Comment on Flomenberg et al, page 1867
Addition of AMD3100, a selective CXCR4 antagonist, to a standard G-CSF–based progenitor cell mobilization regimen increases the number of CD34+ cells collected by apheresis, allowing patients with poor hematopoietic reserve to donate a sufficient number of cells for autologous transplantation.
AMD3100 is a bicyclam derivative that competitively inhibits the binding of stromal cell factor 1 (SDF-1) to its cognate receptor CXCR4 present on CD34+ progenitor and other mononuclear cells.1 CXCR4 is a transmembrane G1-coupled receptor, whereas SDF-1 is a constitutively expressed cytokine produced by marrow stromal cells. Binding of CXCR4 to SDF-1 activates signal transduction pathways that mediate progenitor cell proliferation, trafficking, and homing. Recent reports suggest that cell signaling through the CXCR4 receptor may be necessary to retain stem and progenitor cells in the marrow, and that disruption of this interaction by a CXCR4 antagonist such as AMD3100 leads to rapid egress of progenitor cells into the circulation. By contrast, the CD34+-mobilizing effect of G-CSF takes 4 to 5 days to reach full effect. Elastase and other proteases may be released by G-CSF–induced granulocyte proliferation and activation, and these proteases are thought to degrade SDF-1 and CXCR4, thus indirectly severing the SDF-1–CXCR4 bond that keeps hematopoietic progenitor cells (HPCs) anchored in the marrow.
Since CXCR4 serves as a coreceptor for HIV entry into host cells, AMD3100 was initially developed for its potent inhibition of HIV replication in vitro.2 In clinical trials, AMD3100 did not control HIV replication in vivo, but a 3-fold leukocytosis and a 10-fold increase in circulating CD34+ HPCs were seen within a few hours of administration. Prospective studies in healthy volunteers given a single dose of up to 240 μg/kg of AMD3100 subcutaneously showed a dose-dependent increase in circulating CD34+ cells, with a 15-fold increase above baseline counts at 6 to 9 hours.3 Although AMD3100 as a single agent was not as potent as the conventional mobilization regimen of 5 days of G-CSF at 10 μg/kg/day, the effects of the 2 agents were additive. A single dose of AMD3100 given on the fifth day of G-CSF resulted in a 2- to 3-fold increase in circulating CD34+ cells above what could be achieved with G-CSF alone. Adverse effects of AMD3100 included mild abdominal bloating, nausea, and diarrhea.
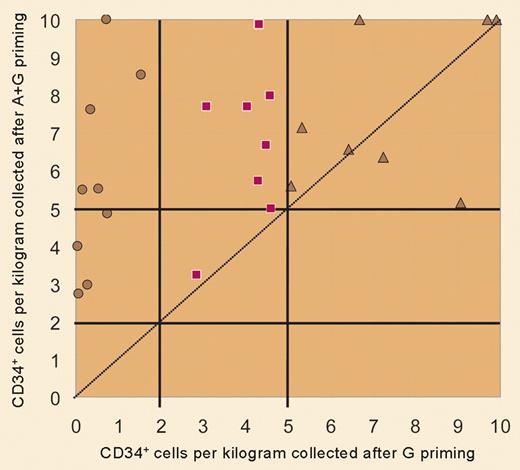
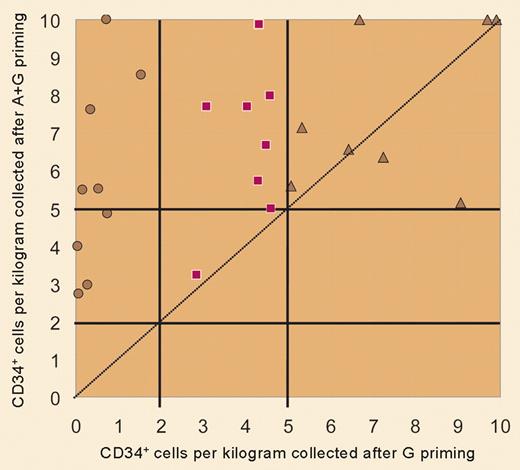
In this issue of Blood, Flomenberg and colleagues extend these findings to chemotherapy-treated patients with myeloma and non-Hodgkin lymphoma. Mobilized blood progenitor cells are the preferred graft source for autologous transplantation in such patients, yet the patients often have a suboptimal response to mobilizing agents, due to insufficient marrow reserve. In a crossover format, each patient underwent 2 mobilization cycles, one using conventional G-CSF for 5 days and the other adding a single dose of AMD3100 at 160-240 μg/kg/day, starting on the fifth day of G-CSF. Addition of AMD3100 was associated with greater apheresis cell yields, a higher rate of success in achieving both a minimal and an optimal CD34 transplantation dose, and a reduction in the number of apheresis procedures needed to collect a minimum HPC dose (see figure). Furthermore, with the exception of one subject with delayed platelet engraftment, neutrophil and platelet recovery were prompt and sustained following transplantation with AMD3100–G-CSF mobilized components.
This is indeed good news for all patients contemplating autologous HPC transplantation and is the first major advance in progenitor cell mobilization for “poor mobilizers” since G-CSF became available. Optimization of the timing of AMD3100 administration has not yet been worked out. Peak mobilization effect is seen at 6 to 9 hours after a single dose, which makes the logistics of starting apheresis and processing the collected component cumbersome. But when given as a supplement to a fifth dose of G-CSF, peak CD34+ cell levels in the blood are sustained for 10 to 18 hours, suggesting that apheresis can commence in the morning following an evening dose of AMD3100 plus G-CSF. Last, since as many as 14% of autologous donors demonstrate a poor mobilization response to G-CSF, AMD3100 is likely to be of maximum benefit in the autologous setting.4 Components collected using the combination of AMD3100 plus G-CSF are known to have higher CD4, CD8, and CD19 cell content than standard G-CSF–mobilized blood cell grafts. Whether this will translate into a higher incidence of graft-versus-host disease in the allogeneic setting is not known. AMD3100 has had landmark effects, both in advancing our understanding of stem cell signaling and trafficking, and in enlarging the pool of patients for whom we can offer safer, more effective progenitor cell grafts. ▪FIG1