Abstract
The difficulty of conducting electrophysiologic recordings from the platelet has restricted investigations into the role of ion channels in thrombosis and hemostasis. We now demonstrate that the well-established synergy between P2Y1 and P2Y12 receptors during adenosine diphosphate (ADP)–dependent activation of the platelet αIIbβ3 integrin also exists in murine marrow megakaryocytes, further supporting the progenitor cell as a bona fide model of platelet P2 receptor signaling. In patch clamp recordings, ADP (30 μM) stimulated a transient inward current at –70 mV, which was carried by Na+ and Ca2+ and was amplified by phenylarsine oxide, a potentiator of certain transient receptor potential (TRP) ion channels by phosphatidylinositol 4,5-bisphosphate depletion. This initial current decayed to a sustained phase, upon which repetitive transient inward cation currents with pre-dominantly P2X1-like kinetics were super-imposed. Abolishing P2X1-receptor activity prevented most of the repetitive currents, consistent with their activation by secreted adenosine triphosphate (ATP). Recordings in P2Y1-receptor–deficient megakaryocytes demonstrated an essential requirement of this receptor for activation of all ADP-evoked inward currents. However, P2Y12 receptors, through the activation of PI3-kinase, played a synergistic role in both P2Y1 and P2X1-receptor–dependent currents. Thus, direct stimulation of P2Y1 and P2Y12 receptors, together with autocrine P2X1 activation, is responsible for the activation of nonselective cation currents by the platelet agonistADP.
Introduction
Platelets express three P2 receptor subtypes—P2X1, P2Y1, and P2Y12—each of which plays important roles in hemostasis and thrombosis.1-5 P2X1 receptors are cation channels that allow significant Na+ and Ca2+ entry and are activated by adenosine triphosphate (ATP) but not adenosine diphosphate (ADP).1,6-8 P2Y1 and P2Y12 receptors are G-protein–coupled receptors at which ADP is the physiologic ligand during platelet activation.2,4 P2Y1 receptors are principally coupled through Gαq proteins, leading to the activation of phospholipase-Cβ (PLCβ) and thus inositol 1,4,5 triphosphate (IP3)–dependent Ca2+ mobilization.9-11 The signaling pathway of Gαi-coupled P2Y12 receptors is less clear, with roles proposed for phosphatidylinositol-3 (PI-3) kinase, Rap1B, K+ channels, and Akt.12-16 P2Y12 receptors also cause the inhibition of adenylate cyclase, though the importance of this pathway is unclear because it is not essential for the activation of functional responses by ADP in vitro.17,18 One possibility is that, in the circulatory system, this P2Y12-receptor signal serves to counteract the stimulation of cyclic adenosine monophosphate (cAMP) formation by endothelial-derived prostacyclin, relieving protein kinase A–dependent inhibition of IP3-mediated Ca2+ release.19
An important feature of P2-receptor–dependent platelet activation is the synergy that exists between purinergic signaling pathways, leading to the amplification of functional responses. For example, coactivation of P2Y1 and P2Y12 receptors is required for full ADP-dependent exposure, and thus aggregation, of fibrinogen-binding sites on the αIIbβ3 integrin.20-22 Furthermore, P2X1 receptors can amplify ADP-evoked Ca2+ increases and thereby amplify platelet activation during co-release of ATP and ADP from dense granules.1,23,24 The underlying mechanisms whereby P2-receptor signaling pathways interact in the platelet are important issues that remain poorly resolved.
Ion channels play fundamental roles in all cell types; however, their study has proven difficult in the tiny, fragile platelet. Because the platelet has limited capacity to synthesize its own proteins, the mature megakaryocyte (MK) is likely to express all ion channels of its daughter cell. Indeed, patch clamp studies of MKs were crucial in the identification of P2X1 as the sole ionotropic receptor underlying ATP-evoked Ca2+ influx in the platelet.1,25 P2Y-receptor activation in the MK also stimulates cation-permeable ion channels that are candidates for store-dependent and store-independent Ca2+ influx in the platelet/MK lineage.1,26-29 However, the extent to which MKs express P2Y1 and P2Y12 receptors is unclear, as is the ability of these receptors to couple to Ca2+-permeable conductances and downstream functional responses in the progenitor cell. The present study has investigated these issues in freshly isolated MKs from murine marrow with the aid of receptor-deficient models and pharmacologic reagents. We demonstrate that the marrow-derived MK is a high-fidelity model of platelet P2Y-receptor interactions and that all 3 platelet P2 receptors are involved in the activation of cation influx currents during ADP stimulation. Furthermore, we provide the first direct recordings under physiologic Ca2+ buffering conditions of a Ca2+ current linked directly to G-protein–coupled receptor activation in the MK/platelet lineage. The MK, therefore, provides a means to identify the underlying channel that contributes to agonist-evoked Ca2+ influx in the platelet.
Materials and methods
Megakaryocyte isolation
Reagents and salines
The saline used for marrow isolation, storage, and confocal experiments contained 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 10 mM glucose, pH 7.35 (NaOH). Type VII apyrase (0.32 U/mL) (Sigma-Aldrich, Poole, United Kingdom) was also included in the preparation of cells for electrophysiologic experiments. Electrophysiologic salines were designed to eliminate K+ currents25,26,28 ; the standard bath saline contained 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM glucose, pH 7.35 (NaOH), and the standard pipette saline contained 150 mM CsCl, 2 mM MgCl2, 0.05 mM Na2 guanosine triphosphate (GTP), 0.05 mM K5Fura-2, 10 mM HEPES, and 0.1 mM EGTA (ethylene glycol-bis (β-aminoethyl ether)N,N,N ′,N ′-tetraacetic acid), pH 7.2 (CsOH). For studies of Ca2+ permeability, Na+ was replaced by the large, impermeant cation N-methyl-d-glucamine (NMDG+), and the saline pH was adjusted with HCl. The absence of voltage-gated outward K+ currents following a depolarizing step from –70 to 0 mV was used to confirm that adequate dialysis had occurred to eliminate K+-selective currents.31,32 We have previously demonstrated that Cl– conductances do not contribute to the nucleotide-evoked currents under these conditions.26,28,29 K5fura-2 and Oregon green–labeled fibrinogen were from Molecular Probes (Leiden, Netherlands). The latter was centrifuged at 16 100g for 5 minutes before use. Unless otherwise stated, all other reagents were from Sigma-Aldrich. ADP was treated with hexokinase to remove contaminating ATP, as previously reported,7 and a luciferin/luciferase assay was used to test that treated samples contained negligible levels of ATP (C. Cendana and M.P.M.-S., unpublished observations, July 2000). Cells were incubated with LY294002 (Tocris Cookson, Bristol, United Kingdom) for 15 minutes before experiments. All other pretreatments were applied 2 minutes before ADP application. Vanadate was prepared either by dissolving sodium orthovanadate directly in the standard bath saline or as described previously33 ; no difference was observed between these 2 preparations. AR-C69931MX was a kind donation from AstraZeneca (Moindal, Sweden).
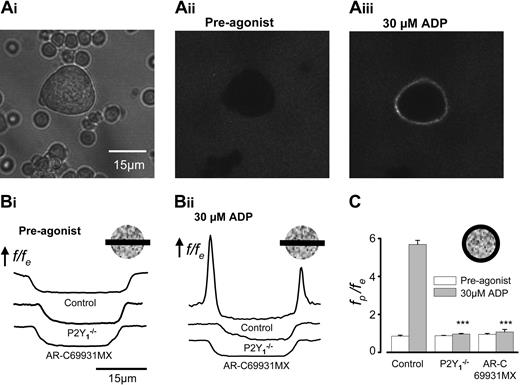
Single-cell measurements of fibrinogen binding demonstrate functional synergy between P2Y1 and P2Y12 receptors in primary murine megakaryocytes. (A) Mouse marrow cells (Ai) were bathed in Oregon green–labeled fibrinogen, and fluorescence images were acquired within a 3-μm z-section at the midplane of a megakaryocyte by confocal microscopy before (Aii) and after (Aiii) the addition of 30 μM ADP. Images were analyzed as described in detail in “Materials and methods”; f is the fluorescence level, fe is the average extracellular fluorescence, and fp is the average fluorescence measured around the cell periphery. (B) f/fe across the center of control, P2Y1–/– and AR-C69931MX–treated MKs before (i) and 2 minutes after (ii) the addition of 30 μM ADP. (C) Average fp/fe ratios before (□) and after (▦) the addition of 30 μM ADP for control, P2Y1–/–, and AR-C69931MX-treated MKs (n = 10 for each). ***Statistical significance of P < .001 compared with control.
Single-cell measurements of fibrinogen binding demonstrate functional synergy between P2Y1 and P2Y12 receptors in primary murine megakaryocytes. (A) Mouse marrow cells (Ai) were bathed in Oregon green–labeled fibrinogen, and fluorescence images were acquired within a 3-μm z-section at the midplane of a megakaryocyte by confocal microscopy before (Aii) and after (Aiii) the addition of 30 μM ADP. Images were analyzed as described in detail in “Materials and methods”; f is the fluorescence level, fe is the average extracellular fluorescence, and fp is the average fluorescence measured around the cell periphery. (B) f/fe across the center of control, P2Y1–/– and AR-C69931MX–treated MKs before (i) and 2 minutes after (ii) the addition of 30 μM ADP. (C) Average fp/fe ratios before (□) and after (▦) the addition of 30 μM ADP for control, P2Y1–/–, and AR-C69931MX-treated MKs (n = 10 for each). ***Statistical significance of P < .001 compared with control.
Electrophysiology
Conventional whole-cell patch clamp recordings were carried out using an Axopatch 200B amplifier (Axon Instruments, Union City, CA) in voltage clamp mode, as described in detail elsewhere.25,27 Membrane currents were filtered at 1 kHz and acquired to computer at a rate of 5 kHz using a Digidata 1200 series A/D converter and Axotape software (Axon Instruments, Union City, CA). All experiments were carried out at room temperature.
[Ca2+]i recordings
[Ca2+]i was measured by single-cell fluorescence photometry, as described in detail elsewhere.34,35 Briefly, fura-2 was excited at 340- and 380-nm wavelengths and emitted light detected at 480 to 600 nm. Data were sampled at 60 Hz and averaged to give a final sample rate of 15 Hz. Background fluorescence was subtracted, and [Ca2+]i was calculated from the 340- to 380-nm fluorescence ratios, as described previously using a viscosity correction factor of 0.85.34-36
Fluorescence imaging of fibrinogen-receptor activation
Confocal fluorescence imaging was conducted at room temperature using a Zeiss LSM 510 (Carl Zeiss, Welwyn Garden City, United Kingdom) with Plan-Neofluar 40 × 1.3 oil and Plan-Apochromat 63 × 1.4 oil DIC objectives, excitation and emission wavelengths of 488 and more than 505 nm, respectively, and an optical section of 3 μm or less. Cells were mixed with 30 μg/mL Oregon green–labeled fibrinogen before they were added to the experimental chamber. Images acquired before and after the addition of ADP were analyzed using either Image J 1.32 (a public domain program developed at the National Institutes of Health by Wayne Rasband and available at http://rsb.info.nih.gov/ij/) or in-house custom-designed software (LSM Toolbox, written by Dr C. J. Schwiening, University of Cambridge). For comparison of spatial fluorescence profiles (Figure 1B), background-subtracted fluorescence levels (f) were expressed relative to the average extracellular fluorescence (fe). ADP promoted the binding of exogenous fibrinogen preferentially to the periphery of the megakaryocyte, which was further quantified as the ratio of the peripheral fluorescence (fp) relative to fe (Figure 1C). In control, time-matched experiments, this ratio remained constant in the absence of added agonist (G.T. and M.P.M.-S., unpublished observations, March 2004).
Analysis
Membrane currents were analyzed in Clampfit version 9.0 (Axon Instruments). Electrophysiologic data and fura-2 fluorescence were also exported, for analysis and presentation, within Microcal Origin (Microcal Software, Northampton, MA). Where applicable, current decays were fitted with a single exponential decay (y = y0 + A1e–(x–x0)/t1). The activation time for both the [Ca2+]i increase and the membrane current were taken as the point at which the trace first increased above (or decreased below) the noise level of the steady state recording under basal conditions. Data are expressed as the mean ± SEM, and statistical analysis was performed using a Student paired or unpaired t test.
Results
Synergy between P2Y1 and P2Y12 receptors at the level of fibrinogen-receptor activation in single-marrow megakaryocytes
To investigate the ability of P2Y receptors to couple to functional platelet responses in marrow-derived MKs, we examined ADP-dependent αIIbβ3 integrin activation using confocal fluorescence measurements of fibrinogen binding. Before the addition of agonist, negligible levels of fibrinogen were bound to the MK (Figure 1Aii, fluorescence image; Figure 1Bi, line profile plots). ADP (30 μM) induced a marked increase in fluorescence predominantly at the periphery of the MK (Figure 1Aiii-Bii, control; Video S1; see the Supplemental Video link at the top of the online article, on the Blood website). The peripheral-external fluorescence ratio (fp/fe) increased from 0.85 ± 0.05 to a peak of 5.69 ± 0.22 (n = 10) (Figure 1C). This response was virtually eliminated by the P2Y12-receptor inhibitor AR-C69931MX (1 μM) or in P2Y1–/– MKs (Figure 1B-C). Compared with control cells, the peak fp/fe increase was only 3% in AR-C69931MX (an increase from 0.95 ± 0.05 to 1.08 ± 1.3; n = 10; P < .001) and 2% in P2Y1–/– MKs (an increase from 0.88 ± 0.02 to 0.96 ± 0.03; n = 10; P < .001). Thus, coactivation of P2Y1 and P2Y12 is essential for ADP-evoked fibrinogen-receptor activation in the primary mouse MK, as previously shown in human and murine platelets.20-22,37,38 Together with previous reports that megakaryocytes, like platelets, have only P2X1 receptors within this family of ATP-gated ion channels,25 these data support the use of the MK as a fully functional model for investigations of platelet P2 receptor signaling.
ADP evokes multiple inward cationic currents through mechanisms that require P2Y1 receptors. Simultaneous whole-cell patch clamp and [Ca2+]i recordings at –70 mV under conditions that eliminate K+-selective currents. (A) Typical biphasic inward current and [Ca2+]i increase activated by 30 μM ADP. Dashed line shows the background current level. (B) Another cell displaying typical repetitive inward currents observed in many recordings during exposure to ADP. These repetitive events had rapid kinetics, as shown in the expanded section. (C) Inward currents and [Ca2+]i increases were not observed in response to 30 μM in P2Y1–/– MKs. (D) Frequency of occurrence (events per minute) of the repetitive transient inward currents for WT and P2Y1–/– MKs before (□) and during (▦) exposure to 30 μM ADP. Error bars represent SEM. *Statistical significance of P < .05 compared to WT.
ADP evokes multiple inward cationic currents through mechanisms that require P2Y1 receptors. Simultaneous whole-cell patch clamp and [Ca2+]i recordings at –70 mV under conditions that eliminate K+-selective currents. (A) Typical biphasic inward current and [Ca2+]i increase activated by 30 μM ADP. Dashed line shows the background current level. (B) Another cell displaying typical repetitive inward currents observed in many recordings during exposure to ADP. These repetitive events had rapid kinetics, as shown in the expanded section. (C) Inward currents and [Ca2+]i increases were not observed in response to 30 μM in P2Y1–/– MKs. (D) Frequency of occurrence (events per minute) of the repetitive transient inward currents for WT and P2Y1–/– MKs before (□) and during (▦) exposure to 30 μM ADP. Error bars represent SEM. *Statistical significance of P < .05 compared to WT.
ADP elicits multiple phases of cation influx currents dependent on activation of P2Y1 receptors
Under conditions that eliminate K+-selective currents and thereby allow measurements of cation influx currents, 30 μM ADP activated an early transient inward current that coincided with the initial increase in intracellular Ca2+ concentration ([Ca2+]i) (Figure 2A). This current corresponds to the P2Y-receptor–evoked transient inward current we reported in MKs from wild-type (WT) rodents and P2X1 knock-out mice25 and is largely carried by Na+ in physiologic saline.25,28,29 During maintained exposure to 30 μM ADP, the initial current declined to a sustained phase and followed a time course that mirrored the plateau phase of the [Ca2+]i increase. In addition, many cells displayed repetitive transient inward currents superimposed on the sustained phase of the response (Figure 2B). Although similar transient currents were also observed spontaneously, their frequency markedly increased during the application of ADP (Figure 2D). ADP (30 μM) failed to activate detectable [Ca2+]i increases and membrane currents in MKs from P2Y1–/– mice (n = 7) (Figure 2C). Spontaneous repetitive-type transient currents were occasionally detected in P2Y1–/– MKs, though the frequency of these events was also unaffected by the application of ADP (P > .05) (Figure 2D). Thus, P2Y1 receptors are essential for all membrane currents activated in the MK by this high concentration of ADP. In the absence of ADP, the repetitive transient currents were less frequent in P2Y1–/– than in WT MKs (n = 7; P > .05) (Figure 2D). Thus, this receptor is also involved in the spontaneous activation of inward cation currents.
Repetitive transient currents are predominantly caused by autocrine activation of P2X1 receptors
Repetitive inward currents during the plateau phase of the ADP-stimulated [Ca2+]i increase exhibited a range of kinetics, though most (84%) had a rise time (time to peak) of less than 100 milliseconds and a decay constant of less than 200 milliseconds (Figure 3A). These rapid kinetics are characteristic of P2X1 receptors in platelets and other cell types.6,39,40 Consistent with this interpretation, most transient events were absent in MKs from P2X1–/– mice (n = 11; Figure 3B). A similar effect was observed after the desensitization of P2X1 receptors with 10 μM α,β-meATP and after their inhibition with 1 μM NF4496,41 (n = 7; P < .001; Figure 3B). As expected, the remaining repetitive transient currents under these conditions had slower kinetics; they decayed either with a single exponential (range, 395-1314 milliseconds), or with a more complex time course (not shown), and followed a rise time of 340 to 2060 milliseconds (P2X1–/– MKs; Figure 3C). As we have previously shown, these slower ADP-evoked repetitive transient currents were associated with a small transient increase in [Ca2+]i in the presence or absence of extracellular Ca2+,26 suggesting that they may be dependent on the cytosolic free [Ca2+]i.
A similar mixture of rapid P2X1 and slower non-P2X1 inward currents was also detected in the absence of exogenous agonist but at a lower frequency (Figure 3D-E). As shown for ADP stimulation, conditions that remove P2X1-receptor activation (P2X1–/– or preexposure to either 1 μM NF449 or 10 μM α,β-meATP) abolished most of the spontaneous membrane currents (Figure 3E). The remaining spontaneous slower inward currents often coincided with a small spontaneous increase in [Ca2+]i (Figure 3D) and were absent in P2Y1–/– MKs (Figure 2C). Together these data suggest that the repetitive inward transient currents under spontaneous and ADP-stimulated conditions are predominantly mediated by P2X1 receptors, with a contribution from another pathway dependent on P2Y1-receptor activation. Because ADP is not an agonist at P2X1 receptors7,42 and the MKs were moved away from other cells in these experiments, activation of the ionotropic receptor must be mediated through an autocrine mechanism after the secretion of ATP or diadenosine polyphosphates, as suggested previously by our group1,43 and by Kawa.44
P2X1-receptor–dependent priming of the ADP-evoked current
Genetic deletion of P2X1 receptors had no significant effect on the amplitude or kinetics of the [Ca2+]i increase or the amplitude of the initial membrane current evoked by 30 μM ADP (P > .05; n = 8) (Figure 4A). However, one noticeable effect of a loss of P2X1 receptors was a small but significant shift in the onset of the initial inward current relative to the [Ca2+]i increase (Figure 4A-B). In control cells, the inward current commenced, on average, before the first detectable [Ca2+]i increase (–37 ± 89 milliseconds; n = 9). In P2X1–/– MKs, the current shifted to 224 ± 64 milliseconds relative to the first detectable [Ca2+]i increase (P < .05; n = 8) (Figure 4C). This increased latency was also observed after preincubation with 1 μM NF449 (894 ± 297 milliseconds; n = 5; P < .05) or 10 μM α,β-meATP (280 ± 84 milliseconds; n = 5; P < .05) (Figure 4C). We have previously reported that P2X1 receptors potentiate the peak amplitude of the P2Y-dependent cation current during costimulation of the ionotropic and G-protein–coupled purinoceptors.25 The present study, therefore, suggests an additional synergistic role for the ionotropic receptor because of its acceleration of the onset of the P2Y-evoked current relative to the [Ca2+]i increase. One explanation for this effect is that the spontaneous activation of P2X1 receptors leads to a basal elevation of [Ca2+]i levels immediately under the plasma membrane, thereby accelerating the onset of the P2Y-receptor–evoked current by acting directly on the channel or on an earlier Ca2+-dependent component of the cascade, such as phospholipase C.25,26,45 This “priming” of a Ca2+-permeable conductance may represent one mechanism whereby P2X1 receptors can accelerate P2Y-mediated Ca2+ signals and potentiate platelet activation.1,3,25
Transient repetitive currents in the presence and absence of exogenous ADP are mainly caused by P2X1 receptors. (A) Scatter plot of activation and inactivation kinetics for the repetitively activated inward currents observed during application of 30 μM ADP. Each point shows the time to peak current and the inactivation time constant for a single transient event when this could be fitted by a single exponential decay. Data from 15 cells. (B) Frequency of occurrence (events per minute) of all transient currents during exposure to 30 μM ADP for control, P2X1–/– MKs, after preexposure to 10 μM α,β-meATP and in the presence of 1 μM NF449. (C) Example of the slower transient events and concurrent [Ca2+]i transient events evoked by ADP in the absence of P2X1 receptors. (D) Example of the spontaneous [Ca2+]i increases and transient inward currents displaying a mixture of rapid and slow current events. (E) Frequency of all spontaneous current transient events in control cells, P2X1–/– MKs (n = 11), after exposure to 10 μM α,β-meATP (n = 7), and in the presence of 1 μM NF449 (n = 7). Error bars represent SEM. Statistical significance compared with control (*P < .05; **P < .01; ***P < .001).
Transient repetitive currents in the presence and absence of exogenous ADP are mainly caused by P2X1 receptors. (A) Scatter plot of activation and inactivation kinetics for the repetitively activated inward currents observed during application of 30 μM ADP. Each point shows the time to peak current and the inactivation time constant for a single transient event when this could be fitted by a single exponential decay. Data from 15 cells. (B) Frequency of occurrence (events per minute) of all transient currents during exposure to 30 μM ADP for control, P2X1–/– MKs, after preexposure to 10 μM α,β-meATP and in the presence of 1 μM NF449. (C) Example of the slower transient events and concurrent [Ca2+]i transient events evoked by ADP in the absence of P2X1 receptors. (D) Example of the spontaneous [Ca2+]i increases and transient inward currents displaying a mixture of rapid and slow current events. (E) Frequency of all spontaneous current transient events in control cells, P2X1–/– MKs (n = 11), after exposure to 10 μM α,β-meATP (n = 7), and in the presence of 1 μM NF449 (n = 7). Error bars represent SEM. Statistical significance compared with control (*P < .05; **P < .01; ***P < .001).
Priming of the initial ADP-activated inward current by P2X1 receptors. Membrane currents and intracellular Ca2+ responses were recorded simultaneously at –70 mV in response to 30 μM ADP. (A) Representative recordings of the initial increase in intracellular [Ca2+] (top panel) and whole-cell current (lower panel) in WT and P2X1–/– MKs. (B) Expanded section of the traces showing current activation before the [Ca2+]i increase in a WT MK but at or later than the [Ca2+]i response in a P2X1–/– MK. (C) Average onset of the ADP-stimulated current relative to the first detectable [Ca2+]i increase in control (n = 9), P2X1–/– MKs (n = 8), in the presence of 1 μM NF449 (n = 5), and after preexposure to 10 μM α,βmeATP (n = 5). Error bars represent SEM. *Statistical significance of P < .05 when compared with control.
Priming of the initial ADP-activated inward current by P2X1 receptors. Membrane currents and intracellular Ca2+ responses were recorded simultaneously at –70 mV in response to 30 μM ADP. (A) Representative recordings of the initial increase in intracellular [Ca2+] (top panel) and whole-cell current (lower panel) in WT and P2X1–/– MKs. (B) Expanded section of the traces showing current activation before the [Ca2+]i increase in a WT MK but at or later than the [Ca2+]i response in a P2X1–/– MK. (C) Average onset of the ADP-stimulated current relative to the first detectable [Ca2+]i increase in control (n = 9), P2X1–/– MKs (n = 8), in the presence of 1 μM NF449 (n = 5), and after preexposure to 10 μM α,βmeATP (n = 5). Error bars represent SEM. *Statistical significance of P < .05 when compared with control.
Synergistic role for P2Y12 receptors in the P2Y1- and P2X1-dependent membrane currents
In contrast to the complete loss of responses in P2Y1–/– MKs, ADP was still able to induce [Ca2+]i increases and inward currents after the inhibition of P2Y12 receptors by a supramaximal concentration of AR-C69931MX (1 μM).46 No significant effect of this compound was observed on the amplitude or time to peak of the [Ca2+]i increase (n = 8; P > .05) (Figure 5C), although given the marked single-cell heterogeneity of the Ca2+ responses, we cannot rule out small effects on ADP-evoked Ca2+ mobilization. However, AR-C69931MX significantly reduced the amplitude of the initial ADP-evoked inward current from 0.72 ± 0.19 pA/pF (n = 9) to 0.17 ± 0.04 pA/pF (n = 9; P < .05) (Figure 5A-B). P2Y12-receptor inhibition also delayed the onset of the current relative to the first detectable [Ca2+]i increase (from –37 ± 89 milliseconds [n = 9] to 374 ± 77 milliseconds [n = 8]; P < .05), although this may simply reflect an increased time to detection above background noise levels for the smaller amplitude current. The ability of the P2Y12 receptor to potentiate this ADP-evoked conductance was largely the result of signaling by PI3-kinase because LY294002 (30 μM; n = 5), a blocker of this enzyme, decreased the current amplitude to an extent similar to that for AR-C69931MX (Figure 5A-B). The average ADP-evoked Ca2+ increase was slightly suppressed compared with control (Figure 5C), but, as observed for AR-C69931MX, this was not statistically significant (P > .05).
AR-C69931MX (1 μM) and LY294002 (30 μM) also caused a marked, significant reduction in the repetitive transient currents evoked by sustained ADP application, from 8.85 ± 2.04 events per minute (n = 15) to 1.87 ± 0.48 (n = 6; P < .01) and 0.46 ± 0.30 (n = 7; P < .01) events per minute, respectively, for AR-C69931MX and LY294002 (Figure 5D). Inhibition of P2Y12 and PI3-kinase using these compounds also decreased the basal activity of the transient currents, although the reduction was only significant in the presence of LY294002 when compared with control (0.32 ± 0.21 [n = 5] and 2.63 ± 0.73 [n = 15] events per minute [P < .001], respectively). As described in “Results,” these transient currents represent the autocrine activation predominantly of P2X1 after secretion of ATP under basal and ADP-stimulated conditions. Taken together, these data show that in addition to a synergistic effect on the main P2Y1-receptor–evoked conductance, P2Y12 receptors contribute, through PI3-kinase, to the autocrine activation of P2X1 receptors during ADP stimulation.
Synergistic role for P2Y12 receptors through PI3-kinase in the activation of inward cation currents by ADP. Membrane currents and intracellular Ca2+ responses were recorded simultaneously at –70 mV in response to 30 μM ADP in control MKs (n = 9) and after exposure to the P2Y12 antagonist AR-C69931MX (1 μM; n = 8) or the PI3-kinase blocker LY294002 (30 μM; n = 5). (A) Timing of the initial ADP-evoked membrane current relative to the intracellular Ca2+ increase. (B) Average initial peak ADP-evoked currents. (C) Normalized ADP-evoked Ca2+i peak amplitude (light boxes) and time to peak (dark boxes), expressed as a percentage of control. (D) Frequency of occurrence of repetitive transient inward currents. Error bars represent SEM. *Statistical significance compared with control (saline or ADP, as appropriate) (*P < .05; **P < .01).
Synergistic role for P2Y12 receptors through PI3-kinase in the activation of inward cation currents by ADP. Membrane currents and intracellular Ca2+ responses were recorded simultaneously at –70 mV in response to 30 μM ADP in control MKs (n = 9) and after exposure to the P2Y12 antagonist AR-C69931MX (1 μM; n = 8) or the PI3-kinase blocker LY294002 (30 μM; n = 5). (A) Timing of the initial ADP-evoked membrane current relative to the intracellular Ca2+ increase. (B) Average initial peak ADP-evoked currents. (C) Normalized ADP-evoked Ca2+i peak amplitude (light boxes) and time to peak (dark boxes), expressed as a percentage of control. (D) Frequency of occurrence of repetitive transient inward currents. Error bars represent SEM. *Statistical significance compared with control (saline or ADP, as appropriate) (*P < .05; **P < .01).
The main P2Y-receptor–evoked conductance is Ca2+ permeable and markedly amplified by phenylarsine oxide, an agent known to amplify TRP ion channels through PIP2 depletion
Previous work has shown that Na+ conducts most of the initial P2Y-receptor–evoked inward current in physiologic external saline.28,29 Detection of Ca2+ permeability through this conductance has proven difficult because the cell viability and responses to ADP are both reduced as external Ca2+ levels are increased.28 More recent work has demonstrated the presence of 3 members of the transient receptor potential family of ion channels—TRPC1, TRPC4, and TRPC6—in platelets and MKs,47-49 all of which are nonselective cation channels and are activated by G-protein–coupled receptors linked to phospholipase C.50,51 Therefore, we further examined the extent to which Ca2+ can permeate the P2Y-receptor–dependent channel. External Na+ was replaced with the large impermeant cation NMDG+, Mg2+ ions were omitted, and Ca2+ was increased to as high a concentration as the cell could withstand (2.5 mM) yet still generate a robust P2Y-receptor–evoked [Ca2+]i increase. These conditions greatly suppressed the current amplitude, as expected for an Na+-permeable conductance; however, we were still able to detect a small initial transient current with a time course similar to that observed in Na+-containing saline and mirroring the initial Ca2+ spike (Figure 6A, representative of 6 cells). This residual current was carried by Ca2+ and not carried by NMDG+ because no detectable ADP-evoked inward current was observed in divalent cation–free NMDG Cl saline (n = 9). Furthermore, the current was also not due to P2X1 receptors because it was observed in the presence of the P2X1 blocker NF449 (1 μM; n = 6).
Phenylarsine oxide (PAO; 15 μM), an agent shown to potentiate a phospholipase C–dependent TRP channel through phosphatidylinositol 4,5-bisphosphate (PIP2) depletion,52 greatly amplified the ADP-evoked inward current in the MK (Figure 6B). This action of PAO was not the result of inhibition of tyrosine phosphatases53 because vanadate (100 μM), another blocker of such enzymes, had no significant effect on the ADP-evoked current (n = 5; P > .05; Figure 6C). PAO also amplified the ADP-evoked current in Na+- and Mg2+-free, 2.5 mM Ca2+ medium (n = 4; P < .05; Figure 6C), further indicating underlying Ca2+ permeability.
Discussion
Activation of platelets by extracellular ADP plays a key role in thrombosis and hemostasis. It is well established that costimulation of P2Y1 and P2Y12 receptors is required for ADP-dependent exposure of fibrinogen-binding sites on the platelet integrin αIIbβ3, leading to the main functional response of aggregation.2,4 However, the mechanism whereby these 2 receptors interact remains poorly resolved. We now show that the functional synergy between P2Y1 and P2Y12 receptors also exists in the primary MK, further supporting direct use of these large cells for studies of platelet signaling. In particular, the MK provides an important tool for investigations of ionic conductances during platelet activation. In the present study, we have focused on understanding the relative role of different P2 receptors in the activation of pathways that conduct cations into the cell during stimulation by ADP. The complete absence of ADP-evoked cation influx currents in the P2Y1–/– MKs demonstrates an essential role for this receptor, but the present work also provides evidence for synergistic roles for P2Y12 and P2X1 receptors. Another important observation within this study is that the P2Y-receptor–evoked conductance, previously shown to be mainly Na+ influx, is also permeable to Ca2+, a key second messenger in the platelet. Therefore, this conductance provides a means whereby P2Y12, P2Y1, and P2X1 receptors can positively interact in the stimulation of Ca2+ influx.
P2Y-receptor–dependent conductance is permeable to Ca2+ and is amplified by phenylarsine oxide. (A) Representative ADP-evoked [Ca2+]i (top panel) increase and whole cell membrane currents at –70 mV in 0 Na+ (NMDG+), 0 Mg2+, 2.5 mM Ca2+ saline with 1 μM NF449. (B) Effect of a 2-minute preexposure to 15 μM phenylarsine oxide on the ADP-evoked membrane currents. (C) Average peak ADP-evoked currents in normal saline (150 mM Na, 1 mM Ca2+) and 2.5 mM Ca2+ saline (0 Na+, 0 Mg2+) with and without pretreatment of 15 μM PAO or 100 μM vanadate. Error bars indicate SEM. *Statistical significance of P < .05 when compared with control.
P2Y-receptor–dependent conductance is permeable to Ca2+ and is amplified by phenylarsine oxide. (A) Representative ADP-evoked [Ca2+]i (top panel) increase and whole cell membrane currents at –70 mV in 0 Na+ (NMDG+), 0 Mg2+, 2.5 mM Ca2+ saline with 1 μM NF449. (B) Effect of a 2-minute preexposure to 15 μM phenylarsine oxide on the ADP-evoked membrane currents. (C) Average peak ADP-evoked currents in normal saline (150 mM Na, 1 mM Ca2+) and 2.5 mM Ca2+ saline (0 Na+, 0 Mg2+) with and without pretreatment of 15 μM PAO or 100 μM vanadate. Error bars indicate SEM. *Statistical significance of P < .05 when compared with control.
The mechanism whereby G-protein–coupled receptors can activate Ca2+-permeable channels remains highly controversial in a variety of nonexcitable cell types, including the platelet. One extensively studied system is the activation of TRP and TRP-like channels by rhodopsin in the Drosophila photoreceptor.54 Considerable note has been taken of this pathway because of the relative ease of manufacturing specific mutants and because TRP channel homologues are the principal candidates for non–voltage-gated Ca2+ influx pathways in mammalian cells. A crucial requirement has been demonstrated for phospholipase C activity in the gating of Drosophila TRP channels by rhodopsin; however, the specific mechanism of channel activation is still unclear.55 In the Drosophila photoreceptor and in mammalian cells, roles have been proposed for second messengers generated by PIP2 hydrolysis, particularly diacylglycerol and products of its own metabolism,56,57 although it is becoming increasingly apparent that multiple signaling modalities are involved.50,58 Indeed, we have previously provided evidence that an increase in cytosolic IP3 (or a metabolite of IP3) can activate a whole-cell cationic conductance and that cytosolic Ca2+ has at least a modulatory role in the ADP-evoked cation channel.26,28,29 Of particular note, it has recently been postulated that a decrease in PIP2 may act as a signal to open TRP channels in the Drosophila photoreceptor.55 This is not surprising given the considerable number of other membrane proteins known to be modulated by the level of PIP2 in the membrane.59 In mammalian cells, the activation of TRPV1 channels by receptors coupled to phospholipase C is greatly potentiated by the depletion of PIP2 levels with PAO.52 We also show that this compound potentiates the initial ADP-evoked cation current (Figure 6) and that, therefore, PIP2 depletion may also play a major role in the activation of P2Y-evoked cation currents in the MK/platelet. Given that a major effect of P2Y12 receptors is the conversion of PIP2 to phosphatidylinositol 3,4,5 triphosphate (PIP3) by PI3-kinase, PIP2 depletion provides a mechanism whereby this Gαi-coupled receptor can potentiate the P2Y1-induced conductance. Other P2Y12 events may contribute, including an increase in PIP3,60 diacylglycerol,48 modulation of the cytoskeleton,60 and activation of lipid kinases such as Btk.60,61 Thus, further studies are required to clarify the relative roles of these different signals and the specific mode of action by PAO. Nevertheless, a role for PIP2 depletion seems plausible, especially given the onset of the current before the increase in cytosolic Ca2+ under control conditions (Figure 4).
The molecular identity of the Ca2+-permeable ion channel underlying the main ADP-evoked conductance in the MK/platelet also remains unknown, but its ability to be amplified by PAO should provide a useful tool in future studies. Evidence to date suggests that Ca2+ influx triggered by P2Y and other Gαq-coupled receptors can occur through second messenger–dependent and store depletion–dependent pathways (termed store-independent and store-dependent Ca2+ influx).62 Previous work has proposed roles for TRPC1 and TRPC6 in Ca2+ influx in the platelet and megakaryocyte,47-49 although the relative contribution by these and other TRP channels remains controversial.63 The main electrophysiologic candidate for the ionic conductance activated by store depletion in the platelet/MK, as in many other nonexcitable cells, is the highly Ca2+-selective CRAC (Ca2+-release-activated Ca2+) conductance.27,28,64 However, this current can only be recorded in the megakaryocyte when the intracellular Ca2+ levels are highly buffered with a chelator such as EGTA or BAPTA (1,2-Bis(2-aminophenoxyl)ethane-N,N,N′,N′-tetraacetic acid)28 ; it is unclear whether this current is active under normal conditions. Furthermore, though the direct depletion of intracellular Ca2+ stores by agents such as thapsigargin leads to Ca2+ influx and thus is a pathway assumed to occur in response to agonists, recent work65 suggests that activation of IP3 receptors, rather than store depletion, may be the crucial transducing event required for activation of this influx component. Thus, considerably more work is required to identify the agonist-evoked Ca2+-permeable ion channels in the platelet. The present study provides the first direct electrophysiologic evidence for a Ca2+ current linked to a G-protein–coupled receptor in the platelet/MK under physiologic Ca2+ buffering conditions. Because of the small Ca2+ permeability, the Ca2+ current was only clearly visible when the conductance was at its largest, during the large initial [Ca2+]i increase. In physiologic salines in which Na+ is the main current carrier, this conductance also continued as a smaller sustained current in many recordings throughout the plateau phase of the [Ca2+]i increase. We interpret this as evidence for a pathway that provides biphasic Ca2+ and Na+ influx during stimulation of P2Y receptors, with initial and sustained phases.
Although we observed marked effects of P2Y12 and PI3-kinase blockade on the ADP-evoked membrane currents, we did not observe significant effects on the intracellular Ca2+ increase evoked by ADP in the MK. This is in contrast to the recent findings by Hardy et al,54 who report a reduction in ADP-evoked Ca2+ increases after the inhibition of P2Y12 receptors or PI3-kinase in human platelets. In part, this may reflect the heterogeneity of the MK Ca2+ responses and the inability to detect small changes in responses averaged from single-cell experiments compared with measurements in platelet populations. However, it may also result from a greater contribution of Ca2+ release relative to influx in the MK because of the larger cellular volume compared with the platelet.28,66 In the platelet, it is likely that P2Y12/PI3-kinase–dependent potentiation of a nonselective cation conductance could significantly contribute to the synergy reported between P2Y1 and P2Y12 during ADP-stimulated Ca2+mobilization54 and is worthy of further investigation.
In comparison to the well-established roles for P2Y1 and P2Y12 receptors in platelet activation, the importance of the P2X1 receptor has been less clear. Recent work has shown that P2X1 receptors contribute to the risk of thrombosis, particularly in small arteries,1,3,67 but the mechanisms by which this receptor can influence platelet activation is unclear. The P2X1-dependent priming of the P2Y1 membrane current (Figure 4) may represent a means of accelerating P2Y1-receptor responses, as we have observed in platelet suspensions.25 Furthermore, the costimulation of P2Y1 and P2Y12 induces repetitive autocrine activation of P2X1 receptors, with a role for PI3-kinase. Because ADP is not directly an agonist at the P2X1 receptor7 and our ADP samples were treated to remove ATP contamination (confirmed by luciferin/luciferase measurements), the P2X1 responses we observed must have been caused by ATP secretion. It was interesting that multiple P2X1 responses could occur in the same cell; this must reflect focal activation by released quanta of ATP. This type of local activation has also been observed in PC12 cells68 and human platelets,43 and it provides a means whereby P2X1 stimulation can be sustained. Autocrine activation may allow the P2X1 receptor to signal in a prolonged and more efficient manner compared with the on-off activation observed after exogenous α,β-meATP.1,3,25,26,45
Proposed mechanisms for P2-receptor–dependent activation of nonselective cation channels in the megakaryocyte and platelet. ADP activates an Na+- and Ca2+-permeable cation channel, likely to be a member of the TRP family of ion channels, by combined stimulation of P2Y1 and P2Y12 receptors. (1) P2Y1 activation of PLCβ hydrolyzes PIP2, resulting in the removal of an inhibitory action on the cation channel. (2) Simultaneously, P2Y12 activates PI3-kinase by its Gβγ subunits, resulting in the phosphorylation of PIP2 to PIP3, further decreasing the inhibition by PIP2. (3) PIP3 may be an activator of this or another cation channel.60 (4) Both diacylglycerol (DAG) and IP3 (or an IP3 metabolite) have been reported to activate cation channels in the platelet/MK.26,28,29,48 IP3 results in the release of stored Ca2+, which further modulates the cation channel.26 (5) P2Y12 receptors, through PI3-kinase, stimulate dense granule release into the open canalicular system in the platelet/demarcation membrane system in the MK (OCS/DMS); P2Y1-dependent protein kinase C (PKC) stimulation is also likely to potentiate this secretion. (6) Released ATP can repetitively activate P2X1 receptors and further modulate the P2Y current through Ca2+ influx. MLCK indicates myosin light-chain kinase.
Proposed mechanisms for P2-receptor–dependent activation of nonselective cation channels in the megakaryocyte and platelet. ADP activates an Na+- and Ca2+-permeable cation channel, likely to be a member of the TRP family of ion channels, by combined stimulation of P2Y1 and P2Y12 receptors. (1) P2Y1 activation of PLCβ hydrolyzes PIP2, resulting in the removal of an inhibitory action on the cation channel. (2) Simultaneously, P2Y12 activates PI3-kinase by its Gβγ subunits, resulting in the phosphorylation of PIP2 to PIP3, further decreasing the inhibition by PIP2. (3) PIP3 may be an activator of this or another cation channel.60 (4) Both diacylglycerol (DAG) and IP3 (or an IP3 metabolite) have been reported to activate cation channels in the platelet/MK.26,28,29,48 IP3 results in the release of stored Ca2+, which further modulates the cation channel.26 (5) P2Y12 receptors, through PI3-kinase, stimulate dense granule release into the open canalicular system in the platelet/demarcation membrane system in the MK (OCS/DMS); P2Y1-dependent protein kinase C (PKC) stimulation is also likely to potentiate this secretion. (6) Released ATP can repetitively activate P2X1 receptors and further modulate the P2Y current through Ca2+ influx. MLCK indicates myosin light-chain kinase.
In conclusion, we have established that the marrow-derived MK displays fully functional interactions between platelet-P2Y receptors, thereby supporting use of the progenitor cell for studies of platelet signaling pathways, particularly ion channels. We show that both P2Y1 and P2Y12 are required for complete activation of a nonselective cation channel that results in Ca2+ and Na+ entry. Furthermore, P2X1 receptors, after the secretion of ATP, can contribute repetitively to the ADP-evoked currents and can act to accelerate the P2Y-receptor currents. This complex interplay, as described in the model (Figure 7), may allow all 3 receptors to cooperate in the generation of Ca2+ influx during platelet activation.
Prepublished online as Blood First Edition Paper, May 24, 2005; DOI 10.1182/blood-2005-02-0725.
Supported by the British Heart Foundation (FS/02/057/14377) and the Wellcome Trust.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. ADP evokes multiple inward cationic currents through mechanisms that require P2Y1 receptors. Simultaneous whole-cell patch clamp and [Ca2+]i recordings at –70 mV under conditions that eliminate K+-selective currents. (A) Typical biphasic inward current and [Ca2+]i increase activated by 30 μM ADP. Dashed line shows the background current level. (B) Another cell displaying typical repetitive inward currents observed in many recordings during exposure to ADP. These repetitive events had rapid kinetics, as shown in the expanded section. (C) Inward currents and [Ca2+]i increases were not observed in response to 30 μM in P2Y1–/– MKs. (D) Frequency of occurrence (events per minute) of the repetitive transient inward currents for WT and P2Y1–/– MKs before (□) and during (▦) exposure to 30 μM ADP. Error bars represent SEM. *Statistical significance of P < .05 compared to WT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-02-0725/6/m_zh80170583530002.jpeg?Expires=1768047345&Signature=G9i9Ed7oFYmyUfqtv3j0vn4nKaM2jcWeJmoZyvcurhm~fktrkAqPAoqT0my4AoeB4ipEZxI3XyQYFOEtSGNAMJuL4a1fZXejnB6OP~WB3OmaRzHwNJskDR38E0J6CCfkNAeI-fsgznkFgX-1gAiHxw7~bu7-9btbwSIrHUXYWlpXhmBm7LDCAWaMgDWCQ8Uze1C1rKpNso17WhVS9Izzn0cfG8E2DZ42~57cN7v~IfBCW7lj9pxyhHXMllQ5LEEmI9JJxkS~33vb-vOUh2Vl-ZgXatYi9ZRPbryvSj6Qz97hANCL14z1Iu6M-IIh2M6HOKpc5BilUWaDtBbYn-iawQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Transient repetitive currents in the presence and absence of exogenous ADP are mainly caused by P2X1 receptors. (A) Scatter plot of activation and inactivation kinetics for the repetitively activated inward currents observed during application of 30 μM ADP. Each point shows the time to peak current and the inactivation time constant for a single transient event when this could be fitted by a single exponential decay. Data from 15 cells. (B) Frequency of occurrence (events per minute) of all transient currents during exposure to 30 μM ADP for control, P2X1–/– MKs, after preexposure to 10 μM α,β-meATP and in the presence of 1 μM NF449. (C) Example of the slower transient events and concurrent [Ca2+]i transient events evoked by ADP in the absence of P2X1 receptors. (D) Example of the spontaneous [Ca2+]i increases and transient inward currents displaying a mixture of rapid and slow current events. (E) Frequency of all spontaneous current transient events in control cells, P2X1–/– MKs (n = 11), after exposure to 10 μM α,β-meATP (n = 7), and in the presence of 1 μM NF449 (n = 7). Error bars represent SEM. Statistical significance compared with control (*P < .05; **P < .01; ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-02-0725/6/m_zh80170583530003.jpeg?Expires=1768047345&Signature=D2ViH3qObTIbW3OSfTtHbVdIcIel47BE79gYnuENSy3hubtb0J4mmt1hS-8l5hW~ZAbMAkAbRepPRUy-08fgGYtoJEetrZsnvKTU5JDwRaCJeXMuRe6XZAvUDWwAWDiJoI7V-SB1JcHwPrw8Cxvay3wogFswUT-kzih5HMWuADL45K9gZVJySBbxbGnJA9cIcZEOuDcUc9y02brdbhGxknndc06sQkLoCuFRyxpbrnZpb~jpQVfCrTVHbJApFTL3GzqoucmcnbaIbnfCb1GNzxq7jSmmkUNaXVee~XYEGEsnG2ETQEISksjIvyL1OsozYXahrYHxJAnC64GrlLAApw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Priming of the initial ADP-activated inward current by P2X1 receptors. Membrane currents and intracellular Ca2+ responses were recorded simultaneously at –70 mV in response to 30 μM ADP. (A) Representative recordings of the initial increase in intracellular [Ca2+] (top panel) and whole-cell current (lower panel) in WT and P2X1–/– MKs. (B) Expanded section of the traces showing current activation before the [Ca2+]i increase in a WT MK but at or later than the [Ca2+]i response in a P2X1–/– MK. (C) Average onset of the ADP-stimulated current relative to the first detectable [Ca2+]i increase in control (n = 9), P2X1–/– MKs (n = 8), in the presence of 1 μM NF449 (n = 5), and after preexposure to 10 μM α,βmeATP (n = 5). Error bars represent SEM. *Statistical significance of P < .05 when compared with control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-02-0725/6/m_zh80170583530004.jpeg?Expires=1768047345&Signature=b7clJdmb20OYMsgSjC3z7EUZW-S6pugEZ5FrzACn4q0QqKR~XTbi2NElWAQlqnzad5TtEFpFAY3C4uvEmc4-FXcB2-pFPVQWYfDvE8AP6zkGBl73~wwXYxDEwD-jKq8nLhNshlTnTrHqwem8sMxfBQFFEqgxxAkSuiM6DIakzxmSH~rdf3tXUVemqPVS88xtX8CDumM8xPTSxK6wAOgdKzsw7OWLIWf4iCzgtmCUKXlhBhsjVYaYRLOPblKAU-QSim508VL16nNMIL~GAYGpeHjkjDkPRuHJnJJloWh6Bj2mFasF4HA-wseNKXMYr3NScz77JDznhAqItriTlxdaTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. P2Y-receptor–dependent conductance is permeable to Ca2+ and is amplified by phenylarsine oxide. (A) Representative ADP-evoked [Ca2+]i (top panel) increase and whole cell membrane currents at –70 mV in 0 Na+ (NMDG+), 0 Mg2+, 2.5 mM Ca2+ saline with 1 μM NF449. (B) Effect of a 2-minute preexposure to 15 μM phenylarsine oxide on the ADP-evoked membrane currents. (C) Average peak ADP-evoked currents in normal saline (150 mM Na, 1 mM Ca2+) and 2.5 mM Ca2+ saline (0 Na+, 0 Mg2+) with and without pretreatment of 15 μM PAO or 100 μM vanadate. Error bars indicate SEM. *Statistical significance of P < .05 when compared with control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-02-0725/6/m_zh80170583530006.jpeg?Expires=1768047345&Signature=eYa4UXbZv-1xdo6vgxEbRgEal7NEHuMn0U23vUAiVr2mT8OirPRxPFtv-tvOpLSQBvVYIGweaNWqUwCHOZCA5NAAFMuO06r3KGN5j~Iy4iK6ke2JDhVsO5rbnuVZvcBKH8fwJ8oZXdIpAMZ6i3LFRWDoIHuXvfqPJ66oe49c3~ELUt9rCayx~SMAKCPaqindXZIzNCmYQobKIk50CrNP-xotY5U1DtcQtcythmghdCjfz5WKMKiW0X~fXgoOBFuzjsBYUGq3F~BvTcPytxTZjA-4YD9A8Dh9UrOxi-8Z~T2D5EJ6BHb2oeyJ4c1KNv-L7mwGFQn8z0D3EMhqBLP44g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

