Comment on Han et al, page 1240
In this issue of Blood, Han and colleagues are the first to report a novel role for cyclooxygenase inhibitor-2 in osteoclastogenesis as an intermediate in the RANKL-induced differentiation of monocytes to osteoclasts.
Cyclooxygenase 2 (Cox-2), which catalyzes the generation of prostaglandins to further modulate the inflammatory response, is best known as a longstanding target to alleviate pain. For diseases such as rheumatoid arthritis, in which the inflammatory response aids in the destruction of the articular cartilage and results in significant pain, inhibition of Cox-2 seems a logical target.1 However, after years of developing molecules to block its actions, we have yet to unravel all of its functions.FIG1
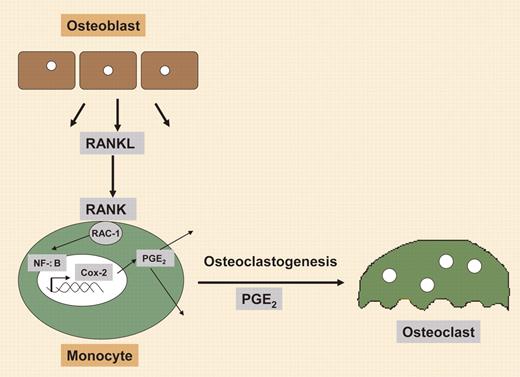
Regulation of osteoclastogenesis by RANKL involves synthesis of Cox-2.
Regulation of osteoclastogenesis by RANKL involves synthesis of Cox-2.
Here, Han and colleagues show a novel functional role for Cox-2 in the differentiation of monocyte precursors into osteoclasts. The authors demonstrate a mechanism in which Cox-2 is an essential mediator for receptor activator of nuclear factor (NF)–κB ligand (RANKL)–induced osteoclastogenesis via a transient increase in Cox-2 expression in a monocytic cell line, after stimulation with RANKL. This enhanced Cox-2 expression was absent in RANKL-stimulated monocytic cells that possessed a dominant-negative form of Rac1, suggesting that this up-regulation was Rac1 dependent. The authors identified a small 27-bp region of the Cox-2 promoter that was essential for RANKL-induced activity. This region was found to have an NF-κB binding site that when mutated completely abolished RANKL-induced NF-κB DNA binding. Collectively, the data suggest a mechanism by which RANKL binds RANK on the monocyte/preosteoclast surface, thereby stimulating the guanosine triphosphatase (GTPase) Rac1 to induce NF-κB binding to the Cox-2 promoter, resulting in a rapid but transient increase in Cox-2 expression.2 Further, with induction of Cox-2, the prostaglandin (PG) E2 is rapidly synthesized, which the authors demonstrate leads to osteoclast differentiation (see figure).
In these studies, RANKL-induced osteoclastogenesis in bone marrow–derived monocyte/macrophage precursors could be completely abolished by addition of the Cox-2 inhibitor celecoxib, but restored by inclusion of exogenous PGE2, demonstrating for the first time a physiologic role for Cox-2 in osteoclastogenesis. The authors suggest that this may be in part what leads to inflammatory bone destruction in rheumatoid arthritis and other bone diseases; however, more importantly, it provides another link between bone formation and resorption. With identification of a key role for Cox-2 in the generation of osteoclasts, another missing link to understanding bone formation and repair is now found. Taken together, the data provide a new model by which inflammatory responses generated during bone injury may lead to PGE2-stimulated angiogenesis,3 chondrogenesis,4 osteogenesis,4 and now osteoclastogenesis via differentiation of resident monocytic precursors. Since osteoclasts are essential factors in the transition of cartilage to bone, this may be the key to understanding how this process is coordinately regulated. Thus, the study by Han et al provides significant insights to our understanding of bone tissue engineering. ▪