphonuclear neutrophil (PMN) counts were greater than 1.5 × 109/L and platelet (PLT) levels were greater than 100 × 109/L. At recovery from the third consolidation course, patients who tested PCR– for the PML-RAR alpha hybrid gene were randomized to 4 maintenance arms: (1) oral 6-mercaptopurine (6-MP) 90 mg/m2/d and weekly intramuscular methotrexate (MTX) 15 mg/m2; (2) ATRA 45 mg/m2/d for 15 days, every 3 months; (3) arm 1 for 3 months, followed by arm 2 for 15 days; and (4) no therapy. Each maintenance arm had to be repeated for a total of 2 years. After April 1997, arms 1 and 4 were closed, and patients were randomized into maintenance arms 2 and 3, which included ATRA. Patients who proved PCR+ at the end of consolidation, if eligible, were given either allogeneic hematopoietic stem-cell transplantation (SCT) if a fully matched sibling was available or autologous transplantation (Figure 1). As of April 1997, a new amendment was made to the protocol: patients in HCR who converted to positive PCR, confirmed in 2 consecutive bone marrow samples, were considered eligible for salvage treatment and taken off study. In addition, the use of autologous SCT was discouraged for patients who remained PCR+.
Due to the severe bleeding diathesis frequently associated with the disease, lumbar punctures (LPs) were not performed at diagnosis; central nervous system (CNS) prophylaxis with intrathecal chemotherapy was not given to patients included in this study.
Laboratory studies
Bone marrow samples were collected and morphologically evaluated at diagnosis, after induction, before each consolidation course, at recovery from the third consolidation cycle, every 3 months during the first year of maintenance, and every 4 months from the second to the fifth year from the end of consolidation. Bone marrow samples were also processed for PML-RAR alpha rearrangement by reverse transcriptase (RT)–PCR at diagnosis, at recovery from the end of consolidation, and at each morphologic evaluation during maintenance, and at 5 years after the completion of therapy. In cases of doubtful or positive PCR, during HCR, a further bone marrow sample was required within 2 to 4 weeks to confirm the result. RT-PCR analyses were performed in 2 reference molecular biology laboratories (Hematology, “La Sapienza” of Rome and Clinica Pediatrica, University of Milan, Monza). Confirmation of a positive test at the end of consolidation or during follow-up was carried out in the same laboratory that performed the previous analysis. The RT-PCR technique for the identification of the PML-RAR alpha fusion transcript has been reported elsewhere.3 The sensitivity of the RT-PCR assay was determined by amplifying serially diluted RNA mixtures of a diagnostic sample with 100% of blasts and the t(15;17)-negative myeloid cell line GF-D8. The PML-RAR alpha transcript was still detectable at a final dilution of 10–3/10–4. Such a detection level was repeatedly obtained in several subsequent experiments.
GIMEMA-AIEOP protocol: treatment schedule. CHT indicates chemotherapy; NO, no further treatment.
GIMEMA-AIEOP protocol: treatment schedule. CHT indicates chemotherapy; NO, no further treatment.
Immunophenotypic and cytogenetic analyses were systematically performed at diagnosis and in case of relapse.
Definition and study endpoints
HCR and hematologic relapse were defined according to the National Cancer Institute (NCI) criteria.3 Molecular remission and relapse were defined as the disappearance and reappearance of RT-PCR positivity for the PML-RAR alpha fusion transcript.4
ATRA syndrome was defined as “definitely present” in the presence of the following 5 signs and symptoms: fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates on chest radiograph, and weight gain. We defined as “indeterminate” ATRA syndrome a combination of 2 to 4 of the just mentioned signs and symptoms with or without edema and/or hypotension of the lower extremities.5
Supportive therapy, treatment of the ATRA syndrome, and prevention and control of coagulopathy were implemented as reported elsewhere.6 During the hypoplastic period after induction chemotherapy, all patients received oral antifungal and antibiotic prophylaxis until PMNs were greater than 0.5 × 109/L. All febrile episodes were treated with a cephalosporin and an aminoglycoside. At the earliest manifestation of symptoms associated with the ATRA syndrome, ATRA treatment was promptly discontinued and was replaced by the use of intravenous dexamethasone at a dose of 10 mg twice a day for a minimum of 3 days with or without furosemide. ATRA treatment was resumed following disappearance of the symptoms associated with the ATRA syndrome. Supportive PLT transfusions were administered in the presence of overt hemorrhages or if the PLT count was less than 20.0 × 109/L with or without laboratory signs of severe coagulopathy (fibrinogen less than 4.4 μM [150 mg/dL] and fibrinogen degradation products [FDPs] greater than 40 pg/mL or D-dimer [XDP] greater than 400 pg/mL). Prophylactic heparin was not recommended. Packed red blood cell units were transfused to maintain hemoglobin levels greater than 80 g/L (8 g/dL).
The overall and event-free survival (OS and EFS) durations were calculated from the date of diagnosis; hematologic disease-free survival (DFS) was calculated from the day of HCR achievement. Death at any time and hematologic relapse were considered events for the EFS curve, while death in HCR and hematologic relapse were considered for hematologic DFS. Molecular relapses were censored for the EFS and for the hematologic DFS curves. Since all patients randomized were PCR–, the comparison between the different randomization arms has been performed using a “molecular” DFS where, beyond death in CR, both molecular and hematologic relapses, the first which occurred, were considered events.
Statistical methods
Survival, EFS, and DFS were calculated according to the Kaplan-Meier method. The log-rank test was adopted for group comparisons.
Results
Accrual and patient characteristics
Between January 1993 and June 2000, 124 consecutive patients aged younger than 18 years from 42 Italian pediatric centers were registered based on a morphocytochemical diagnosis of acute myeloid leukemia (AML)–M3 according to the French-American-British (FAB) classification. Nearly all (110) of them were eligible for treatment (10 patients had no available molecular and cytogenetic data; 2 PML-RAR alpha–negative patients, 1 with hystiocytosis, and 1 with a performance status of 4 were not eligible for treatment). The main clinical and biological characteristics of the 110 patients are shown in Table 1. Median age was 11.6 years (range, 1.4-17.9 years) with only 1 child younger than 2 years. The WBC count, at diagnosis, was below or equal to 10 × 109/L in 72 patients and greater than 10 × 109/L in the other 38. Eighty-seven children presented with a PLT count below or equal to 40 × 109/L and 20 children presented with PLTs greater than 40 × 109/L (in 3 children the PLT count at diagnosis was not available). Ninety-eight children were classified as hypergranular M3, while 12 showed the microgranular variant form (M3v) on morphologic examination. All patients were genetically diagnosed by the demonstration of the specific translocation (86 patients), the PML-RAR alpha hybrid gene (104 patients) or both (80 patients). The type of PML-RAR alpha transcript, as detected by RT-PCR, was bcr1 in 55 patients, bcr2 in 5 patients, bcr3 in 36 patients, and not available in the remaining 14 patients.
Induction therapy and toxicity
Of the 110 eligible patients, 107 were evaluable for induction response. Three patients were excluded because of major protocol violation consisting of use of ARA-C during induction. A total of 110 patients (96%) achieved an HCR, and 4 died during induction. Of the 4 early deaths, 3 were due to intracerebral hemorrhage occurring at days 1, 9, and 16, respectively, from the beginning of treatment, and 1 occurred on day 34 due to severe infection. All 4 children presented with a WBC count greater than 10 × 109/L at diagnosis (Table 2). All 3 patients who died of hemorrhage also had laboratory signs of severe coagulopathy at presentation. No child failed to respond to induction therapy. ATRA was administered for a median of 32 days (range, 1-56 days) during induction. A total of 7 patients received ATRA for less than 15 days. These included the 3 patients who died early of hemorrhage, 3 patients with the ATRA syndrome, and 1 who developed pseudotumor cerebri. The other 4 patients were alive and in first HCR with a follow-up ranging from 53 to 111 months.
PML-RAR alpha re-evaluation at the end of induction or before consolidation was available in 69 cases; 32 patients (46%) tested PCR+ and 37 (54%) PCR–.
Univariate analysis demonstrated no significant relationship between different clinicobiological parameters (PLT count 40 × 109/L or less, or greater than 40 × 109/L; FAB subtype M3 or M3v) and response to induction treatment (98% vs 89%, P = .1; and 97% vs 91%, P = .3, respectively). However, a WBC count less than or equal to and more than 10 × 109/L at presentation influenced the HCR rate significantly (100% vs 89%; P = .01).
Induction toxicity is listed in Table 3. Of the 107 children evaluable for induction therapy, 29 experienced at least 1 toxicity. Two children (2%) developed a “definitely present” ATRA syndrome that was not fatal. The ATRA syndrome occurred on days 4 and 11 from start of treatment, respectively, requiring in both children therapy discontinuation and the prompt administration of dexamethasone. ATRA therapy was resumed only in the first patient, without further toxicity. Both patients achieved HCR. In 6 additional cases, an “indeterminate” ATRA syndrome was observed. Other ATRA-related adverse reactions included pseudotumor cerebri (10 cases), headache (14 cases), severe bone pain (5 cases), mucosal and skin dryness (6 cases), hypercholesterolemia (10 cases), and cheilitis (15 cases).
With regard to the other therapy-related toxicities, these were represented by infections in 27 patients (35 episodes), including 7 cases of sepsis (fatal in 1), 3 cases of bacterial pneumonias, and 3 cases of enteritis; stomatitis (11 cases); hemorrhages (7 cases, fatal in 3); hepatic (3 cases), and renal (1 case) and cardiac (2 cases) toxicities. Cardiac toxicity consisted in both cases of transient sinus tachycardia (pulse rate higher than 160 beats/min) occurring during septic fever and requiring treatment.
Consolidation, maintenance, and toxicity
Of the 103 children who entered HCR, 2 received other consolidation therapies due to local medical decisions; both children were alive and leukemia-free at 73 and 103 months from HCR, respectively. A total of 101 children proceeded to consolidation treatment as scheduled; 6 of them did not complete the 3 chemotherapy courses because of therapy-related toxicity (4 severe infections, 1 severe gastrointestinal toxicity, and 1 intracranial hemorrhage after the first and second courses). No toxic death was recorded among these 6 patients and all of them were currently alive, therapy-free, and in continuous molecular and HCR between 46 and 109 months (median, 79 months) from HCR. The other 95 children completed consolidation therapy and 94 were tested by RT-PCR at recovery from the third consolidation course (1 child, not tested by RT-PCR, was given an allograft by the decision of the medical staff and was alive and well at 97 months). Three of these tested PCR+; the other 91 patients (97%) were found to be PCR–. Two PCR+ patients received transplants (1 autologous, 1 allogeneic); both were alive and in continuous molecular remission at 57 and 111 months from SCT, respectively. The other PCR+ patient refused a bone marrow grafting procedure and died from disease progression 20 months later. Of the 91 PCR– patients, 3 refused maintenance randomization, 2 received other forms of maintenance, and 1 discontinued chemotherapy by the decision of the medical staff. Five of these latter 6 children were alive and well after a median of 64 months (range, 57-121 months); 1 developed a hematologic relapse after 40 months, was retreated and was alive and leukemia-free 26 months later. The other 85 PCR– children were randomized for the maintenance arm.
Twenty-one of the 85 randomized patients relapsed during follow-up. Prior to April 1997, 14 children underwent hematologic relapse at a median time of 26 months (range, 6 to 72 months); conversion to PCR positivity had been documented in the 9 children between 2 and 5 months prior to hematologic relapse for whom molecular data were available. These 14 patients were treated with a combination of MTZ and high-dose ARA-C, associated to ATRA in 10 of them. Thirteen children achieved a second HCR and 1 child died during reinduction therapy. Following reinduction, 11 patients were consolidated with SCT, 1 received the anti-CD33 monoclonal antibody/calicheamicin conjugate gemtuzumab-ozogamicin (Mylotarg, Wyeth Pharmaceuticals, Aprilia, Italy) and 1 underwent no further treatment. Nine responders were alive and in second molecular CR at 9 to 99 months from SCT (5 autologous; 3 allogeneic; 1 Mylotarg); 1 patient died in second CR due to allogeneic transplantation-related toxicity and the other 3 patients (2 who had received an autologous SCT and 1 only reinduction) relapsed after a median of 7 months (range, 6-25 months); 1 of these 3 children received an allogeneic SCT in third HCR and was alive in fourth HCR, while the other 2 died of disease progression.
OS and EFS probability for the whole cohort of patients. C.I. 95% indicates confidence interval 95%.
OS and EFS probability for the whole cohort of patients. C.I. 95% indicates confidence interval 95%.
As of April 1997, conversion to RT-PCR positivity for the PML-RAR alpha fusion transcript in 2 consecutive marrow samples collected 2 to 4 weeks apart was considered a molecular relapse. Following activation of this amendment, 5 children developed molecular relapse at a median time of 31 months (range, 10-66 months). One child received an allogeneic SCT from an identical sibling and relapsed 16 months later. The other 4 children were treated with chemotherapy and ATRA followed by an allogeneic SCT, and they all remained alive and in second molecular CR at 23 to 55 months. Extrahematologic relapse, occurring in the middle ear, was observed in 2 children at 57 and 70 months from HCR. Both received reinduction chemotherapy and ATRA, and were alive in HCR at 24 and 29 months, respectively.
Considering both hematologic and molecular relapses, a total of 15 patients received a SCT in second remission after reinduction. Eight children underwent an allogeneic SCT; 7 of them were alive and in second HCR at the time of the statistical analysis, while 1 died of transplantation-related toxicity. The remaining 7 children were submitted to an autologous transplantation: 2 presented a hematologic relapse after 7 and 25 months, respectively, while 5 were alive in second HCR at the time of the statistical analysis.
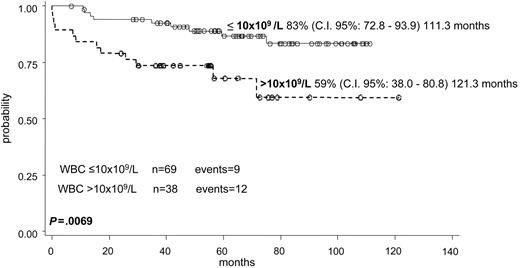
The OS and EFS for the whole series of 107 children were 89% (95% c.i.: 83.0%-95.3%) and 76% (95% c.i.: 65%-85%) at 121 months, respectively (Figure 2). Including all 110 patients in the survival curve, the OS probability at 10 years remains the same (89% c.i.: 83.4%-95.4%). Univariate analysis showed that only the presenting WBC count significantly affected EFS: EFS was 83% for patients with a WBC count 10 × 109/L or less at diagnosis, compared with 59% for those with a WBC count higher than 10 × 109/L (P = .069) (Figure 3). A PLT count at diagnosis of 40 × 109/L or less, or more than 40 × 109/L, did not influence significantly the EFS (73% and 84%, respectively; P = .56).
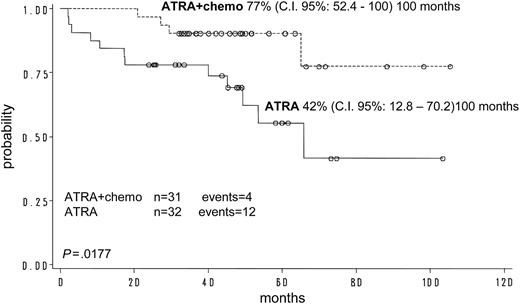
For the 103 children who achieved an HCR, the hematologic DFS was 78% (c.i.: 68%-88%) at 120 months. The “molecular” DFS for children randomized to receive ATRA plus chemotherapy for maintenance was significantly better compared with that of children who received ATRA alone (77% vs 42%, respectively; P = .0177) (Figure 4). Due to the fact that the other 2 maintenance arms were closed early, this comparison was not feasible in the 4 groups because of the low numbers.
“Molecular” DFS probability from randomization according to the maintenance arm assigned: ATRA vs ATRA + chemotherapy (chemo).
“Molecular” DFS probability from randomization according to the maintenance arm assigned: ATRA vs ATRA + chemotherapy (chemo).
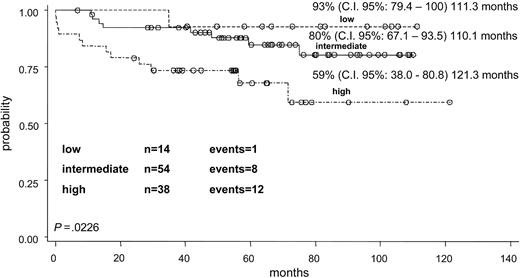
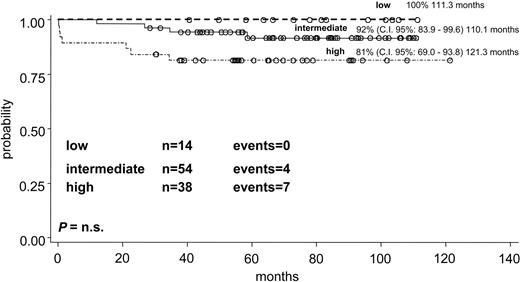
The whole group of 106 children with all clinical data available and evaluable for response was divided into the 3 risk categories according to the prognostic model proposed by Sanz et al.7 Fourteen children were defined as low-risk group (WBC count 10 × 109/L or less and PLT counts greater than 40 × 109/L), 54 as intermediate-risk group (WBC and PLT levels 10 × 109/L or less and 40 × 109/L or less, respectively) and 38 as high-risk group (WBC count greater than 10 × 109/L). The differences in EFS curves (Figure 5) of the 3 risk groups were significant: the EFS, at more than 110 months, was 93%, 80%, and 59%, respectively, for the low-, intermediate-, and high-risk categories (P = .0226). The OS in the 3 prognostic groups at more than 110 months was 100%, 92%, and 81%, respectively (P = .02) (Figure 6).
The incidence and type of toxicity associated with each consolidation course are reported in Table 4. Neutropenic fevers were mainly observed after the first 2 cycles (20 and 17 episodes, respectively); these led to a definitive discontinuation of therapy in 4 children. One child developed a severe gastrointestinal toxicity and 1 developed an intracranial hemorrhage without sequelae after the second consolidation course; both such complications required definitive chemotherapy discontinuation. No toxic deaths in HCR were recorded. All patients randomized for maintenance received therapy as scheduled. Mild neutropenia and slight abnormalities of liver function tests were the major side effects reported during maintenance. One child developed acute viral hepatitis B and discontinued chemotherapy temporarily. The ATRA syndrome was never observed in patients randomized to the ATRA-including maintenance arms. None of the 101 long-term survivors developed symptomatic anthracycline-related cardiotoxicity. In this group of patients, the evaluation of late complications, including asymptomatic cardiomiopathy, is still ongoing.
Two patients developed therapy-related myelodysplasia (tMDS) after 36 and 80 months from initial diagnosis. Both patients were in first HCR and were PML-RAR alpha PCR– when MDS was diagnosed. The first patient developed chronic myelomonocytic leukemia with monosomy 7 8 months after discontinuation of maintenance therapy. She received a transplant from an unrelated donor and was alive and well 16 months after the diagnosis of tMDS. The other patient had already undergone an SCT because of PML-RAR alpha PCR positivity at the end of the AIDA consolidation. She developed a refractory anemia with excess of blasts (RAEB) with complex karyotypic abnormalities and received a second transplant from the same family donor. This patient died of MDS recurrence 14 months after the second allogeneic SCT.
Discussion
Scant information is available in the literature on pediatric APL, and most cooperative studies have reported few cases of APL included in AML polychemotherapy trials. The combination of ATRA and intensive chemotherapy has proven very effective in childhood APL compared with previously adopted chemotherapy-based regimens.8-11 However, ATRA-related side effects, including headache, fever and, in particular, pseudotumor cerebri, seem to be more pronounced in children than in the adult patients.12-17
The present study represents the largest pediatric APL cohort homogeneously diagnosed (all patients had genetic evidence of the t(15;17) in the leukemic cells) and treated with a specific disease-tailored protocol. The induction combination of ATRA plus Ida proved highly effective with a hematological response rate of 96% and none of the patients showing evidence of resistance to treatment.
It is worth noting that, while 4 patients experienced early death during the first 2 years from the opening of the protocol, no other induction deaths were recorded thereafter.
In an attempt to reduce ATRA-related neurotoxicity, more frequently observed in children, the daily dosage of ATRA was 25 mg/m2/d, a dose that had proven effective in a previous adult APL dose-reduction trial.18 In spite of such reduction, both headache and pseudotumor cerebri were observed in 14 and 10 children, respectively, while an overt ATRA syndrome occurred only in 2 cases. All these side effects were transient, reversible, and were never a cause of death in our patients.
With regard to the toxicity related to consolidation, febrile neutropenia, infections, and mucositis were frequently observed, although these episodes led to a definitive treatment withdrawal in only 6 children and no deaths in remission were recorded. Therefore, consolidation toxicity was less severe in children compared with adult patients. In fact, among the 229 adult patients treated with the AIDA protocol and in HCR after induction, 5 died of complications and 16 did not complete the 3 consolidation courses because of therapy-related toxicity mainly consisting of infections and/or prolonged marrow hypoplasia.3
A less intensive postremission treatment could be considered in the future. The Spanish Programa de Estudio Y Tratamiento de las Hemopatias Malignas (PETHEMA) group, using an anthracycline-based consolidation treatment, has shown a reduction in consolidation-related toxicity without compromising the antileukemic effect.7,19 Furthermore, using a risk-adapted protocol19 in which reinforced anthracyclines and no ARA-C were given to patients defined as intemediate and high risk, according to the criteria of Sanz et al, the PETHEMA group recently reported improved outcome results, particularly evident in the intermediate-risk category, while the improvement in the high-risk group was less significant. Both the Spanish PETHEMA APL-967 and PETHEMA APL-9919 studies included children. It is conceivable that ARA-C may have a role in patients with hyperleukocytic disease (a subgroup more represented in the pediatric population). In the presently ongoing risk-adapted GIMEMA trial (AIDA-2000), ARA-C was indeed maintained for patients with hyperleukocytosis, while the anthracycline-based PETHEMA APL-96 approach was chosen for intermediate- and low-risk patients. The results are currently under evaluation.
When applying the criteria of Sanz et al7 to our childhood series, the presenting WBC count significantly influenced all outcome estimates (EFS and OS), due to an impact on both early death and relapse risk.
Also, the role of ATRA in the postremission phase of patients with APL is still controversial. The combination of intermittent maintenance ATRA and chemotherapy appeared to be particularly useful for patients presenting with a high WBC count at diagnosis.1,20 Furthermore, some ongoing risk-adapted protocols for adult APL are now exploring the potential synergistic effect of ATRA and chemotherapy given simultaneously in consolidation. Preliminary data suggest that higher postconsolidation molecular remission rates and improved outcome may be obtained using this strategy.19,21
With regard to maintenance results, our study allowed us to establish an advantage in terms of improved “molecular” DFS for patients receiving ATRA plus chemotherapy compared with those treated with ATRA alone. These results are in agreement with those reported by the French APL 93 study.17 Due to the fact that the 2 other arms were terminated early, this comparison was not feasible in the 4 groups because of the low number of cases randomized to chemotherapy alone or observation.
Finally, a special consideration is deserved for the risk of cardiomyopathy that may occur in children treated with regimens including high-doses of anthracyclines.22 No severe acute cardiotoxicity was observed in our patients, in spite of the high cumulative anthracycline dosage (650 mg/m2). However, a longer follow-up is needed to draw definitive conclusions. A total of 61 patients on follow-up after completion of the standard protocol, who were still in first CR and had not developed tMDS, are being monitored echocardiographically and electrocardiographically at least once a year. The results of this monitoring of the cardiac function are currently under central revision and evaluation.
In conclusion, the results of our study indicate that a specifically designed APL protocol including the simultaneous administration of ATRA and anthracyclines for the induction phase is feasible, well tolerated, and highly effective in children. A risk-adapted postremission therapy, modulated according to risk factors influencing outcome, persistence, and/or recurrence of molecular disease, needs to be explored in future pediatric multicenter APL trials.
Appendix
The following institutions enrolled children in the GIMEMA-AIEOP AIDA protocol: Ancona, Clinica Pediatrica, Centro Regionale Oncoematologia Pediatrica, Ospedale dei Bambini G. Salesi (Dr. P. Pierani); Avellino, Servizio di Ematologia, Azienda Ospedaliera S.G. Moscati (Dr. N. Cantore); Bari, Dipartimento Biomedicina Età Evolutiva (Dr. D. De Mattia, Dr N. Santoro); Bari, Clinica Pediatrica II (Prof. N. Rigillo); Bari, Cattedra di Ematologia (Prof. V. Liso. Prof.ssa G. Specchia); Bergamo, Divisione Ematologia (Prof. T. Barbui); Bologna, Dipartimento di Scienze Pediatriche Mediche e Chirurgiche (Prof. G. Paolucci, Prof. A. Pession, Dr R. Rondelli); Bologna, Istituto Ematologia e Oncologia Medica (Prof. M. Baccarani); Bolzano, Ematologia, Ospedale Regionale (Dr. P. Coser); Cagliari, Servizio di Oncoematologia Pediatrica, Ospedale Regionale Microcitemie (Prof. P. Biddau, Dr.ssa R. Mura); Catania, Oncoematologia Pediatrica (Prof. G. Schilirò, Dr L. Lo Nigro); Catania, Cattedra di Ematologia, Ospedale Ferrarotto (Prof. R. Giustolisi); Catanzaro, Divisione di Ematologia (Prof. S. Magro); Firenze, Ospedale Meyer, Dipartimento di Pediatria, U.O. Oncoematologia Pediatrica (Prof.ssa G. Bernini, Dr.ssa A. Lippi); Genova, Dipartimento di Ematologia ed Oncologia Pediatrica, Istituto G. Gaslini (Dr. G. Dini, Dr.ssa C. Micalizzi); Latina, Divisione Ematologia, Ospedale S. Maria Goretti (Dr. A. De Blasio); Milano, Servizio di Ematologia, Istituto Scientifico San Raffaele (Dr. M. Bregni); Monza, Clinica Pediatrica, Ospedale S. Gerardo (Prof. G. Masera, Prof. A. Biondi, Dr C. Rizzari); Napoli, Ematologia e Oncologia, Ospedale Pausilipon (Prof. V. Poggi, Dr G. Menna); Napoli, II Università, Dipartimento di Pediatria, Servizio Autonomo di Oncoematologia Pediatrica (Prof.ssa M.T. Di Tullio, Dr.ssa F. Casale); Napoli, Clinica Pediatrica II (Prof. S. Auricchio); Napoli, Ematologia, Università Federico II (Prof. B. Rotoli); Napoli, Divisione di Eamatologia, Ospedale San Giovanni Bosco (Dr. E. Miraglia); Nuoro, Servizio di Ematologia Clinica (Dr. A. Gabbas); Padova, Dipartimento di Pediatria, Cattedra di Oncoematologia Pediatrica (Prof. L. Zanesco, Dr.ssa C. Putti); Palermo, Oncoematologia Pediatrica, Ospedale dei Bambini (Dr. M. Aricò); Parma, Pediatria ed Oncoematologia, Azienda Ospedaliera (Dr. G. Izzi); Pavia, Oncoematologia Pediatrica, IRCCS Policlinico S. Matteo, (Prof. F. Locatelli, Dr M. Zecca); Perugia, Divisione di Oncoematologia Pediatrica, Ospedale Silvestrini (Dr A. Amici, Dr P. Zucchetti); Pescara, Divisione di Ematologia (Prof. G. Fioritoni); Pisa, Clinica Pediatrica III (Prof. P Macchia, Dr C. Favre); Reggio Calabria, Divisione di Ematologia, Ospedali Riuniti (Dr. F. Nobile, Dr M. Comis); Roma, Divisione di Ematologia, Ospedale Bambino Gesú (Prof. G. De Rossi, Dr M. Luciani); Roma, Dipartimento Biotecnologie Cellulari ed Ematologia (Prof. F. Mandelli, Prof. R. Foà, Dr.ssa A. M. Testi); Roma, Dipartimento di Biopatologia e Diagnostica per Immagini (Prof. S. Amadori, Prof. F. Lo Coco); S. Giovanni Rotondo, Divisione di Pediatria, Ospedale Casa Sollievo della Sofferenza (Dr. S. Ladogana); Sassari, Clinica Pediatrica (Dr. D. Galisai); Siena, Dipartimento di Pediatria Ostetricia e Medicina della Riproduzione (Prof. G. Morgese, Dr A. D'Ambrosio); Taranto, Ematologia, Ospedale S.S. Annunziata (Dr. P. Mazza); Torino, Scienze Pediatriche e Adolescenza, Ospedale Infantile Regina Margherita (Prof. E. Madon, Dr.ssa E. Barione, Dr.ssa F. Fagioli); Trieste, Clinica Pediatrica (Prof. P. Tamaro); and Vicenza, Divisione di Ematologia (Dr. F. Rodeghiero)
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-05-1971.
Supported by Istituto Superiore di Sanità and Ministero dell'Istruzione, Università e Ricerca, Rome, Italy.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge the competent contribution of Dr Paola Fazi and Dr Francesca Paoloni to the statistical analysis of this study. Finally, we are indebted to Dr Daniela Diverio, Dr Andrea Biondi, and all the members of the referral molecular biology laboratories of Hematology, “La Sapienza” of Rome and Clinica Pediatrica, University of Milan, Monza, Italy.