Abstract
Common variable immunodeficiency (CVID) is a primary immune disorder characterized by impaired antibody production, which is in many instances secondary to defective T-cell function (T-CVID). We have previously identified a subset of patients with T-CVID characterized by defective T-cell receptor (TCR)-dependent protein tyrosine phosphorylation. In these patients, ZAP-70 fails to be recruited to the TCR as the result of impaired CD3ζ phosphorylation, which is, however, not dependent on defective Lck expression or activity. Here we show that neither Fyn nor CD45 is affected in these patients. On the other hand, T-CVID T cells show dramatic defects in the Vav/Rac pathway controlling F-actin dynamics. A significant deficiency in Vav protein was indeed observed; in 3 of 4 patients with T-CVID, it was associated with reduced VAV1 mRNA levels. The impairment in Vav expression correlated with defective F-actin reorganization in response to TCR/CD28 coengagement. Furthermore, TCR/CD28-dependent up-regulation of lipid rafts at the cell surface, which requires F-actin dynamics, was impaired in these patients. The actin cytoskeleton defect could be reversed by reconstitution of Vav1 expression in the patients' T cells. Results demonstrate an essential role of Vav in human T cells and strongly suggest Vav insufficiency in T-CVID. (Blood. 2005;106:626-634)
Introduction
Common variable immunodeficiency (CVID) is a primary dysfunction of the immune system, encompassing a heterogenous group of disorders whose unifying feature is late-onset hypogammaglobulinemia. As a consequence of the impaired antibody production, CVID patients have increased susceptibility to bacterial infections and show a trend to autoimmune disorders and cancer.1 Establishing the molecular basis of CVID has to date been hampered by the heterogeneity in phenotypic features of the disease. Major progress toward elucidating CVID has been achieved with the identification of defects in lymphocyte populations other than B cells. Although B-cell dysfunctions account for a significant proportion of CVID, B cells from many patients are functional in vitro but are not activated in vivo into mature antibody-secreting plasma cells because of defective T-cell help. Defects in cytotoxic T cells and in antigen-presenting cells have also been identified in a subset of CVID patients, suggesting that all cells implicated in the orchestration of the immune response are potential targets of the genetic lesion(s) in this disease.2,3
In patients who have CVID with defective T-cell function (T-CVID), decreased proliferative responses to mitogens, impaired production of cytokines such as interleukin-2 (IL-2), IL-4, IL-5, IL-10, and interferon-γ (IFN-γ)4-11 and defective expression of activation markers, including CD40 ligand,12 attractin,13 and L-selectin,14 have been observed. Furthermore, homozygous loss of ICOS, an inducible costimulator expressed on activated T cells, has been recently documented in a subset of patients with T-CVID.15 Although distinct genetic lesions affecting different stages of T-cell activation and differentiation may underlie the impairment in helper T-cell function, defects in the molecular machinery that controls the initiation of TCR signaling could account for many of the abnormalities described in T-CVID. Impairment of early signaling events, such as calcium mobilization and phospholipid breakdown, has indeed been reported in a subset of patients with T-CVID.7,16 Furthermore, a decrease in the levels of the tyrosine kinase Lck, resulting from aberrant splicing of the gene transcript, has been described in 1 T-CVID patient.17
We have previously shown a correlation between impaired proliferative responses to TCR engagement and defective triggering of the early tyrosine phosphorylation cascade in T cells from patients with T-CVID,18 suggesting a defective coupling of the TCR to downstream tyrosine kinases. ZAP-70 activation, an event crucial to productive TCR signaling,19 is indeed impaired in these patients as the result of defective ZAP-70 recruitment to the activated TCR, which appears, in turn, secondary to a defect in CD3ζ phosphorylation.20 Here we present a structural and functional analysis of the molecular components implicated in the initiation of TCR signaling, characterized by defective ZAP-70/CD3ζ interaction, in 4 patients with T-CVID. The data indicate that impaired reorganization of the actin cytoskeleton, resulting from a reduction in Vav expression, underlies the TCR signaling defect in these patients.
Patients, materials, and methods
Patients and cells
Patients were classified as having CVID according to the World Health Organization (WHO) classification of primary immunodeficiencies.21 Hematologic and immunophenotypical characterization of patients T-CVID1, T-CVID2, dis.ctr1, and dis.ctr2 has been previously presented.18,20 (Patients T-CVID1, T-CVID2, dis.ctr.1, and dis.ctr2 were included in previous studies.18,20 For clarity, they have been renamed T-CVID1 and T-CVID2 and correspond, respectively, to patients 1 and 2 in both studies. Patient dis.ctr1 corresponds to patient 3 in Boncristiano et al20 and to patient 5 in Majolini et al.18 Patient dis.ctr2 corresponds to patient 4 in Boncristiano et al20 and to patient 6 in Majolini et al.18 Patients T-CVID3 and T-CVID4 were newly recruited. They were classified as having T-CVID based on their impaired proliferative responses to TCR/CD3 ligation (control, 100% ± 2.9%; T-CVID3, 50.2% ± 2.7%; T-CVID4, 41.4% ± 1.9%). As with patients T-CVID1 and T-CVID2,20 immunoblot analysis of ZAP-70-specific immunoprecipitates of peripheral blood lymphocytes (PBLs) with antiphosphotyrosine antibodies revealed a defective interaction of ZAP-70 with phospho-ζ and an impairment in ZAP-70 activation in response to TCR engagement in these patients, which correlated with defective CD3ζ phosphorylation (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article), whereas CD3ζ, Lck, and ZAP-70 expression was unimpaired (Figure S2; Table 1). Controls were sex- and age-matched healthy donors. The experiments were performed after approval by the institutional review board of the University of Siena for these studies. Peripheral blood was obtained after informed consent according to the Declaration of Helsinki, and sample size was kept small according to the guidelines of the ethics committee. No overt infectious disease was present at the time of blood sampling. To limit sampling from the patients, phytohemagglutinin (PHA) T-cell lines were derived according to standard procedures and used as a source of RNA for cDNA sequencing. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density centrifugation on Ficoll-Hypaque (Amersham Biosciences Europe, Milan, Italy) and subsequently were depleted of monocytes by adherence.
Cell lines included Jurkat T-lymphoma cells, the Lck-defective Jurkat variant JCaM1,22 and the CD45-defective variant J45.01.23 Mammalian expression vectors encoding either F505Lck24 or F528Fyn25 and carrying the genes encoding neomycin and hygromycin resistance, respectively, were introduced into JCaM1 cells by electroporation. Stably transfected cells were selected in medium containing 1 mg/mL G418 (Gibco BRL, Life Technologies Italia srl, Milan, Italy) or 50 μg/mL hygromycin (Sigma Italia srl, Milan, Italy). Stable transfectants were checked for CD3 expression by flow cytometry. Peripheral blood T cells were transiently transfected using the Amaxa nucleofector device (Amaxa Biosystems, Cologne, Germany) and the conditions for T-cell transfection recommended by the manufacturer. Endotoxin-free plasmid DNA was purified using a kit from Qiagen GmbH (Hilden, Germany). Flow cytometric analysis of PBLs transfected with the Amaxa control green fluorescent protein (GFP) reporter and subsequently labeled with fluorochrome-conjugated anti-CD3 monoclonal antibody (mAb) confirmed that only CD3+ cells had been transfected and showed that the transfection efficiency was consistently 50% or greater. PBLs from the 4 patients with T-CVID and from 4 matched healthy donors were cotransfected with 1 μg GFP reporter/sample and 3 μg of empty vector (pEF-BOS) or of the same vector encoding myc-tagged Vav1.26 Twenty-four hours after transfection, cells were processed for confocal microscopy as described in “Flow cytometry and confocal microscopy.” Lysates of transfected cells were tested for recombinant Vav expression by immunoblotting with anti-myc mAb.
Antibodies and reagents
Phosphospecific polyclonal antibodies against the active forms of p38, JNK, and ZAP-70 (Y493) were purchased from Cell Signaling Technologies (Beverly, MA). Antiphosphotyrosine, anti-ZAP-70, anti-Vav, anti-Lck, anti-Shc, anti-LAT, and anti-Rac polyclonal and monoclonal antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Polyclonal anti-ZAP-70, anti-p38 and monoclonal anti-CD3ζ antibodies, and antibody specific for phosphorylated CD3ζ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Fyn, anti-Grb2, and anti-myc mAb were purchased from BD Biosciences (Heidelberg, Germany). Antitubulin and antiactin mAb were purchased from Amersham Biosciences. A mouse mAb suitable for immunoprecipitation of tyrosine-phosphorylated CD3ζ was kindly provided by M. Banyiash. Anti-CD3 immunoglobulin G (IgG) from OKT3 hybridoma supernatants were affinity purified on Mabtrap (Amersham Biosciences) and were titrated by flow cytometry. Anti-human CD28 mAb was purchased from BD Biosciences. Fluorochrome-labeled anti-human CD3, CD22, CD4, CD8, CD45RA, and CD45RO were purchased from BD Biosciences, fluorochrome-labeled anti-mouse immunoglobulin was purchased from DAKO SpA (Milan, Italy), and fluorochrome-labeled phalloidin was purchased from Sigma Italia. Secondary unlabeled antibodies were purchased from Cappel (Durham, NC), and secondary peroxidase-labeled antibodies were purchased from Amersham Pharmacia Biotech. Cholera toxin B was purified from Vibrio sp 60 harboring pATA13, as reported.27 The anti-CtxB mouse mAb was previously described.28
Activations, immunoprecipitations, immunoblots, and in vitro kinase assays
Activations by cross-linking of anti-CD3 mAb in solution were carried out as described.29 Cells (1-5 × 106/sample for immunoblot analysis, 1-2 × 107/sample for immunoprecipitations) were lysed in 1% (vol/vol) Triton X-100 in 20 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 8, 150 mM NaCl (in the presence of 0.2 mg/mL Na orthovanadate, 1 μg/mL pepstatin, leupeptin, and aprotinin, and 10 mM phenylmethylsulfonyl fluoride [PMSF]), and postnuclear supernatants were probed as such or were immunoprecipitated using the appropriate polyclonal or monoclonal antibodies and either proteinA-Sepharose or agarose-conjugated anti-mouse immunoglobulin (Sigma-Aldrich srl, Milan, Italy). Immunoblots were carried out using a chemiluminescence detection system (Pierce, Rockford, IL). In vitro autophosphorylation assays of Lck-specific immunoprecipitates were carried out in 20 μL of 20 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 10 mM MnCl2, and 10 μCi (370 KBq) γ-[32P] adenosine triphosphate (ATP) at room temperature for 10 minutes. Reaction products were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and exposed to a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The filters were subsequently probed with anti-Lck mAb as immunoprecipitation control.
CD3/CD28 costimulation for confocal microscopy and flow cytometry was carried out by cell incubation on ice for 30 minutes in the presence of saturating amounts of OKT3 and 5 μg/mL anti-CD28 mAb, followed by transfer to plate-bound secondary antibodies.
Flow cytometry and confocal microscopy
Immunophenotyping was carried out by 2- or 3-color flow cytometry using fluorochrome-conjugated mAb from BD Biosciences. To analyze GM1 expression, cells were incubated on ice for 1 hour with 15 μg/mL CtxB, then washed, added with a saturating amount of anti-CtxB mAb, and incubated on ice for another 30 minutes. Bound mAb was revealed using fluorochrome-conjugated anti-mouse immunoglobulin. Samples were processed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Calcium flux was measured using Fluo-4 FF (Molecular Probes Europe BV, Leiden, The Netherlands). Cells (106/sample) were suspended in 200 μL RPMI without phenol Red (Invitrogen srl, Milan, Italy) but with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 5 μM Fluo-4, and 500 μM sulfinpyrazone (Sigma-Aldrich srl), incubated for 45 minutes at 37°C, pelleted by centrifugation, and resuspended in 500 μL of the same medium without Fluo-4. Baseline fluorescence was recorded for 40 seconds at 37°C. Cells were subsequently added with saturating amounts of anti-CD3 mAb and 50 μg/mL secondary antibody. Fluorescence was continuously recorded for 512 seconds at 37°C.
To analyze cortical actin reorganization, cells were fixed and permeabilized in Orthopermeafix (Orthodiagnostic Systems, Raritan, NJ) for 45 minutes at room temperature (RT). After washing, cells were incubated in the dark for 40 minutes with 0.77 μM fluorescein isothiocyanate (FITC)-labeled phalloidin. GM1 surface expression was analyzed using CtxB/anti-CtxB, as described earlier. Cells were resuspended in PBS, and confocal microscopy was carried out on a Leica TCS 4D laser scanning confocal microscope equipped with argon-Krypton laser and coupled to a Leica DMRBE microscope equipped with a 63×/1.4 PL Apo objective lens (Leica Microsystems, Heidelberg, Germany). Images collected at several focal planes were superimposed and merged into a single file using Confocal Assistant software 4.02 (Bio-Rad Laboratories srl, Milan, Italy) and subsequently imported into Adobe Photoshop (Adobe Systems Europe, Edinburgh, United Kingdom).
RT-PCR, cDNA sequencing, genomic DNA PCR
Total RNA was extracted from T-cell lines or from PBLs using the RNA WIZ isolation reagent (Ambion, Austin, TX). Reverse transcription-polymerase chain reaction (RT-PCR) was carried out using AMV reverse transcriptase and the Expand High Fidelity PCR system from Roche Diagnostics SpA (Milan, Italy). The primer for the first strand was oligo-dT (Promega Italia srl, Milan, Italy), whereas pairs of specific primers were used for cDNA amplification. cDNA was amplified in an Eppendorf Mastercycler Thermal Cycler (Eppendorf srl, Milan, Italy). PCR products were separated by agarose gel electrophoresis and recovered using the Nucleo Spin extraction kit from Macherey-Nagel Srl (Hoerdt, France). Automatic sequencing was performed on both DNAstrands on RT-PCR products from the 4 patients with T-CVID, 1 or both disease control patients and at least 1 healthy control. The nucleotide sequence of VAV-1 was identical to the published sequence. A conserved substitution (C-to-T transition at position 3 of the codon for alanine 1137) compared with the published sequence was found in CD45 cDNA from the 4 patients with T-CVID, 1 disease control patient and 2 healthy controls.
Semiquantitative RT-PCR analysis of VAV1, VAV2, and VAV3 expression was carried out using RNA from purified PBLs from the 4 patients with T-CVID, 1 disease control patient (dis.ctr.1), and a pool of RNA from 3 healthy controls. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as housekeeping control. RT-PCR products were separated by agarose gel electrophoresis, and the identity of the band amplified with the VAV-specific primers was checked by sequencing. Intensities of the VAV- and GAPDH-specific bands were quantitated by laser densitometry (Kodak [Eastman Kodak, Rochester, NY] Digital Science Electrophoresis Documentation and Analysis System 120), and the relative levels of VAV mRNA were normalized to the levels of GAPDH mRNA.
Genomic DNA was extracted from purified PBLs from the 4 patients with T-CVID, 1 disease control patient (dis.ctr.1), and a pool of 3 healthy controls. Cells were washed twice in phosphate-buffered saline (PBS), resuspended in 500 μL lysis buffer (see “Activations, immunoprecipitations, immunoblots, and in vitro kinase assays”) containing 3% Triton X-100, and incubated on ice for 5 minutes. Nuclei were recovered by centrifugation and lysed in 0.5% SDS and 20 μg/mL RNase in TE (10 mM Tris-HCl, 1 mM EDTA [ethylenediaminetetraacetic acid], pH 8). After 1-hour incubation at 37°C, the nuclear extract was added with 100 μg/mL proteinase K and was incubated for another 3 hours at 50°C. DNA was purified by standard phenol-chloroform extraction and ethanol precipitation. The regions of genomic DNA spanning positions -312 to +137 of human VAV1 and exons 1, 3 to 4, 5 to 6, 7, and 27 of VAV1 were amplified by PCR. In the latter case, the primers were designed to pair with noncoding sequences flanking the exons. The identities of the resultant fragments were confirmed by automatic sequencing.
Results
Normal expression and cDNA sequence of Fyn and CD45 in T-CVID T cells
The failure of ZAP-70 to be recruited to the activated TCR in the 4 patients with T-CVID who participated in this study appears secondary to a defect in CD3ζ phosphorylation; however, the cDNA sequence, protein expression, activity, and subcellular localization of Lck are unaffected in these patients (Figures S1 and S3; Boncristiano et al20 ). We have assessed the integrity of 2 other components implicated in the regulation of CD3ζ phosphorylation, the tyrosine kinase Fyn and the tyrosine phosphatase CD45.
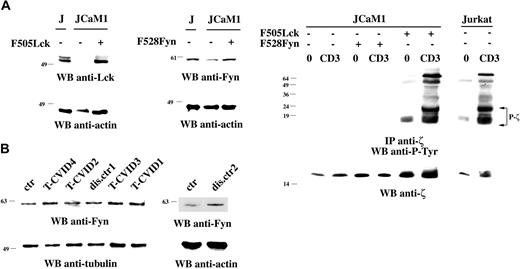
Although Lck and Fyn can phosphorylate CD3ζ in heterologous systems, genetic evidence indicates that this function is principally subserved by Lck.22,30-32 Nevertheless, to rule out a contribution of Fyn to this process, the Lck-defective Jurkat line JCaM1, where CD3ζ fails to become phosphorylated after TCR engagement, was stably transfected with mammalian expression constructs encoding constitutively active mutants of Lck (F505Lck) or Fyn (F528Fyn). As shown in Figure 1A (top), the resultant transfectants expressed significant levels of Lck and showed an approximately 2-fold increase in levels of Fyn, respectively. As expected, expression of F505Lck resulted in full recovery of TCR-dependent CD3ζ phosphorylation and coprecipitation of tyrosine-phosphorylated ZAP-70. Conversely, no significant effect on CD3ζ phosphorylation was observed in JCaM1 cells expressing F528Fyn (Figure 1A, bottom), notwithstanding increased protein tyrosine phosphorylation in total cell lysates (data not shown), indicating that Fyn cannot replace Lck in CD3ζ phosphorylation and supporting the notion that CD3ζ is not a major substrate of Fyn in vivo. It is unlikely, therefore, that a defect in Fyn underlies the phosphorylation defect in our patients with T-CVID. This is further supported by the finding that Fyn expression, as evaluated by immunoblot analysis of PBL lysates from the 4 patients with T-CVID and the 2 disease control patients, was indistinguishable from that observed in T cells from healthy donors (Figure 1B). The possibility of point mutations was ruled out by sequence analysis of the entire cDNA encoding Fyn.
Src kinases are the earliest components of the TCR signaling machinery, and their activity is positively controlled by the tyrosine phosphatase CD45, which is responsible for dephosphorylation of their C-terminal regulatory tyrosine residue.33 Accordingly, TCR engagement fails to trigger CD3ζ phosphorylation in CD45-deficient T cells (Koretzky et al,23 Stone et al,34 and data not shown). The possibility that alterations in CD45 expression underlie the T-cell defect in our patients with T-CVID was ruled out by immunophenotypic analysis of CD45RA/CD45RO expression on T cells from these patients (Table 2). Furthermore, no mutation was found in the nucleotide sequence of the cDNA encoding the transmembrane and intracellular domains of CD45. Collectively, though it has not been possible to measure the activities of CD45 and Fyn because of the limited size of the biologic samples available, these data, together with our finding that Lck is unaffected in the same patients (Figure S3 for patients T-CVID3 and T-CVID4 and Boncristiano et al20 for the other patients), suggest that the kinases/phosphatases directly responsible for CD3ζ phosphorylation are normal in T-CVID T cells.
Defective Vav expression in T-CVID T cells
A number of essential components of the intracellular machinery implicated in TCR signal transduction are constitutively segregated to plasma membrane microdomains, known as lipid rafts, including Src kinases, small GTPases such as Ras, and transmembrane adaptors such as LAT.35 On the other hand, the TCR/CD3 complex itself is only weakly associated with rafts.36 TCR triggering results in stabilization of the TCR in lipid rafts, possibly as the result of raft clustering. In this location, the CD3/ζ immunoreceptor tyrosine-based activation motifs (ITAMs) are phosphorylated by the resident Src kinases and become competent to initiate the full signaling cascade.35,37 Others38 and we39 have shown that reorganization of the actin cytoskeleton is essential for raft coalescence. A defect in this step might underlie the failure of T-CVID T cells to initiate TCR signaling.
Fyn fails to restore correct CD3ζ phosphorylation in Lck-deficient cells and is normally expressed in T-CVID T cells. (A, left) Immunoblot analysis of Lck and Fyn expression in parental JCaM1 cells and in stable transfectants expressing F505Lck or F528Fyn. (Right) Immunoblot analysis using antiphosphotyrosine antibodies of CD3ζ-specific immunoprecipitates from lysates of Jurkat cells, JCaM1 cells, or stable JCaM1 transfectants expressing F505Lck or F528Fyn. A small amount of tyrosine-phosphorylated CD3ζ in JCaM1 cells expressing Fyn was detectable at longer exposure times (data not shown). 0 indicates nonstimulated; CD3, activated by CD3 cross-linking with OKT3 mAb for 30 seconds at 37°C. The filter was stripped and reprobed with anti-CD3ζ mAb. (B) Anti-Fyn immunoblots of postnuclear supernatants of PBLs from patients with T-CVID (T-CVID1-4), 2 disease control patients (dis.ctr1 and 2), and a representative healthy control (ctr; n = 3). Filters were stripped and reprobed with antitubulin or antiactin mAb. Migration of molecular mass markers is indicated.
Fyn fails to restore correct CD3ζ phosphorylation in Lck-deficient cells and is normally expressed in T-CVID T cells. (A, left) Immunoblot analysis of Lck and Fyn expression in parental JCaM1 cells and in stable transfectants expressing F505Lck or F528Fyn. (Right) Immunoblot analysis using antiphosphotyrosine antibodies of CD3ζ-specific immunoprecipitates from lysates of Jurkat cells, JCaM1 cells, or stable JCaM1 transfectants expressing F505Lck or F528Fyn. A small amount of tyrosine-phosphorylated CD3ζ in JCaM1 cells expressing Fyn was detectable at longer exposure times (data not shown). 0 indicates nonstimulated; CD3, activated by CD3 cross-linking with OKT3 mAb for 30 seconds at 37°C. The filter was stripped and reprobed with anti-CD3ζ mAb. (B) Anti-Fyn immunoblots of postnuclear supernatants of PBLs from patients with T-CVID (T-CVID1-4), 2 disease control patients (dis.ctr1 and 2), and a representative healthy control (ctr; n = 3). Filters were stripped and reprobed with antitubulin or antiactin mAb. Migration of molecular mass markers is indicated.
F-actin reorganization is regulated in hemopoietic cells primarily by Vav, which promotes guanine nucleotide exchange on Rho family GTPases.40 The expression of Vav and Rac was evaluated by immunoblot analysis of PBL lysates from patients with T-CVID and control patients with disease (CVID patients without T-cell defects). Although no differences in Rac expression were detectable (Figure 2A), a dramatic impairment in Vav expression, exclusive to T-CVID, was consistently observed (Figure 2B). The expression defect was specific to Vav because T cells from these patients were comparable to control T cells in the expression of a panel of proteins implicated in TCR signaling (Figure S2). Semiquantitative RT-PCR showed that in 3 of the 4 patients with T-CVID, the reduction in Vav protein levels correlated with reduced mRNA levels (Figure 2C). Sequence analysis of full-length VAV1 cDNA from the same patients did not reveal any mutation. The possibility of a compensatory mechanism involving up-regulation of the genes encoding Vav2 or Vav3 in the patients with T-CVID was ruled out by semiquantitative RT-PCR (Figure 2D).
Interestingly, 2 Vav immunoreactive bands, 1 of which was characterized by faster electrophoretic mobility, was detected in patient T-CVID1, suggesting the possibility of a deletion on 1 allele. Because no internal deletions were found in the full-length cDNA amplified by RT-PCR, the faster migrating species may be encoded by an allele deleted at the 5′ or the 3′ end of the coding sequence and, therefore, unable to pair with 1 of the primers. This possibility was tested by PCR amplification of the portion of genomic DNA corresponding to the first and last exons of the VAV1 gene, respectively. Control DNA was from a patient with a single Vav immunoreactive band (T-CVID3) and from a healthy donor. A portion of an unrelated gene (RNA polymerase II) was used as independent control. The amount of template and the PCR conditions were calibrated to obtain an amount of PCR product proportional to the amount of template. As shown in Figure S4, although the abundance of PCR product obtained from all DNAs tested was comparable for the last VAV1 exon, the abundance of PCR product obtained from the DNA from patient T-CVID1 was consistently reduced by approximately 50% compared with the control DNA for the first VAV1 exon, supporting the presence of a deletion at the 5′ end of 1 allele. PCR amplification of exons 3 to 4, 5 to 6, and 7 showed a reduction in PCR product for all amplified regions with the exception of exon 7, placing the 3′ boundary of the deletion, which appeared to extend for at least 50 kb into the VAV1 locus, between exons 6 and 7. The resultant Vav truncation would lack the N-terminal calponin-homology and acid domains required for the activation of IL-2 expression in T cells.41 The fact that the size of the second Vav immmunoreactive band appears larger than predicted suggests an in-frame fusion of the Vav coding sequence with a sequence upstream of the deletion might have occurred.
Defective Vav expression in T-CVID T cells. Anti-Rac (A) or anti-Vav (B) immunoblots of postnuclear supernatants of PBL from patients with T-CVID (T-CVID1-4), 1 or 2 disease control patients (dis.ctr1 and 2), and a representative healthy control (ctr; n ≥ 4). Filters were stripped and reprobed with antiactin mAb. Migration of molecular mass markers is indicated. (C-D) Semiquantitative RT-PCR analysis of VAV1 (C) or of VAV2 and VAV3 (D) mRNA levels in T-CVID PBLs. VAV-specific RT-PCR products from patients with T-CVID (T-CVID1-4), 1 disease control patient (dis.ctr1), and a pool of 3 healthy controls (ctr) were resolved by agarose gel electrophoresis, quantitated by laser densitometry, and normalized to the levels of GAPDH mRNA used as internal control. Data are expressed as percentages of the levels of Vav mRNA in PBLs pooled from 3 healthy controls. Raw data of a representative experiment (n ≥ 2) are shown in the inset. Error bars represent SD.
Defective Vav expression in T-CVID T cells. Anti-Rac (A) or anti-Vav (B) immunoblots of postnuclear supernatants of PBL from patients with T-CVID (T-CVID1-4), 1 or 2 disease control patients (dis.ctr1 and 2), and a representative healthy control (ctr; n ≥ 4). Filters were stripped and reprobed with antiactin mAb. Migration of molecular mass markers is indicated. (C-D) Semiquantitative RT-PCR analysis of VAV1 (C) or of VAV2 and VAV3 (D) mRNA levels in T-CVID PBLs. VAV-specific RT-PCR products from patients with T-CVID (T-CVID1-4), 1 disease control patient (dis.ctr1), and a pool of 3 healthy controls (ctr) were resolved by agarose gel electrophoresis, quantitated by laser densitometry, and normalized to the levels of GAPDH mRNA used as internal control. Data are expressed as percentages of the levels of Vav mRNA in PBLs pooled from 3 healthy controls. Raw data of a representative experiment (n ≥ 2) are shown in the inset. Error bars represent SD.
To understand whether a mutation in the VAV1 gene promoter could underlie the decrease in the levels of VAV mRNA observed in patients T-CVID1, T-CVID2, and T-CVID4, a 450-bp fragment spanning positions -312 to +137, containing the minimal promoter region of VAV1 required for tissue-specific expression42 and the 5′ untranslated portion of exon 1, was amplified from genomic DNA from all patients and from a healthy control. Sequence analysis of this fragment in all the patients did not reveal any difference compared with the published sequence, ruling out a defect in the VAV1 gene promoter as causal to the decrease in VAV1 mRNA in these patients.
Defective F-actin reorganization in T-CVID T cells
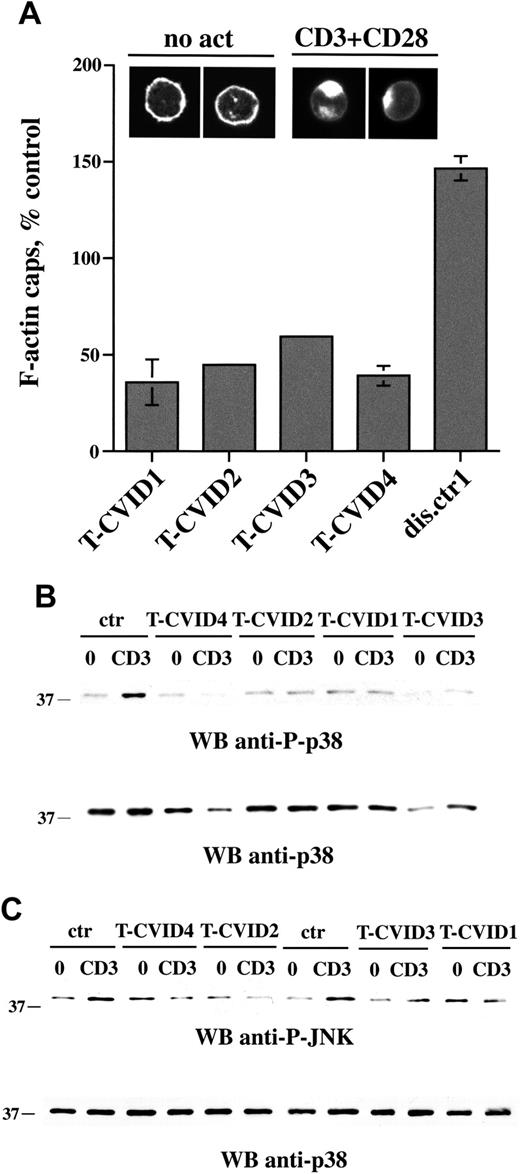
To understand whether the defect in Vav expression could affect Rac activity and, hence, actin cytoskeleton rearrangements triggered by TCR ligation, F-actin capping induced by CD3/CD28 coligation was analyzed by confocal microscopy of PBLs labeled with fluorochrome-conjugated phalloidin. Costimulation was essential to achieve consistent levels of actin capping in these cells. Representative micrographs are shown in Figure 3A (top). F-actin caps after CD3/CD28 coligation were quantitated in the 4 patients with T-CVID and 1 disease control patient (patient dis.ctr1). A significant reduction in F-actin reorganization was reproducibly detected specifically in T-CVID T cells (Figure 3A).
Activation of Rac, which in T cells is essential for F-actin reorganization, also results in the activation of the serine-threonine kinase Pak1, which in turn initiates a serine-threonine kinase cascade leading to the activation of the stress kinases p38 and Jun kinase (JNK).43-45 In agreement with the impairment in F-actin reorganization, which is presumably correlated to defective Rac activation, CD3-dependent p38 activation was significantly reduced in T-CVID T cells (Figure 3B). Furthermore, though the basal phosphorylation levels were relatively high, TCR-dependent increase in JNK phosphorylation was also impaired in patients T-CVID1, T-CVID2, T-CVID4, and, to a lesser extent, in T-CVID3 (Figure 3B). Hence, the defect in Vav expression results in defective activation of Rac and downstream events.
Partial impairment in [Ca2+] mobilization in T-CVID T cells
T cells from Vav-/- mice harbor defects in [Ca2+] mobilization.46-48 To address whether this defect is also observable in T cells with reduced Vav expression, TCR-dependent [Ca2+] mobilization was measured in T-CVID T cells by flow cytometric analysis of PBLs loaded with the fluorescent calcium chelator, Fluo-4 FF. As shown in Figure 4, a partial but consistent attenuation in [Ca2+] flux triggered by CD3 engagement was observed in all patients with T-CVID.
Defective up-regulation of GM1 expression in T-CVID T cells
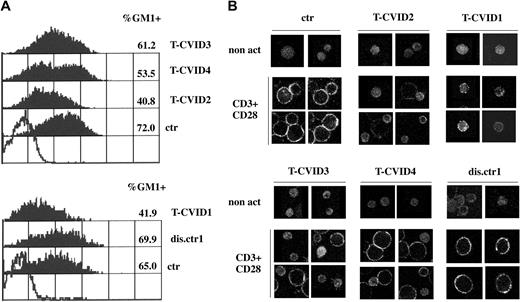
CD3/CD28 costimulation has been shown to increase the levels of surface lipid rafts as the result of de novo synthesis and transport from intracellular stores.49 Defective up-regulation of surface rafts has been reported in T cells from patients with Wiskott-Aldrich syndrome (WAS),50 in whom the genetic defect correlates with impairment of actin reorganization,51 suggesting a role for F-actin in lipid raft mobilization. We analyzed the levels of lipid rafts on T-cell surfaces in the 4 patients with T-CVID and 1 disease control patient using ganglioside GM1 as lipid raft marker. GM1 was measured by indirect immunofluorescence using cholera toxin B (CtxB) and anti-CtxB mAb. Flow cytometric analysis of T-CVID T cells after CD3/CD28 coligation showed reductions in the levels of GM1 and in the relative number of GM1+ T cells compared with T cells from control patients with disease and healthy control (Figure 5A). In all nonstimulated cells, the levels of GM1 were not significantly greater than background levels (data not shown). Differences revealed in the flow cytometric analysis were also observable by confocal microscopy. As shown in Figure 5B, nonstimulated PBLs showed barely detectable levels of diffuse immunoreactivity to CtxB/anti-CtxB complexes in all samples. In control cells and in T cells from a control patient with disease (dis.ctr1), CD3/CD28 costimulation resulted in a significant increase in cell size and a strong increase in GM1 expression, detectable as punctuate staining at the cell surface. In T-CVID T cells consistent differences were observed, particularly in patients T-CVID1 and T-CVID2, with a significant percentage of small cells showing weak, diffuse CtxB-specific immunoreactivity (Figure 5B). Hence, the impairment in F-actin reorganization can be correlated to a defective up-regulation of lipid rafts, an important downstream event in productive TCR signaling.49
F-actin capping is impaired in T-CVID T cells. (A) Relative percentage of F-actin cap formation in PBLs from the 4 patients with T-CVID and a disease control patient (expressed as percentage capping in PBL from healthy controls; n = 6), as measured by confocal microscopy of cells labeled with fluorochrome-conjugated phalloidin. Error bars represent SD. Representative examples of F-actin labeling in cells not stimulated (no act) or activated for 1 hour by CD3/CD28 costimulation are shown at the top. (B-C) Immunoblot analysis of p38 (B) and JNK (C) phosphorylation using phosphospecific antibodies of postnuclear supernatants of PBL from patients with T-CVID and 1 or 2 representative healthy controls (ctr). 0 indicates nonstimulated; CD3, activated by CD3 cross-linking with OKT3 mAb for 5 minutes; and WB, Western blot. Filters were probed with anti-phospho-p38 or anti-phospho-JNK antibodies and, after stripping, with anti-p38 antibodies as loading control.
F-actin capping is impaired in T-CVID T cells. (A) Relative percentage of F-actin cap formation in PBLs from the 4 patients with T-CVID and a disease control patient (expressed as percentage capping in PBL from healthy controls; n = 6), as measured by confocal microscopy of cells labeled with fluorochrome-conjugated phalloidin. Error bars represent SD. Representative examples of F-actin labeling in cells not stimulated (no act) or activated for 1 hour by CD3/CD28 costimulation are shown at the top. (B-C) Immunoblot analysis of p38 (B) and JNK (C) phosphorylation using phosphospecific antibodies of postnuclear supernatants of PBL from patients with T-CVID and 1 or 2 representative healthy controls (ctr). 0 indicates nonstimulated; CD3, activated by CD3 cross-linking with OKT3 mAb for 5 minutes; and WB, Western blot. Filters were probed with anti-phospho-p38 or anti-phospho-JNK antibodies and, after stripping, with anti-p38 antibodies as loading control.
Recovery of F-actin reorganization in T-CVID T cells after reconstitution of Vav expression
The functional defects in Vav-dependent events in T-CVID T cells, particularly the impairment in F-actin reorganization, are consistent with the observed reductions in Vav expression in these patients. To establish whether the decrease in Vav protein is causal to the defect in cortical actin reorganization in T-CVID T cells, a reconstitution experiment was carried out. T cells from the 4 patients with T-CVID and from 4 healthy donors were transiently transfected by electroporation with empty vector or with the same expression vector encoding myc-tagged Vav1. A constitutive GFP reporter was used as transfection control. Immunoblotting with anti-myc mAb showed that recombinant Vav1 was expressed in transfected cells (Figure 6). F-actin capping induced by CD3/CD28 coligation was analyzed by confocal microscopy after cell labeling with fluorochrome-conjugated phalloidin. As shown in Figure 6, the defect in F-actin reorganization was reversed in T-CVID T cells after transfection with the Vav expression construct. Hence, the defect in Vav expression underlies the impairment in actin cytoskeleton reorganization in T cells from the 4 patients with T-CVID included in this study.
[Ca2+] mobilization is partially impaired in T-CVID T cells. Flow cytometric analysis of intracellular [Ca2+] in T-CVID PBLs. Arrow indicates the time of addition of anti-CD3 mAb. Data were analyzed and plotted using FlowJo (Tree Star). Results of representative experiments are shown (n ≥ 2).
[Ca2+] mobilization is partially impaired in T-CVID T cells. Flow cytometric analysis of intracellular [Ca2+] in T-CVID PBLs. Arrow indicates the time of addition of anti-CD3 mAb. Data were analyzed and plotted using FlowJo (Tree Star). Results of representative experiments are shown (n ≥ 2).
Discussion
Cortical actin reorganization is crucial for TCR signaling.52,53 In T cells, this process is primarily regulated by the activation of Rac, which is triggered by the guanine nucleotide exchanger Vav.40,53 T cells from VAV-/- mice and from Vav-deficient Jurkat T cells display defective TCR clustering at the immunologic synapse and impaired formation of actin caps.46,47,54,55 This defect correlates with impaired T-cell activation. Our finding of an impairment in F-actin reorganization in T-CVID T cells resulting from defective Vav expression is fully consistent with the notion that Vav is a master regulator of the actin cytoskeleton in T-cell activation. That defects in F-actin dynamics and TCR signaling are observable in T-CVID T cells even though Vav expression is not abolished indicates, though the levels of Vav are normally sufficient to meet the requirements of the cell, a decrease below these levels is likely to result in Vav insufficiency. This notion is in agreement with the finding that TCR clustering and F-actin accumulation at the T-cell/antigen-presenting cell (APC) interface are impaired in mice heterozygous for a VAV1 null mutation.55 Furthermore, the data suggest that other guanine nucleotide exchangers such as Sos, which has been shown to activate Ras and Rho GTPases,56 cannot substitute for Vav in T cells.
T-CVID T cells fail to up-regulate surface GM1. (A) Flow cytometric analysis of GM1 expression in PBL from the 4 patients with T-CVID (T-CVID1-4), 1 disease control patient (dis.ctr1), and 2 representative healthy controls (ctr; n = 4). Cells were activated for 27 hours by CD3/CD28 costimulation. Empty histogram shows the negative control (anti-CtxB and fluorochrome-labeled anti-mouse immunoglobulin; no CtxB). GM1 labeling in nonstimulated cells was not significantly higher than the negative control (not shown). Relative percentages of GM1+ cells and their mean fluorescence intensity are indicated. Results of representative experiments are shown (n ≥ 2). (B) Analysis of GM1 expression by confocal microscopy of PBL from patients with T-CVID (T-CVID1-4), 1 disease control patient (dis.ctr1), and 1 representative healthy control (ctr; n = 4). Cells were activated for 48 hours by CD3/CD28 costimulation or not (non act). Representative images are shown. Each experiment was performed 2 to 3 times.
T-CVID T cells fail to up-regulate surface GM1. (A) Flow cytometric analysis of GM1 expression in PBL from the 4 patients with T-CVID (T-CVID1-4), 1 disease control patient (dis.ctr1), and 2 representative healthy controls (ctr; n = 4). Cells were activated for 27 hours by CD3/CD28 costimulation. Empty histogram shows the negative control (anti-CtxB and fluorochrome-labeled anti-mouse immunoglobulin; no CtxB). GM1 labeling in nonstimulated cells was not significantly higher than the negative control (not shown). Relative percentages of GM1+ cells and their mean fluorescence intensity are indicated. Results of representative experiments are shown (n ≥ 2). (B) Analysis of GM1 expression by confocal microscopy of PBL from patients with T-CVID (T-CVID1-4), 1 disease control patient (dis.ctr1), and 1 representative healthy control (ctr; n = 4). Cells were activated for 48 hours by CD3/CD28 costimulation or not (non act). Representative images are shown. Each experiment was performed 2 to 3 times.
Recovery of F-actin capping in T-CVID T cells by reconstitution of Vav1 expression. (A) Relative percentage of F-actin cap formation in response to CD3/CD28 costimulation in T cells from the 4 patients with T-CVID (expressed as percentage of capping in T cells from 4 matched healthy controls after transfection with empty vector), as measured by confocal microscopy of cells labeled with fluorochrome-conjugated phalloidin. T cells were cotransfected with a GFP reporter and either the empty vector or the same vector encoding myc-tagged Vav1. Capping was quantitated on GFP+ cells (n = 100). The error bars represent SD. (B) Two representative examples of F-actin labeling (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin) and GFP fluorescence in Vav/GFP cotransfected T-CVID T cells activated for 1 hour by CD3/CD28 costimulation, together with the respective overlays, are shown. (C) An immunoblot analysis with anti-myc mAb of a representative control and T-CVID T-cell lysate transfected with the empty vector or the Vav expression vector is also shown. The arrow indicates the migration of myc-tagged VAV1.
Recovery of F-actin capping in T-CVID T cells by reconstitution of Vav1 expression. (A) Relative percentage of F-actin cap formation in response to CD3/CD28 costimulation in T cells from the 4 patients with T-CVID (expressed as percentage of capping in T cells from 4 matched healthy controls after transfection with empty vector), as measured by confocal microscopy of cells labeled with fluorochrome-conjugated phalloidin. T cells were cotransfected with a GFP reporter and either the empty vector or the same vector encoding myc-tagged Vav1. Capping was quantitated on GFP+ cells (n = 100). The error bars represent SD. (B) Two representative examples of F-actin labeling (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin) and GFP fluorescence in Vav/GFP cotransfected T-CVID T cells activated for 1 hour by CD3/CD28 costimulation, together with the respective overlays, are shown. (C) An immunoblot analysis with anti-myc mAb of a representative control and T-CVID T-cell lysate transfected with the empty vector or the Vav expression vector is also shown. The arrow indicates the migration of myc-tagged VAV1.
Coalescence of lipid rafts, which is essential for full triggering of the TCR signaling cascade,39,57-59 is impaired in Vav-/- T cells38 and in T cells treated with pharmacologic inhibitors of actin polymerization,39 suggesting a role for Vav-dependent reorganization of cortical actin in this process. Clustering is likely to stabilize the preexisting weak association of the TCR with lipid rafts36 and to promote its juxtaposition to Lck, which is primarily responsible for CD3ζ phosphorylation and subsequent initiation of the TCR signaling cascade as the result of recruitment and activation of ZAP-7022,31,32 (Figure 1A). Although T cells from patients with T-CVID displayed normal expression of Lck and Fyn and of their positive regulator CD45, ZAP-70 failed to be recruited to the activated TCR as the result of defective CD3ζ phosphorylation.20 We propose that a defect in the Vav/Rac pathway controlling lipid raft clustering, attributed to impaired Vav expression and F-actin reorganization, may underlie the failure of T cells from our patients with T-CVID to correctly initiate TCR signaling. The signaling defects observed in T-CVID T cells from our patients are largely in agreement with the defects reported for T cells from Vav-/- mice and for Vav-deficient Jurkat T cells, including defective actin capping, impaired Ca2+ mobilization, and—albeit more controversial—impaired stress kinase activation.46-48,54 In contrast, as opposed to our patients, early protein tyrosine kinase (PTK)-dependent signaling appears unaffected in VAV1-/- T cells. The possibility of compensation for the loss of Vav1 by up-regulation of Vav2 or Vav3 in VAV1-/- T cells has, however, not been addressed. Furthermore, a different phenotypic outcome of Vav deficiency in mice and humans cannot be excluded. On the other hand, the alternative possibility that the impairment in CD3ζ phosphorylation and ZAP-70 activation in T-CVID T cells may result from a defect affecting early events and Vav expression cannot at this stage be ruled out.
The impairment in Vav protein expression can, in 3 of the 4 patients with T-CVID, be correlated to a reduction in the levels of Vav-specific mRNA. No mutation was found in any of the patients in the region upstream of the VAV1 gene which has been identified as essential and sufficient for the tissue-specific expression of Vav1,42 ruling out promoter-sequence defects. Alternative intrinsic defects, including decreased mRNA stability or VAV1 haploinsufficiency (as appears to be the case for patient T-CVID1), may underlie the reduction in VAV1-specific mRNA. Alternatively, transcriptional regulation of the VAV1 gene may be impaired in these patients. Vav is primarily expressed in hematopoietic cells.40 It has been proposed that the regulation of Vav expression is achieved by stage- and tissue-specific transcriptional activators rather than by epigenetic modifications of the gene promoter.60 This notion is supported by the finding that VAV1 expression can be reactivated in mouse 3T3 fibroblasts by fusion with human monocytic U937 cells, which express Vav.61 Analysis of the VAV1 gene promoter has identified a 23-bp segment essential for tissue-specific regulation, which includes a 7-bp potential core binding factor/acute myeloid leukemia 1 (CBF/AML-1) binding site.42 The absence of Vav expression in fetal liver of CBP/AML-1-/- mice supports a role for this transcription factor in the regulation of Vav, at least in primitive hemopoiesis.62 Whether the defect in Vav expression in T-CVID T cells is dependent on a defect in the transcriptional machinery remains to be determined. In this context, it is noteworthy that T cells from 1 of the patients with T-CVID (T-CVID3) show a significant impairment in Vav expression, notwithstanding normal levels of VAV1 mRNA, which suggests a posttranscriptional defect affecting VAV1 mRNA translation or Vav protein stability. This feature indicates that different genetic lesions may underlie Vav deficiency in T-CVID.
Vav plays a key role in sustaining TCR signaling after lipid raft clustering and formation of the immunologic synapse,46-48,54 suggesting that, in addition to preventing initiation of the TCR signaling cascade, the reduction of Vav in T-CVID T cells might also affect later signaling events downstream of Rac. In support of this notion, the up-regulation of lipid rafts at the cell surface triggered by CD3/CD28 costimulation in T-CVID T cells (Figure 5) and their proliferative response to TCR ligation18 (see “Patients, materials, and methods”) were significantly impaired in our patients with T-CVID. Collectively, the defect in Vav expression shown by our data not only contributes a significant step forward in the understanding of the molecular basis of T-CVID, but also underlines the essential role of Vav in human T-cell activation.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2004-05-2051.
Supported by Telethon (grant E.1161) and by the Italian Association for Cancer Research and the European Union (grant QLK2-CT-2002-00620).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Denis Alexander for the gift of CD45-deficient Jurkat cells; Frédérique Michel for generously providing myc-tagged Vav1; Nagaja Capitani, Giacomo Spinsanti, and Francesco Nardi for support and advice; and Sonia Grassini for technical assistance.




![Figure 4. [Ca2+] mobilization is partially impaired in T-CVID T cells. Flow cytometric analysis of intracellular [Ca2+] in T-CVID PBLs. Arrow indicates the time of addition of anti-CD3 mAb. Data were analyzed and plotted using FlowJo (Tree Star). Results of representative experiments are shown (n ≥ 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-05-2051/6/m_zh80140581380004.jpeg?Expires=1763461053&Signature=KPEP5SHCnHyl6uoKQjqYoDAfp1sLTJtaREuEi842YHlIjd8ElnDC8M5piKWJ4pDothCngIq4mcRfMgf7be3ot978zGPLTeFGxihJ8-AZmky-upiEHy2FkWVqvW~VK73mFSOSLRKFVSGbnOwE9Z3bMcJ5eRoeRJyaC~xuOaj1gBEufK0LfxZav-Xct7oTEC-pK6M0nj0FVkzCP2DSVUEdAyypolV24oGsdfu~FtCmuxilOS~EREKMsgZ2coZnBPFBfxy2q~WQITZZiBRlhsnL0Qm4u9GVVHrRVUZgUwCEF6vkCSNC0JD-j7W7vHPHATtTFaRd6cVAl6i1Qb7CmvsXPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Recovery of F-actin capping in T-CVID T cells by reconstitution of Vav1 expression. (A) Relative percentage of F-actin cap formation in response to CD3/CD28 costimulation in T cells from the 4 patients with T-CVID (expressed as percentage of capping in T cells from 4 matched healthy controls after transfection with empty vector), as measured by confocal microscopy of cells labeled with fluorochrome-conjugated phalloidin. T cells were cotransfected with a GFP reporter and either the empty vector or the same vector encoding myc-tagged Vav1. Capping was quantitated on GFP+ cells (n = 100). The error bars represent SD. (B) Two representative examples of F-actin labeling (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin) and GFP fluorescence in Vav/GFP cotransfected T-CVID T cells activated for 1 hour by CD3/CD28 costimulation, together with the respective overlays, are shown. (C) An immunoblot analysis with anti-myc mAb of a representative control and T-CVID T-cell lysate transfected with the empty vector or the Vav expression vector is also shown. The arrow indicates the migration of myc-tagged VAV1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-05-2051/6/m_zh80140581380006.jpeg?Expires=1763461053&Signature=ogYkvRBpO7~ZRS4BIRXG1xtnfWfrCimxrzDUFPTqnoNSipAKfn4tGnBRVMl4vUHh1QSx8e5BXnn8Hsq2fa1qUhGs9EZOZrY8ys7YhKvPA6x4bTW7T1RNGv~qy6wijYq6r3lG8ndQNUX5tw13Gm0vuzUFSGLBgBaCSa9HdKj4hH1R0UOXKUOk32DlX1mdCIoVluVkbcukNbCy6eDWrDEYnlLvrJGcMGYx4vQWBujwls2zbWHjH0xOpl5Ekq-CAzAb2dxLvJ1XIrFXunASsK2mUmHklJRHQe3fL9Mcg6YlKEVZzD3fhoYd5uMaEneMQ13cmUaXmX5J7SK5wQraQfSjFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. [Ca2+] mobilization is partially impaired in T-CVID T cells. Flow cytometric analysis of intracellular [Ca2+] in T-CVID PBLs. Arrow indicates the time of addition of anti-CD3 mAb. Data were analyzed and plotted using FlowJo (Tree Star). Results of representative experiments are shown (n ≥ 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-05-2051/6/m_zh80140581380004.jpeg?Expires=1763461054&Signature=O2UbPHJBDiDWu4bSeMx8emDxPj6Iv0wZD-IohCFRCJ~fIthUbzgqOZpIlXSAIAqPgoFEpSoJVWHEQwrf41JcCV61hkzGg8X3glw4gOw1clWtScnMOzyKRkJ5lBT2N5OHb3bPkkv3B1Vrhvh9LisNw5DDTKbpj~HOmqd3r9k9xPxPXjHaK2KwhlPSyLDkt8GEfgKy0b9c~6GNudu58PFqjhn~iCDeRXpC~0x0b6LD2xR73lMxaskjJrg7EoQxsYujMP6JkcKxwbYXcpQLR2gArlgE-9T38VE7XnZ2m3~0C3EjfkRtq5sqPs48S~r2FcXVGkaXQ~NMjS534k8LwmZsJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Recovery of F-actin capping in T-CVID T cells by reconstitution of Vav1 expression. (A) Relative percentage of F-actin cap formation in response to CD3/CD28 costimulation in T cells from the 4 patients with T-CVID (expressed as percentage of capping in T cells from 4 matched healthy controls after transfection with empty vector), as measured by confocal microscopy of cells labeled with fluorochrome-conjugated phalloidin. T cells were cotransfected with a GFP reporter and either the empty vector or the same vector encoding myc-tagged Vav1. Capping was quantitated on GFP+ cells (n = 100). The error bars represent SD. (B) Two representative examples of F-actin labeling (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin) and GFP fluorescence in Vav/GFP cotransfected T-CVID T cells activated for 1 hour by CD3/CD28 costimulation, together with the respective overlays, are shown. (C) An immunoblot analysis with anti-myc mAb of a representative control and T-CVID T-cell lysate transfected with the empty vector or the Vav expression vector is also shown. The arrow indicates the migration of myc-tagged VAV1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-05-2051/6/m_zh80140581380006.jpeg?Expires=1763461054&Signature=OGUNzswdTq9wcXXWuYuDTzFxToQRsb73nBFRn77jIQnZsCAY778THy6x9icqqgwFog34LucH7XUqrScxJaQqx5Yf9LC4lq3Y5yb9CBjughooYtV4Ulz5tu9Gc-~tHcZJzjc6z9SsnxiaSPxPYK0yTNAjp8tyOBac-LZxsEwyI~w26DXg6t3jivfRctaXx1~7P7BhMiwIygxBNusBGk92VN97ciIPreVlcj6kg6fC18xprNMXuP9pG5DA5DSO8rmGq9j7pPG27xE1o21y6r40iuGb1Mr3WqwQwpCyA1jw5nDboKACO1Cf4u0HGDE54K87lx6OO~vasFC5GtQ9IL9rng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)