Abstract
Decreased bone formation contributes to the development of bone lesions in multiple myeloma (MM) patients. In this study, we have investigated the effects of myeloma cells on osteoblast formation and differentiation and the potential role of the critical osteoblast transcription factor RUNX2/CBFA1 (Runt-related transcription factor 2/core-binding factor Runt domain α subunit 1) in the inhibition of osteoblastogenesis in MM. We found that human myeloma cells suppress the formation of human osteoblast progenitors in bone marrow (BM) cultures. Moreover, an inhibitory effect on osteocalcin, alkaline phosphatase, collagen I mRNA, protein expression, and RUNX2/CBFA1 activity by human preosteoblastic cells was observed in cocultures with myeloma cells. The inhibitory effect was more pronounced in the cell-to-cell contact conditions compared with those without the contact and involved the very late antigen 4 (VLA-4) integrin system. Among the soluble osteoblast inhibitors screened, we show the potential contribution of interleukin-7 (IL-7) in the inhibitory effect on osteoblast formation and RUNX2/CBFA1 activity by human myeloma cells in coculture. Finally, our in vitro results were supported in vivo by the finding of a significant reduction in the number of Runx2/Cbfa1-positive cells in the BM biopsies of patients with MM who had osteolytic lesions compared with those who did not have bone lesions, suggesting the critical involvement of RUNX2/CBFA1 in the decreased bone formation in MM. (Blood. 2005;106:2472-2483)
Introduction
Multiple myeloma (MM) is a plasma-cell malignancy characterized by the high capacity to induce osteolytic bone lesions.1-3 Bone destruction in MM depends mainly on the increase of osteoclast formation and activity that occurs in close contact with myeloma-cell infiltration.3,4 The histomorphometric studies, performed in patients with MM, have demonstrated that patients with MM who have high plasma-cell infiltrate are also characterized by a lower number of osteoblasts and decreased bone formation that contributes to the development of bone lesions.3,5 Unbalanced bone remodeling with increased bone resorption and low bone-formation rate is the characteristic feature of the majority of patients presenting with lytic bone lesions.5 On the contrary, the presence of balanced bone remodeling with increased osteoclastogenesis and normal or increased bone formation is the usual feature of patients without bone osteolysis.5,6 On the basis of the histomorphometric studies, the inhibitory effect of myeloma cells on osteoblast formation seems to be a critical point in the development of lytic bone lesions.
The biologic mechanisms by which human myeloma cells inhibit osteoblast formation and differentiation have not been completely understood. Recently, the involvement of the Wnt signaling inhibitor Dickkopf1 (DKK1) has been postulated in this process, as suggested by the relationship between DKK1 production by myeloma cells and the presence of bone lesions in patients with MM.7 However, other molecules and/or mechanisms could be involved in the inhibition of bone formation in MM. Several factors have been identified as potential osteoblast inhibitors in both the mouse and human systems, such as the secreted Frizzled-related protein 1 (sFRP-1), sFRP-2, sFRP-3, sFRP-4,8,9 noggin,10,11 gremlin,12 and interleukin-7 (IL-7).13 Recently, we have demonstrated the presence of high IL-7 levels in the bone marrow (BM) plasma of patients with MM and the involvement of IL-7 in osteoclast activation through the up-regulation of receptor activator of nuclear factor-κ B ligand (RANKL) by T cells14 ; however, the role of IL-7 in the inhibition of bone formation in MM is not known.
In human BM models, pluripotential fibroblast colony-forming units (CFU-Fs) have long been recognized as the early cell source of osteoblast progenitors, which then give rise to cells capable of forming mineralized nodules in vitro (bone-nodule CFUs [CFU-OBs]).15,16 CFU-F colonies are able to differentiate in vitro to osteoblastic cells as well as to adipocytes, endothelial cells, and chondrocytes in the presence of specific growth factors.15 In physiologic condition, the formation and differentiation of osteoblastic cells from BM mesenchymal/stromal cells is critically regulated by the transcription factor RUNX2/CBFA1 (Runt-related transcription factor 2/core-binding factor Runt domain α subunit 1).17-21 Indeed, the activation of RUNX2/CBFA1 in human BM stromal and preosteoblastic cells (PreOBs) induces the expression of the osteoblast markers collagen, alkaline phosphatase, and osteocalcin during the different steps of osteoblast maturation.20-22 Moreover, RUNX2/CBFA1-deficient mice (Runx2-/-) completely lack osteoblast and bone formation,21,23 demonstrating that Runx2/Cbfa1 has a critical role in this process.
On the basis of the evidence in this study, we have investigated the effect of human myeloma cells on osteoblast formation and differentiation in human BM cultures and the potential role of Runx2/Cbfa1 in the myeloma-induced inhibition of bone formation. We have also identified potential osteoblast inhibitors involved in the osteoblastogenesis suppression induced by myeloma cells.
Patients, materials, and methods
Reagents and antibodies
Recombinant human (rh) IL-6 and rhIL-7 were obtained by Endogen (Woburn, MA). rhDKK1 and sFRP-3 were purchased from R&D Systems (Minneapolis, MN). Dexamethasone (Dex), ascorbic acid, and beta-glycerophosphate were obtained from Sigma Aldrich (St Louis, MO). RPMI-1640 and α-modified essential medium (α-MEM) culture media, glutamine, penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Invitrogen Life Technologies (Milan, Italy).
Cells and cell-culture conditions
Cell lines. Human myeloma cell lines (HMCLs) XG-6, XG-1, and JJN3 were obtained from Dr Regis Bataille (Nantes, France). U266 and the Epstein-Barr virus (EBV)-infected B95.8 cells were obtained from the American Type Culture Collection (Rockville, MD). OPM2 and RPMI-8226 were purchased from DSM (Braunschweig, Germany). Human trabecular simian virus 40 (SV-40)-transfected osteoblasts (HOBITs) were a generous gift from Dr B. Lawrence Riggs (Mayo Clinic, Rochester, MN). Immortalized mesenchymal/stromal cell line was kindly gifted from Dr Giuseppe Gaipa (Monza, Italy) and Dr Dario Campana (Memphis, TN). These cells were developed from unfractionated BM mononuclear cells (BMMNCs) from a healthy donor and were transduced with telomerase reverse transcriptase. They maintain the capacity to grow for more than 2 years without alterations in the cell behavior and gene expression.24
Human BM cultures, osteoprogenitors cells (PreOBs), and cocultures. BMMNCs were obtained from healthy subjects by aspiration from the iliac crest and isolated by Ficoll-Hypaque density sedimentation. BM cells were seeded in 6-well plates at the density of 106/well in 4 mL α-MEM medium with 15% fetal calf serum (FCS), ascorbic acid (50 μg/mL), and Dex (10-8 M). The cells were then cocultured in the presence or absence of several HMCLs (XG-1, XG-6, RPMI-8226, U266, OPM2, and JJN3; 2.5 × 105/well); freshly purified CD138+ MM cells or normal purified BM CD20+ B lymphocytes, with or without a transwell insert (0.45-μM pore size, Falcon; Becton Dickinson, San Jose, CA); or BM plasma obtained from patients with MM (dilution 1:3). Blocking polyclonal anti-IL-7 antibody (Ab, 0.5 μg/mL; Peprotech, London, United Kingdom), blocking anti-DKK1 monoclonal antibody (mAb, 5 μg/mL; R&D Systems), or anti-immunoglobulin G (IgG) control Ab was added to the cocultures in some experiments. BM cocultures were maintained for 14 days for CFU-F formation and for 21 days for CFU-OB formation (adding 10 mM beta-glycerophosphate); half of the medium was replaced with fresh medium every 3 days. In other experiments, BMMNCs were maintained in the presence or absence of rhIL-7 (20 pg/mL-20 ng/mL) or rhDKK1 (20-100 ng/mL) in α-MEM medium for 14 to 21 days for CFU-F and CFU-OB evaluation, respectively.
PreOBs were obtained as previously described25 either from primary BM adherent cells after the attachment period (3-5 days) or from an immortalized human mesenchymal/stromal cell line. Briefly, cells were incubated in α-MEM medium with 15% FCS and 2 mM glutamine in the presence of ascorbic acid (50 μg/mL) and Dex (10-8M) for 2 weeks. A series of coculture experiments were performed with confluent adherent PreOBs (2 × 106) and HMCLs with the Epstein-Barr virus (EBV)-positive cell line B95.8, freshly purified MM CD138+ cells, or normal BMMNCs (10 × 106 cells) added directly or placed in a transwell insert for 24 to 48 hours; the experiments were performed in RPMI-1640 medium with 2% FCS or pretreated for 20 minutes in the presence of blocking anti-very late antigen 4 (VLA-4) mAb (10 μg/mL; R&D Systems) or anti-IgG control. The antibodies were maintained for the entire coculture period. In some experiments, at the end of culture period, cocultures were depleted of myeloma cells using a negative immunoselection with anti-CD138 mAb (magnetic activated cell sorting [MACS]).
Moreover, confluent PreOBs were incubated in the presence or absence of rhIL-7 (20 pg/mL-20 ng/mL), rhDKK1 (20-100 ng/mL), and sFRP-3 (30-300 pg/mL) for 24 to 48 hours.
CFU-F and CFU-OB assays. For CFU-F evaluation, cells were fixed with cold formalin at 4°C, washed 3 times with PBS, and stained with alkaline phosphatase semiquantitative histochemical kit (Sigma Aldrich) according to the manufacturer's procedures. Each colony was defined by the presence of at least 50 alkaline phosphatase-positive cells and quantified by direct counting of all positively stained colonies. In some experiments, the quantification of alkaline phosphatase staining was performed using 1D Image Analysis Software (Kodak Digital Science, Rochester, NY). For CFU-OB evaluation, cells were fixed with a 1:1 mixture (vol/vol) of 37% formaldehyde and ethanol for 5 minutes, washed 3 times with PBS, and stained for 10 minutes with a solution of 2% of alizarin red (Sigma Aldrich) at pH 4.2. Colonies were quantified by direct counting of all stained bone nodules positive for alizarin red under light microscopy. Images were visualized under an Eclipse TE 300 microscope with a digital DS-U1 sight (Nikon Instruments, Florence, Italy). Alizarin red staining identifies osteoblast-differentiated cells able to mineralize as previously described.25
Cytotoxicity and apoptotic assay. Cell viability of BM-adherent cells in long-term (2-3 weeks) cocultures and of PreOBs in short-term (24-48 hours) cocultures was assessed by a colorimetric method (Cell Counting Kit-8; Alexis Biochemicals, Lausen, Switzerland) using tetrazolium salt according to the manufacturer's procedure. The number of deaths and apoptotic PreOB cells in short-term coculture has also been evaluated by flow cytometry with the intercalating DNA-dye 7-aminoactinomycin D (7-AAD) and the Apo 2.7 mAb (Becton Dickinson, San Jose, CA) gating on the CD138- cells.
Osteocalcin and alkaline phosphatase quantitative detection. Soluble osteocalcin levels in cocultures were detected using an enzyme-linked immunosorbent assay (ELISA) purchased from Nordic Biosciences Diagnostic (Herlev, Denmark). Alkaline phosphatase levels in the cultures were detected by a fluorimetric assay on cell lysates (StarBright Alkaline Phosphatase Detection kit; MT-1000; Sigma Aldrich) according to the manufacturer's procedures.
Patients
We studied 40 patients with newly diagnosed or relapsed MM in stages I to III. All patients underwent total-body x-rays to identify the presence of osteolytic bone lesions and/or diffuse osteoporosis. Patients whose standard x-rays were negative underwent magnetic resonance imaging (MRI) scans. Bone disease was defined on the basis of the presence of one or more lesions that appeared on the x-rays or MRI scan. BM aspirates and bone biopsies were obtained from the iliac crest of all patients with MM at diagnosis or in relapse after informed consent according to the Declaration of Helsinki. Study protocols were approved by the University of Parma institutional review board (Parma, Italy). Patients in relapse were excluded from the analysis if treated in the last 6 months with steroid or bisphosphonates. BM plasma was obtained from BM aspirate (10 mL) treated with EDTA (ethylenediaminetetraacetic acid) to prevent clotting; BM aspirates were centrifuged at room temperature, and 3 mL plasma was separated from the buffy coat, harvested, and stored in sterile vials at -80°C until the analysis. Before testing plasma in the cultures, an amount of BM plasma was checked for sterility, viral markers, and protein concentration. Only sterile and virus-free samples were used.
Fresh CD138+ plasma cells were purified from isolated mononuclear cells with an immunomagnetic method using anti-CD138 mAb-coated microbeads (MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany) as previously described.14 Only samples with a purity of more than 90%, checked by flow cytometry, were considered. Fresh CD138+ MM cells were analyzed immediately after purification.
RNA isolation and reverse-transcriptase-polymerase chain reaction (RT-PCR) amplification
For RT-PCR analysis, total cellular RNA was extracted from cells using Trizol reagent (Invitrogen Life Technologies). RNA(1 μg) was reverse-transcribed with 400 U Moloney murine leukemia virus reverse transcriptase (Invitrogen Life Technologies) according to the manufacturer's protocol. cDNAs were amplified by PCR using specific primer pairs. For Noggin amplification, mRNA was pretreated using DNasi (Invitrogen Life Technologies) to avoid DNA contamination because the gene has no intron regions. PCR reactions were performed in a thermal cycler (MiniCycler; MyResearch, Watertown, MA) for 30 cycles. Specific primer pairs have been used as reported in Table 1. The following positive controls have been used for the PCR reactions: sFRP-1, sFRP-2, sFRP-3, sFRP-4; fetal and adult human cardiomiocytes, noggin; human amniotic cells, gremlin; osteoblast-like cell line MG-63 stimulated with rh bone morphogenetic protein 2 (rhBMP-2, 100 ng/mL; Peprotech) and DKK1; and HOBIT. Pictures of the electrophoresed cDNAs were recorded and quantified as previously described.25
Western blot analysis
Western blot analysis was performed as previously described.14 The following primary Abs were used: polyclonal anti-collagen I, anti-sFRP-2, anti-sFRP-3, and anti-DKK1 (all at 1:100; all from Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin mAb (1:5000; Sigma Aldrich). Runx-2/Cbfa1 protein expression was detected on 40 μg nuclear extracts, prepared using the Nuclear Extraction kit (Active Motif, Carlsbad, CA) according to manufacturer's protocol. Either a rabbit polyclonal anti-Runx2/Cbfa1 Ab (sc-10758, dilution 1:100; Santa Cruz Biotechnology) or a goat polyclonal anti-Runx2/Cbfa1 Ab (sc-8566, 1:100; Santa Cruz Biotechnology) was used as the primary Ab.
Gel mobility shift assay (EMSA)
Gel mobility shift assay was performed using a Nushift AML-3/Runx2 kit (Active Motif). Briefly, Runx2/Cbfa1 binding consensus oligonucleotide probes (OSE2: 5-AGCTGCAATCACCAACCACAGCA-3) were annealed by heating to 95°C and cooling to 25°C in 50 mM Tris (tris(hydroxymethyl)-aminomethane)-HCl (pH 8.2), 10 mM MgCl2, and 1 mM EDTA, end-labeled with [32P] deoxycytidine triphosphate (dCTP) and purified through a G-25 purification column (Active Motif). Nuclear extracts (20 μg) were incubated on ice for 10 minutes in binding buffer (20 mM HEPES-HCl, 0.1 M KCl, 1 mM dithiothreitol [DTT], 1 mM EDTA, 0.5 mg/mL bovine serum albumin [BSA], 50 μg/mL poly(dI/dC) (polydeoxyinosinic/deoxycytidylic acid; ____), and 20% glycerol, pH 7.9) with 10 fmol (2 × 104 cpm) 32P-labeled double-stranded oligonucleotide probes in a total volume of 20 μL. In competitive binding reactions, unlabeled wild-type oligonucleotide was added before the 32P probe. Protein-DNA complexes were resolved in 5% nondenaturing polyacrylamide gels in Tris-glycine buffer (25 mM Tris, 192 mM glycine, pH 8.3) at 20°C, 25 mA, and 200 V for 1.5 hours. Supershift experiments contained 0.2 μg Runx2/Cbfa1 polyclonal rabbit Ab supplied with the kit. Gels were dried and analyzed by autoradiography.
Immunohistochemistry
Immunohistochemical staining was performed on BM biopsies, obtained from 40 patients with MM who either had osteolysis (n = 24) or did not have bone lesions (n = 16). All samples were fixed in B5-formalin mixture, decalcified by EDTA, and embedded in paraffin. Serial sections of 3-μm thick were processed for immunohistochemical staining with goat anti-RUNX2/Cbfa1 ligand (CD178) Ab (working dilution, 1:100; DAKO, Glostrup, Denmark) using the immunoperoxidase technique as previously described.24 As negative control, primary antibodies were omitted or replaced with an isotype control-matched mAb with irrelevant specificity. The total trabecular area of each section was measured by a dedicated software analyzer (Image Pro Plus 4.5; Media Cybermetics, Silver Spring, MD) after the acquisition of bioptic images by FOTOVIX (TAMRON; Tamron, Commack, NY). Osteoblastic cells and osteoid surface were identified in the BM biopsies using morphologic criteria, as previously described.5,6
Statistical analysis was performed using Student t test. P values less than .05 were considered significant. Results are expressed as mean plus or minus standard error (SE).
Microscopy was performed with an Olympus B×60 F5 microscope equipped with a UPlan Fi 100×/1.30 oil iris, WH10×/22 (Olympus, Tokyo, Japan). An Olympus DP10 digital microscope camera system was used for image acquisition.
Results
Effect of human myeloma cells on CFU-F and CFU-OB formation in human BM cultures
In long-term cell-to-cell contact coculture system with healthy BMMNCs, we found that HMCLs (RPMI-8226, OPM2, XG-6, and XG-1) significantly inhibited CFU-F formation after 14 days (overall % mean ± SD decrease vs control: -73% ± 13%, P < .001; RPMI-8226: -95% ± 18%, P < .001; OPM2: -80% ± 16%, P < .001; XG-6: -70% ± 14%, P < .01; XG-1: -50% ± 10%, P < .01). A similar inhibitory effect on CFU-F formation was observed with U266 (-62.5 ± 11%, P < .01) and JJN3 (-68 ± 10%, P < .01) (Figure 1A).
Similarly, we found that purified BM MM CD138+ cells significantly inhibited CFU-F formation in coculture (overall % mean ± SD decrease vs control: -55% ± 11%, P < .01). Figure 1B shows the inhibitory effect on the number of CFU-Fs/well observed in cocultures by MM cells of 4 different representative patients (% mean ± SD decrease vs control: MM1: -59% ± 9%, MM2: -61% ± 10%, MM3: -50% ± 13%, MM4: -38% ± 12%; P < .01). On the contrary, purified BM B lymphocytes from healthy donors did not inhibit CFU-F formation.
When in some coculture experiments we compared the effect of myeloma cells on CFU-F formation in the presence and absence of a transwell insert, we found a more pronounced effect in the cell-to-cell contact condition (% mean ± SD decrease vs control: XG-6: -76% ± 10%; RPMI-8226: -92% ± 11%) compared with the condition without the cellular contact (XG-6: -47% ± 9%; RPMI-8226: -54% ± 12%) (P < .05) as shown for XG-6 and RPMI-8226 (Figure 1C).
Figure 1D shows at higher magnification the inhibitory effect of 2 representative HMCLs (RPMI-8226 and XG-6) in coculture using alkaline phosphatase staining.
To further investigate the effect of myeloma cells on osteoblastogenesis, we examined the formation of colonies more differentiated toward the osteoblast phenotype. We found that HMCLs significantly inhibited CFU-OB formation after 21 days of culture (overall % mean decrease ± SD vs control: XG-6: -58% ± 11%, P < .001; RPMI-8226: -64% ± 14%, P < .001; XG-1: -57% ± 10%, P < .01; OPM2: -75% ± 11%, P < .001; XG-1: -36% ± 6%, P < .01) (Figure 2A). As observed for the CFU-Fs, the inhibitory effect of HMCLs on CFU-OB formation was more pronounced in the cell-contact condition compared with the presence of a transwell insert (data not shown). An inhibitory effect on CFU-OB formation was also observed with freshly purified CD138+ MM cells as shown for 2 different representative patients (MM1: -45% ± 10%, P < .05; MM2: -88% ± 18%, P < .01) but not with purified BM CD20+ B lymphocytes from healthy donors (P = not significant [NS]) (Figure 2B).
Figure 2C shows the effect of XG-6 and RPMI-8226 on bone nodule formation, stained with alizarin red for representative fields at high magnification.
Effect of human myeloma cells on CFU-F formation in human BM cultures. Human BM mononuclear cells were cocultured in the appropriate medium with (A) human myeloma cell lines (HMCLs; RPMI-8226, OPM2, XG-6, XG-1, U266, and JJN3) and (B) BM MM cells obtained from 4 different patients with MM (MM1, MM2, MM3, MM4), or with BM B lymphocytes from healthy donors. (C) In some experiments, HMCLs were incubated in the presence or absence of a transwell insert. CFU-F formation was evaluated after 14 days as described in “Patients, materials, and methods” and quantified. Graphs represent the mean number of CFU-Fs/well ± SD of 6 independent experiments performed in triplicate. (D) Alkaline phosphatase staining was performed in BM cocultures at higher magnification (images were obtained on a Nikon Eclipse TE 300 microscope at 10×/18 using a DS-U1 digital sight and a 4×/0.12 objective lens. (Nikon Instruments). (original magnification, × 5). C indicates control; *P < .01; **P < .001.
Effect of human myeloma cells on CFU-F formation in human BM cultures. Human BM mononuclear cells were cocultured in the appropriate medium with (A) human myeloma cell lines (HMCLs; RPMI-8226, OPM2, XG-6, XG-1, U266, and JJN3) and (B) BM MM cells obtained from 4 different patients with MM (MM1, MM2, MM3, MM4), or with BM B lymphocytes from healthy donors. (C) In some experiments, HMCLs were incubated in the presence or absence of a transwell insert. CFU-F formation was evaluated after 14 days as described in “Patients, materials, and methods” and quantified. Graphs represent the mean number of CFU-Fs/well ± SD of 6 independent experiments performed in triplicate. (D) Alkaline phosphatase staining was performed in BM cocultures at higher magnification (images were obtained on a Nikon Eclipse TE 300 microscope at 10×/18 using a DS-U1 digital sight and a 4×/0.12 objective lens. (Nikon Instruments). (original magnification, × 5). C indicates control; *P < .01; **P < .001.
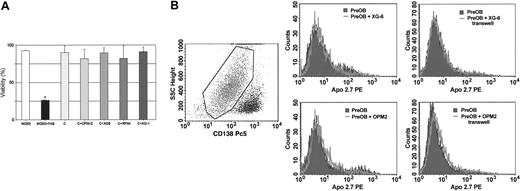
To exclude that the inhibitory effect observed on CFU-Fs and CFU-OBs in our cocultures could be due to toxicity, we have evaluated the viability of BM adherent cells in the presence and absence of myeloma cells using a cytotoxic assay. The viability of BM cells was evaluated after 1, 2, and 3 weeks of coculture. No significant reduction of BM adherent-cell viability was observed at any time point. Figure 3A shows the percent of cell viability at 3 weeks of coculture with OPM2, XG-6, RPMI-8226, and XG-1.
Effect of human myeloma cells on osteoblast differentiation markers in cocultures
The effect of human myeloma cells on the osteoblast differentiation markers has also been investigated. We performed a coculture system with HMCLs and confluent human PreOBs obtained both from primary BMMNCs and from immortalized mesenchymal/stromal cell line. First, we tested the viability of PreOBs by flow cytometry, confirming that no toxic or apoptotic effect was present in PreOBs in our cocultures either in the presence or absence of a transwell insert as shown for XG-6 and OPM2 in Figure 3B-C.
Effect of human myeloma cells on bone nodules formation in BM cultures. Human BM cells were cocultured in the appropriate medium with (A) HMCLs (RPMI-8226, XG-1, XG-6, OPM2) and (B) freshly purified MM cells of 2 different patients (MM1 and MM2), or with normal BM B lymphocytes. Colony-forming bone nodules (CFU-OBs) were stained with alizarin red after 21 days and counted at the light microscopy as described in “Patients, materials, and methods.” Graphs represent the mean number of CFU-Fs/well ± SD of 6 independent experiments performed in triplicate. (C) CFU-OBs stained with alizarin red (original magnification, × 5) of representative fields. Graphs show the mean ± SD of bone nodules/field. C indicates control; *P < .05; **P < .01. Images were obtained as described for Figure 1.
Effect of human myeloma cells on bone nodules formation in BM cultures. Human BM cells were cocultured in the appropriate medium with (A) HMCLs (RPMI-8226, XG-1, XG-6, OPM2) and (B) freshly purified MM cells of 2 different patients (MM1 and MM2), or with normal BM B lymphocytes. Colony-forming bone nodules (CFU-OBs) were stained with alizarin red after 21 days and counted at the light microscopy as described in “Patients, materials, and methods.” Graphs represent the mean number of CFU-Fs/well ± SD of 6 independent experiments performed in triplicate. (C) CFU-OBs stained with alizarin red (original magnification, × 5) of representative fields. Graphs show the mean ± SD of bone nodules/field. C indicates control; *P < .05; **P < .01. Images were obtained as described for Figure 1.
In these coculture systems, we found that XG-6, RPMI-8226, and OPM2, but not the EBV-positive cell line B95.8 or normal BMMNCs, reduced alkaline phosphatase, osteocalcin, and collagen I mRNA expression by PreOBs after 24-hour coculture (Figure 4A). A more pronounced effect on collagen I and OC mRNA expression was observed in the cell-to-cell contact condition as shown for XG-1 in Figure 4A on the left.
Lack of toxic and apoptotic effect on BM cells cocultured with HMCLs. Viability of BM-adherent cells in long-term cocultures with HMCLs (OPM2, XG-6, RPMI-8226, XG-1) was assessed after 21 days of coculture as described in “Patients, materials, and methods.” Graphs represent the percent mean ± SD of viable cells of 6 replicate wells. (A) MG63 cells treated with FAS (anti-CD95 mAb) were used as positive control for death cells. The presence of both death and apoptotic PreOBs has been investigated by flow cytometry after 48-hour coculture with XG-6 or OPM2 either in the presence or absence of a transwell insert using 7-amino-actinomycin D (7AAD) gating on all (B) CD138- cells and (C) Apo 2.7 mAb gating on viable CD138- cells. Any significant difference in the number of death or apoptotic PreOBs was not observed in the control and coculture condition.
Lack of toxic and apoptotic effect on BM cells cocultured with HMCLs. Viability of BM-adherent cells in long-term cocultures with HMCLs (OPM2, XG-6, RPMI-8226, XG-1) was assessed after 21 days of coculture as described in “Patients, materials, and methods.” Graphs represent the percent mean ± SD of viable cells of 6 replicate wells. (A) MG63 cells treated with FAS (anti-CD95 mAb) were used as positive control for death cells. The presence of both death and apoptotic PreOBs has been investigated by flow cytometry after 48-hour coculture with XG-6 or OPM2 either in the presence or absence of a transwell insert using 7-amino-actinomycin D (7AAD) gating on all (B) CD138- cells and (C) Apo 2.7 mAb gating on viable CD138- cells. Any significant difference in the number of death or apoptotic PreOBs was not observed in the control and coculture condition.
Consistently, collagen I protein expression by PreOBs was inhibited by HMCLs (OPM2, XG-1, XG-6, RPMI-8226) after 48-hour coculture, with a more pronounced effect in the cell-to-cell contact condition compared with the transwell condition (Figure 4B). On the contrary, no inhibitory effect on collagen expression was observed with the EBV+ cell line and normal BMMNCs (Figure 4B). Osteocalcin levels were significantly reduced after 48 hours in the cell-to-cell contact cocultures with XG-1, XG-6, RPMI-8226, and OPM2, but not with EBV+ cells or normal BMMNCs in comparison with the control (Figure 4C). Finally, we found a significant reduction in the alkaline phosphatase activity in 48-hour coculture between PreOBs and HMCLs but not with EBV+ cells or normal BMMNCs (Figure 4D). No difference in the inhibitory effect of HMCLs was observed in the presence or absence of a transwell insert (Figure 4D).
Effect of human myeloma cells on osteoblast differentiation. Confluent PreOBs obtained from healthy BMMNCs were cocultured with HMCLs (XG-6, RPMI-8226, OPM2, and XG-1) in the presence or absence of a transwell insert or with healthy BMMNCs or the EBV-positive cell line B95.8 for 24 to 48 hours. After 24 hours mRNA was extracted from either PreOBs alone, in the control condition and transwell coculture condition, or from pooled cells in cell-to-cell contact condition. (A) Alkaline phosphatase (ALP), collagen I, and osteocalcin (OC) mRNA expression was evaluated by RT-PCR. (B) Collagen I protein was assessed by Western blot analysis after 48-hour coculture. Figures are representative of 3 independent experiments. (C) OC levels were measured by ELISA assay in the 48 hours conditioned medium of cell-to-cell contact cocultures. Graphs and bars represent the mean OC levels plus SD of 3 independent experiments performed in duplicate. *P = .01; **P = .001. (D) Alkaline phosphatase (ALP) levels were detected by a fluorimetric method on cell lysates of 48-hour coculture. Graphs and bars represent the mean % minus SD versus the control condition (PreOB alone). *P < .05. (BMMNC: bone marrow mononuclear cells; EBV: EBV-positive cell line B95.8)
Effect of human myeloma cells on osteoblast differentiation. Confluent PreOBs obtained from healthy BMMNCs were cocultured with HMCLs (XG-6, RPMI-8226, OPM2, and XG-1) in the presence or absence of a transwell insert or with healthy BMMNCs or the EBV-positive cell line B95.8 for 24 to 48 hours. After 24 hours mRNA was extracted from either PreOBs alone, in the control condition and transwell coculture condition, or from pooled cells in cell-to-cell contact condition. (A) Alkaline phosphatase (ALP), collagen I, and osteocalcin (OC) mRNA expression was evaluated by RT-PCR. (B) Collagen I protein was assessed by Western blot analysis after 48-hour coculture. Figures are representative of 3 independent experiments. (C) OC levels were measured by ELISA assay in the 48 hours conditioned medium of cell-to-cell contact cocultures. Graphs and bars represent the mean OC levels plus SD of 3 independent experiments performed in duplicate. *P = .01; **P = .001. (D) Alkaline phosphatase (ALP) levels were detected by a fluorimetric method on cell lysates of 48-hour coculture. Graphs and bars represent the mean % minus SD versus the control condition (PreOB alone). *P < .05. (BMMNC: bone marrow mononuclear cells; EBV: EBV-positive cell line B95.8)
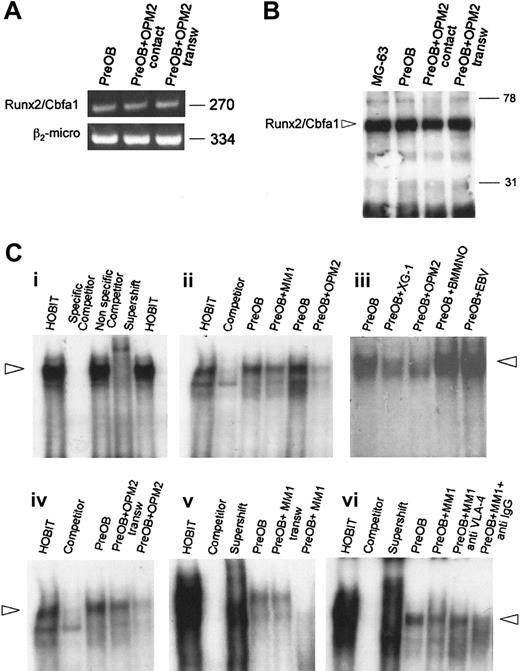
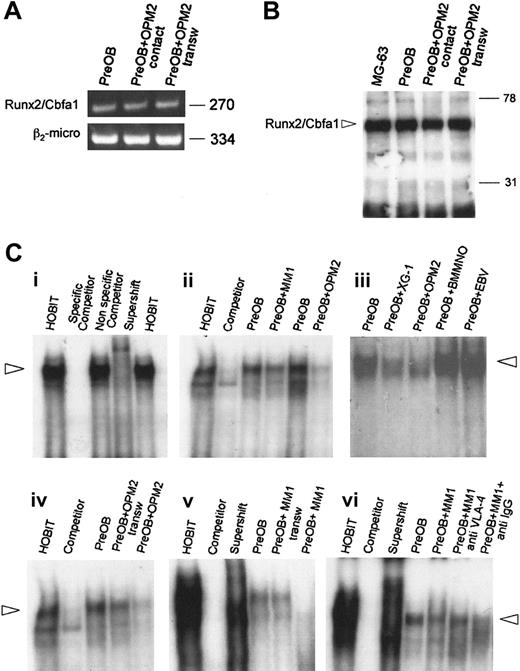
Myeloma-cell effect on Runx2/Cbfa1 expression and activity by PreOBs in cocultures
To investigate whether human myeloma cells could affect the expression and activity of the critical osteoblast transcription factor Runx2/Cbfa1, we performed a coculture system with a mesenchymal/stromal cell line differentiated toward osteoblast phenotype and either OPM2, which we have previously demonstrated to be negative for Runx2/Cbfa1 expression (S.C., F.M., M.L., Rita Rizzato, Paulo Lunghi, S.B., Cristina Mancini, Mario Pedrazzoni, Monica Crugnola, V.R., and N.G. Human myeloma cells express the bone regulating gene Rumx2/Cbfa1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Manuscript submitted to Leukemia June 24, 2005), or Runx2/Cbfa1-positive HMCLs (such as XG-1), depleting the cocultures of myeloma cells before the analysis. Similarly, a coculture system with PreOBs and fresh MM cells has been performed.
First, we found that Runx2/Cbfa1 mRNA expression by PreOBs was not modified after 24 hours by OPM2 either in the presence or absence of a transwell insert (Figure 5A). Similarly, we found that Runx2/Cbfa1 protein expression by PreOBs evaluated by Western blot on nuclear extracts was not modified in the presence of OPM2 with or without a transwell insert (Figure 5B). Thereafter, we investigated whether myeloma cells could modify the activity of Runx2/Cbfa1 in PreOBs after coculture by EMSA using the osteoblastic cells HOBIT as positive control (Figure 5Ci). We found that both XG-1 and OPM2 but not EBV+ cells or normal BMMNCs significantly inhibited Runx2/Cbfa1 activity by PreOBs in a 24-hour coculture. Similarly, freshly purified MM cells, as shown for a representative patient (MM1), inhibited Runx2/Cbfa1 activity (Figure 5Cii). The inhibitory effect of OPM2 and freshly purified MM cells on Runx2/Cbfa1 activity was more pronounced in the cell-to-cell contact condition compared with the transwell condition (Figure 5Ciii). Moreover, we found that a blocking anti-VLA-4 Ab but not an anti-IgG Ab reduced the inhibitory effect of MM cells on Runx2/Cbfa1 activity in coculture as shown for one representative MM patient (MM1; Figure 5Civ). A similar effect was observed testing HMCLs or freshly purified MM cells of other patients (data not shown).
Expression of osteoblast inhibitors by human myeloma cells and in cocultures
In order to identify osteoblast inhibitors that may contribute together with the cell contact to the suppressive effect of myeloma cells in coculture systems, first we screened the mRNA expression of several potential osteoblast inhibitors by HMCLs and freshly purified CD138+ MM cells. As summarized in Table 2, we found that DKK1 mRNA was expressed in 3 of 6 HMCLs and in about 80% of patients with MM, and IL-7 mRNA was expressed in all HMCLs tested and in all patients with MM. Among the sFRPs, we found that sFRP-3 was expressed by XG-6 and by XG-1 and in about 80% of patients with MM, whereas sFRP-2 was expressed at low intensity in U266 and JJN3 but not in purified MM cells. A similar percent of DKK1 and sFRP-3 expression was observed in patients with MM even if the pattern of expression of the 2 molecules was not the same. We found that 20% of patients differed for DKK1 and sFRP-3 expression. Moreover, we failed to find a significant correlation between DKK1 and sFRP-3 expression and the bone lesion status in our cohort of patients, probably due to the lower number of patients analyzed if compared with those reported by Tian et al.7
Effect of myeloma cells on Runx2/Cbfa1 expression and activity by PreOBs. (A) The expression of Runx2/Cbfa1 mRNA was evaluated by RT-PCR in PreOBs after 24-hour coculture with OPM2 in the presence or absence of a transwell insert. Nuclear extracts (80 μg) were obtained from PreOBs, PreOBs cocultured for 48 hours with OPM2 placed in a transwell insert, or from PreOBs pooled with OPM2. The expression of Runx2/Cbfa1 protein was analyzed by Western blot as described in “Patients, materials, and methods.” (B) Runx2/Cbfa1 was identified as a band of a molecular weight of about 65 kDa. (C) Human osteoblast cell line HOBIT was used as positive control. Runx2/Cbfa1 DNA-binding activity was evaluated by EMSA. Nuclear extracts (20 μg) of the cells were incubated with 32P-labeled OSE2 probe. Protein-DNA complexes were resolved in 5% nondenaturating polyacrylamide gel, which was then dried by heating in vacuum and analyzed by autoradiography. (Ci) To validate the EMSA protocol we used HOBIT (positive control), HOBIT plus 100-fold wild-type unlabeled probe (competitor), HOBIT plus 100-fold mutant unlabeled probe (nonspecific competitor), HOBIT plus antibody specific for Runx2/Cbfa1 (supershift). (Cii) A coculture system between PreOBs and the HMCLs OPM2 and XG-1, healthy BMMNCs, EBV cell line B95.8, or freshly purified MM cells of a representative patient (MM1) has been performed for 24 hours, and Runx2/Cbfa1 DNA-binding activity was evaluated by EMSA. (Ciii) In some experiments, OPM2 and fresh MM cells (MM1) were cocultured in the presence or absence of a transwell insert. (Civ) Finally, PreOBs and fresh MM cells (MM1) were cocultured in a cell-to-cell contact system with or without blocking anti-VLA-4 mAb or anti-IgG control mAb. Figures are representative of 3 independent experiments.
Effect of myeloma cells on Runx2/Cbfa1 expression and activity by PreOBs. (A) The expression of Runx2/Cbfa1 mRNA was evaluated by RT-PCR in PreOBs after 24-hour coculture with OPM2 in the presence or absence of a transwell insert. Nuclear extracts (80 μg) were obtained from PreOBs, PreOBs cocultured for 48 hours with OPM2 placed in a transwell insert, or from PreOBs pooled with OPM2. The expression of Runx2/Cbfa1 protein was analyzed by Western blot as described in “Patients, materials, and methods.” (B) Runx2/Cbfa1 was identified as a band of a molecular weight of about 65 kDa. (C) Human osteoblast cell line HOBIT was used as positive control. Runx2/Cbfa1 DNA-binding activity was evaluated by EMSA. Nuclear extracts (20 μg) of the cells were incubated with 32P-labeled OSE2 probe. Protein-DNA complexes were resolved in 5% nondenaturating polyacrylamide gel, which was then dried by heating in vacuum and analyzed by autoradiography. (Ci) To validate the EMSA protocol we used HOBIT (positive control), HOBIT plus 100-fold wild-type unlabeled probe (competitor), HOBIT plus 100-fold mutant unlabeled probe (nonspecific competitor), HOBIT plus antibody specific for Runx2/Cbfa1 (supershift). (Cii) A coculture system between PreOBs and the HMCLs OPM2 and XG-1, healthy BMMNCs, EBV cell line B95.8, or freshly purified MM cells of a representative patient (MM1) has been performed for 24 hours, and Runx2/Cbfa1 DNA-binding activity was evaluated by EMSA. (Ciii) In some experiments, OPM2 and fresh MM cells (MM1) were cocultured in the presence or absence of a transwell insert. (Civ) Finally, PreOBs and fresh MM cells (MM1) were cocultured in a cell-to-cell contact system with or without blocking anti-VLA-4 mAb or anti-IgG control mAb. Figures are representative of 3 independent experiments.
sFRP-1 and sFRP-4 as well as noggin and gremlin were absent in HMCLs and fresh CD138+ MM cells (Table 2). The production of DKK1 and sFRP by HMCLs was confirmed using Western blot analysis, whereas the secretion of IL-7 was confirmed by an ELISA assay (data not shown).
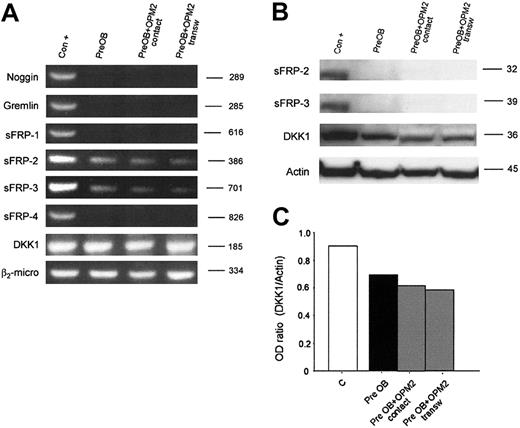
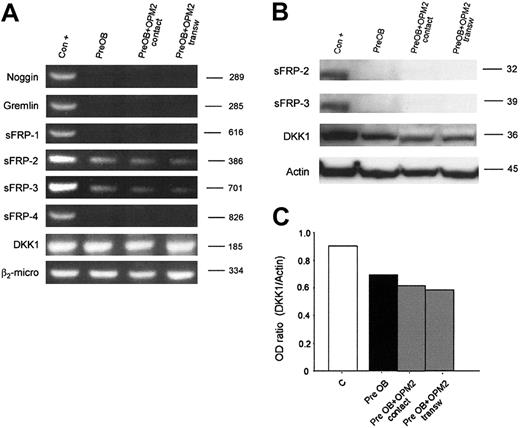
Furthermore, we have evaluated whether human myeloma cells could induce the expression of these osteoblast inhibitors in PreOBs using coculture experiments. No induction of noggin, gremlin, sFRP-1, sFRP-2, sFRP-3, sFRP-4, and DKK1 mRNA expression was observed in coculture with HMCLs as shown for OPM2 in Figure 6A. The lack of a stimulatory effect on sFRP-2, sFRP-3, and DKK1 production in coculture between PreOBs and OPM2 was confirmed by Western blot analysis. Furthermore, a slight inhibitory effect on DKK1 expression was observed in coculture as shown by densitometry (Figure 6B).
Expression of osteoblast inhibitors in coculture. Human BM PreOBs were cocultured in the presence or absence of a transwell insert with OPM2. (A) After 24 hours, mRNA was extracted and the expression of noggin; gremlin sFRP-1, sFRP-2, sFRP-3, and sFRP-4; and DKK1 was evaluated by RT-PCR. (B) sFRP-2, sFRP-3, and DKK1 protein expression was evaluated by Western blot analysis after 48 hours. Figures are representative of 3 independent experiments. Graphs represent the mean optic density (OD ratio) of DKK1 normalized to actin of a representative experiment. The following positive controls (Con +) have been used: noggin, human amniotic cells; Gremlin, osteoblast-like cell line MG-63 stimulated with rhBMP-2 (100 ng/mL), sFRP-1, sFRP-2, sFRP-3, and sFRP-4; fetal and adult human cardiomiocytes, DKK1; and HOBIT.
Expression of osteoblast inhibitors in coculture. Human BM PreOBs were cocultured in the presence or absence of a transwell insert with OPM2. (A) After 24 hours, mRNA was extracted and the expression of noggin; gremlin sFRP-1, sFRP-2, sFRP-3, and sFRP-4; and DKK1 was evaluated by RT-PCR. (B) sFRP-2, sFRP-3, and DKK1 protein expression was evaluated by Western blot analysis after 48 hours. Figures are representative of 3 independent experiments. Graphs represent the mean optic density (OD ratio) of DKK1 normalized to actin of a representative experiment. The following positive controls (Con +) have been used: noggin, human amniotic cells; Gremlin, osteoblast-like cell line MG-63 stimulated with rhBMP-2 (100 ng/mL), sFRP-1, sFRP-2, sFRP-3, and sFRP-4; fetal and adult human cardiomiocytes, DKK1; and HOBIT.
Effect of IL-7 and DKK1 on CFU-F and CFU-OB formation in BM cultures and on Runx2/Cbfa1 activity
Since we have shown that human myeloma cells produce IL-7 and DKK1, and at least in part sFRP-3, we have investigated the effect of these cytokines in our system and their potential contribution to the inhibitory effect of myeloma cells on osteoblast formation and Runx2/Cbfa1 activity observed in the coculture system in the presence of a transwell insert. First we confirmed, as previously reported,14 that PreOBs express IL-7 receptor mRNA and protein by RT-PCR and Western blot, respectively (data not shown). Thereafter, we tested the effect of rhIL-7 in BM cultures, finding that IL-7 significantly inhibited in a dose-dependent manner the CFU-F number (mean decrease ± SD vs control: -36% ± 9% [P < .05] at 20 pg/mL; -80% ± 18% [P < .01] at 20 ng/mL) and CFU-OB formation (-26% ± 11% [P < .05] at 20 pg/mL and -84% ± 15% [P < .01], 20 ng/mL) (Figure 7A, left). On the contrary, DKK1 had no inhibitory effect on CFU-F, and DKK1 (Figure 7A, right) as well as sFRP-3 (data not shown) induced a slight stimulatory effect on CFU-OB formation. In line with this observation, we found that IL-7 inhibited Runx2/Cbfa1 binding activity at both concentrations in a dose-dependent manner, and it did not change Runx2/Cbfa1 protein expression (Figure 7B). On the contrary, DKK1 showed a slight inhibitory effect on Runx2/Cbfa1 activity only at a high concentration (Figure 7B). Consistently, we found that blocking anti-IL-7 Ab but not anti-DKK1 Ab partially reduced the inhibitory effect of XG-6 on CFU-F formation (Figure 7C). Similarly, blocking anti-IL-7 Ab blunted the inhibitory effect of BM MM patient plasma as shown in Figure 7D.
Runx2/Cbfa1 immunostaining in BM biopsies of patients with MM
To support our in vitro data, we have evaluated the number of osteoblastic cells and performed Runx2/Cbfa1 immunostaining on the BM biopsies of 40 patients with MM comparing osteolytic with nonosteolitic patients. A significant reduction of the number of osteoblastic cells/mm2 was observed in patients with MM who had osteolysis compared with those who did not have bone lesions (mean ± SE: 91.4 ± 3.1 vs 131.1 ± 3.8, P = .05) (Figure 8A). As expected, a reduction of the osteoid surface was also observed in osteolytic versus nonosteolytic patients with MM (mean, 22% ± 0.38% vs 16% ± 0.39%, P = .03). A significant reduction of both the number of total stromal/osteoblastic cells positive for Runx2/Cbfa1 (mean ± SE: 97.22 ± 6.6 vs 252.36 ± 8.1, P = .005) and the percent of Runx2/Cbfa1-positive osteoblastic cells (% mean ± SE: 27.8% ± 1.3% vs 48.51% ± 0.7%, P = .001) was observed in patients with MM who had osteolytic lesions in comparison with patients who did not have osteolysis (Figure 8B). Figure 8B shows 2 representative patients, one with extensive bone lesions characterized by a negative Runx2/Cbfa1 staining and a reduced number of osteoblastic cells and the other without bone lesions characterized by the presence of active osteoblastic cells and a strong positive immunostaining for Runx2/Cbfa1.
Discussion
The histomorphometric studies performed in patients with MM have demonstrated that a reduction of the number of osteoblastic cells contributes, together with the increase of osteoclast activity, to the development of bone lesions.5 These studies are supported by clinical studies showing that patients with MM who have bone lesions exhibit a reduction of the osteoblast markers (such as alkaline phosphatase and osteocalcin) together with an increase of the bone resorption markers.26 In addition, a marked osteoblastopenia and reduced bone formation have been reported in murine MM models correlated with the development of bone lesions.27 All of these data underline that the interaction of myeloma cells with osteoblastic cells and the inhibition of bone formation have a critical role in the pathogenesis of MM bone disease.
The biologic mechanisms involved in the pathogenesis of the suppression of bone formation that occurs in patients with MM have not been completely identified. In this study, first we have shown that human myeloma cells suppress the formation of the early osteoblast progenitors CFU-Fs and the more differentiated osteoblast progenitors CFU-OBs in ex vivo BM cultures. This effect was observed using several HMCLs and freshly purified MM cells. Moreover, it has also been confirmed in ex vivo culture by the finding of a reduced CFU-F and CFU-OB formation in patients with MM compared with control subjects (M.G., unpublished data, December 24, 2004). The inhibitory effect of myeloma cells on CFU-F and CFU-OB formation may explain the low number of osteoblastic cells observed in bone biopsies in patients with MM who have osteolytic lesions.5 In contrast with our evidence and histomorphometric results, previously published data have shown that HMCLs promote the recruitment of osteoblast precursors, increasing the number of osteoblastic colonies in vitro.28 The opposite results obtained by these authors could be explained by their different experimental system, which includes murine BM cultures and a lower percentage of HMCL-conditioned media (10%) compared with our system.
The capacity of myeloma cells to block osteoblast formation in vitro was supported by the inhibitory effect observed on the expression of osteoblast differentiation markers such as alkaline phosphatase, osteocalcin, and collagen I in a short-term coculture system performed between myeloma cells and PreOBs. An inhibitory effect on osteocalcin secretion has also been previously reported in osteoblast-like cells.29
The inhibition of both osteoblast formation and differentiation by myeloma cells may be involved in the reduced bone formation observed in patients with MM. However other mechanisms could contribute to the suppression of bone formation in MM. A direct effect on mature osteoblasts by myeloma cells has been also demonstrated, showing that myeloma cells are able to inhibit osteoblast proliferation30 and up-regulate osteoblast apoptosis in coculture.31 Moreover, osteoblasts obtained from patients with MM are also highly prone to apoptosis.32
The mechanism by which human myeloma cells are able to inhibit osteoblast formation and differentiation has been also investigated in this study, showing that myeloma cells reduce Runx2/Cbfa1 activity in PreOBs. We have focused our attention on this transcription factor because several data points demonstrate that Runx2/Cbfa1 has a critical role in the osteoblast formation and differentiation.17-22 It has been demonstrated that the activation of Runx2/Cbfa1 in human BM stromal/osteoprogenitor cells regulates the expression of osteoblast markers such as alkaline phosphatase, osteocalcin, and collagen I.21,22 In contrast to the murine system, where osteoblast differentiation is associated with increased synthesis of Runx2/Cbfa1,17,20,21 in the human system the osteoblast differentiation is primarily associated with increased RUNX2/CBFA1 activity without a change in mRNA or protein level.33 Similarly, in our coculture system we found that human myeloma cells block RUNX2/CBFA1 activity without modifying Runx2/Cbfa1 mRNA and protein expression. The inhibition of Runx2/Cbfa1 activity by myeloma cells observed in our system may also be involved in the reduction of the secretion of the osteoclast inhibitor osteoprotegerin (OPG) by PreOBs,24 because it has been demonstrated that Runx2/Cbfa1 transcription factor contributes to the expression of OPG.34 On the contrary, the production of the critical osteoclastogenic factor RANKL by stromal/osteoblastic cells seems not to be regulated by RUNX2/CBFA1.35 In line with these observations, we have previously demonstrated that myeloma cells up-regulate RANKL and down-regulate OPG in BM PreOBs.24 Finally, the block of the RUNX2/CBFA1 activity by myeloma cells suggests that this transcription factor could have a critical role in the development on MM bone disease, with RUNX2/CBFA1 involved in the regulation of both OPG secretion and osteoblastogenesis.
Effect of IL-7 and DKK1 on osteoblast formation and Runx2 activity. (A) Human BMMNCs were incubated in osteoblast differentiation medium as described in “Patients, materials, and methods” in the presence or absence of rhIL-7 (20 pg/mL-20 ng/mL) and DKK1 (20-100 ng/mL). CFU-F and CFU-OB formation was evaluated after 14 days with alkaline phosphatase staining and 21 days with alizarin red, respectively. Figures are representative of 3 independent experiments (original magnifications, × 1 [top] and × 5 [bottom]). Graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate. (*P < .05; **P < .01). (B) Confluent human PreOBs were stimulated with or without IL-7 (20 pg/mL-20 ng/mL) or DKK1 (100 ng/mL) for 24 hours. After the culture period, nuclear extracts were obtained and Runx2/Cbfa1 binding activity and protein expression were evaluated by EMSA and Western blot, respectively, as described in “Patients, materials, and methods.” (C) Human BM cells were cocultured with XG-6 (on the top) in the presence or absence of blocking anti-IL-7 Ab (0.5 μg/mL) or anti-DKK1 Ab (5 μg/mL). The CFU-Fs were stained using alkaline phosphatase kit after 14 days as described in “Patients, materials, and methods.” Figures are representative of 3 independent experiments and graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate (*P < .05). (D) BM cells were incubated with BM plasma (dilution = 1:2) of patients with MM (MM1, MM2, and MM3) in the presence or absence of a blocking anti-IL-7 Ab and CFU-F colonies were stained after 14 days. On the left is a representative experiment, with MM1 sample quantified by densitometry as total pixel intensity. Graphs on the right represent the mean number of CFU-Fs/well ± SD of an experiment performed in triplicate with 3 different MM samples (*P < .05). Images were obtained as described for Figure 1.
Effect of IL-7 and DKK1 on osteoblast formation and Runx2 activity. (A) Human BMMNCs were incubated in osteoblast differentiation medium as described in “Patients, materials, and methods” in the presence or absence of rhIL-7 (20 pg/mL-20 ng/mL) and DKK1 (20-100 ng/mL). CFU-F and CFU-OB formation was evaluated after 14 days with alkaline phosphatase staining and 21 days with alizarin red, respectively. Figures are representative of 3 independent experiments (original magnifications, × 1 [top] and × 5 [bottom]). Graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate. (*P < .05; **P < .01). (B) Confluent human PreOBs were stimulated with or without IL-7 (20 pg/mL-20 ng/mL) or DKK1 (100 ng/mL) for 24 hours. After the culture period, nuclear extracts were obtained and Runx2/Cbfa1 binding activity and protein expression were evaluated by EMSA and Western blot, respectively, as described in “Patients, materials, and methods.” (C) Human BM cells were cocultured with XG-6 (on the top) in the presence or absence of blocking anti-IL-7 Ab (0.5 μg/mL) or anti-DKK1 Ab (5 μg/mL). The CFU-Fs were stained using alkaline phosphatase kit after 14 days as described in “Patients, materials, and methods.” Figures are representative of 3 independent experiments and graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate (*P < .05). (D) BM cells were incubated with BM plasma (dilution = 1:2) of patients with MM (MM1, MM2, and MM3) in the presence or absence of a blocking anti-IL-7 Ab and CFU-F colonies were stained after 14 days. On the left is a representative experiment, with MM1 sample quantified by densitometry as total pixel intensity. Graphs on the right represent the mean number of CFU-Fs/well ± SD of an experiment performed in triplicate with 3 different MM samples (*P < .05). Images were obtained as described for Figure 1.
Runx2/Cbfa1 immunostaining in BM biopsies of patients with MM. (A) Histomorphometry and Runx2/Cbfa1 immunostaining performed on BM biopsies of 40 patients with MM who either had bone lesions or did not have skeletal involvement as described in “Patients, materials, and methods.” Graphs and bars represent the mean ± SD of the number of osteoblasts (OBs)/mm2, the number of total Runx2/Cbfa1 positive stromal/osteoblastic cells, and the % of Runx2/Cbfa1-positive OBs. (B) Runx2/Cbfa1 immunostaining performed in a trabecula of 2 representative patients with MM who did or did not have bone lesions (original magnification, × 1000). See “Immunohistochemistry” for image acquisition details.
Runx2/Cbfa1 immunostaining in BM biopsies of patients with MM. (A) Histomorphometry and Runx2/Cbfa1 immunostaining performed on BM biopsies of 40 patients with MM who either had bone lesions or did not have skeletal involvement as described in “Patients, materials, and methods.” Graphs and bars represent the mean ± SD of the number of osteoblasts (OBs)/mm2, the number of total Runx2/Cbfa1 positive stromal/osteoblastic cells, and the % of Runx2/Cbfa1-positive OBs. (B) Runx2/Cbfa1 immunostaining performed in a trabecula of 2 representative patients with MM who did or did not have bone lesions (original magnification, × 1000). See “Immunohistochemistry” for image acquisition details.
The inhibitory effect observed in our cocultures by myeloma cells on osteoblast formation and differentiation was more pronounced in the cell-to-cell contact condition compared with the condition without the cellular contact; this suggests that both the cellular contact and the release of soluble factors contribute to the block of osteoblastogenesis in vitro, although the cell contact between myeloma cells and osteoprogenitor cells is the prevalent mechanism. In line with this observation, we have demonstrated a higher inhibitory effect on RUNX2/CBFA1 activity by myeloma cells in the cell-to-cell contact culture conditions compared with the conditions in which the cellular contact was avoided by a transwell insert. Consistently, it has been previously demonstrated that human myeloma cells inhibit osteocalcin secretion by osteoblast-like cells through the cell-to-cell contact.29 Furthermore, we found that the presence of a blocking anti-VLA-4 Ab in the cocultures reduced the inhibitory effect on Runx2/Cbfa1 activity by myeloma cells. This evidence suggests that among the molecules involved in myeloma-cell adhesion to stromal/osteoblastic cells, the VLA-4/vascular-cell adhesion molecule 1 (VCAM-1) interaction could be responsible for the block of osteoblastogenesis by myeloma cells.
The role of the cellular contact and VLA-4/VCAM-1 interaction in the development of bone lesions in MM has been recently underlined.24,36,37 Previous data indicate that myeloma cells induce RANKL up-regulation in human BM stromal cells through the cell contact with the involvement of the VLA-4 molecule.24 Similar data were obtained in a coculture murine system in which the direct contact of myeloma cells with marrow stromal cells via VLA-4 interaction increased the osteoclast activity.36 In murine MM models, it has also been shown that pretreatment with blocking anti-VLA-4 Ab completely prevented osteolysis, reducing the number of osteoclastic cells and increasing the trabecular bone mass.37 Moreover, it has been demonstrated that HMCLs such as JJN3 that strongly expresses VLA-438 are able to induce bone lesions in vivo in mice.39 In this model, the authors show a marked osteoblastopenia with a significant reduction of the bone formation rate, suggesting that the block of osteoblast formation is critical in the development of bone lesions induced by JJN3. Consistently, the data presented in our paper indicate that the VLA-4 system may be involved in the inhibition of osteoblast formation and differentiation.
Clearly, other molecules involved in the myeloma-cell adhesion could be implicated in the inhibition of osteoblastogenesis by human myeloma cells. Recent data indicate that the neural-cell adhesion molecule (NCAM)-NCAM interaction between myeloma cells and stromal/osteoblastic cells may decrease bone matrix production by osteoblastic cells and contribute to the occurrence of bone lesions in patients with MM.29,40
Our coculture experiments indicate that soluble factors may contribute to the suppression of osteoblast formation and differentiation by myeloma cells other than the cellular contact. In order to identify potential molecules involved in this process, we have screened the expression of several osteoblast inhibitors by myeloma cells and evaluated their potential up-regulation in coculture. Among the osteoblast inhibitors, the potential role of DKK1 in MM bone disease has recently been emphasized.7 A tight relationship has been demonstrated between DKK1 production by myeloma cells and the occurrence of lytic bone lesions in patients with MM.7 In line with this observation, we found that HMCLs and freshly purified MM cells produce DKK1 as reported by Tian et al,7 even if we failed to find a significant correlation with the bone lesion status, which was probably due to the lower number of patients analyzed.
DKK1 as well as sFRP-3 are inhibitors of Wnt/β-catenin signaling involved in osteoblast maturation and bone formation9,41 ; however, most of the experimental data regarding the role of this pathway are obtained using murine progenitor cell lines that acquire the osteoblast phenotype in the presence of BMP-2.9,42 Actually, Tian et al7 have demonstrated the role of DKK1 in myeloma-induced inhibition osteoblast formation using the murine mesenchymal cell line C2C12. In addition, it has been demonstrated that Wnt/β-catenin signaling induces the expression of alkaline phosphatase but has no effect on Runx2/Cbfa1 osteocalcin and collagen I expression by murine osteoprogenitor cells.42
In line with these observations, we found that DKK1 did not inhibit CFU-F and CFU-OB formation in human BM culture. On the contrary, we observed a slight stimulatory effect of DKK1 (at 20 ng/mL) and of sFRP-3 on bone nodule formation, as already recently described.43 Moreover, we found that DKK1 did not affect Runx2/Cbfa1 activity in PreOBs. A slight inhibitory effect was observed only at the highest concentration of DKK1 (100 ng/mL) tested, which, however, is not reached in BM plasma of patients with MM.7 Consistently, we found that the blocking anti-DKK1 Ab did not blunt the effect of HMCLs in BM cultures. Altogether these data suggest that the production of DKK1 by myeloma cells does not contribute to the inhibitory effect observed in our coculture system on osteoblast formation, differentiation, and Runx2/Cbfa1 activity, even if DKK1 could be implicated in the inhibition of alkaline phosphatase expression observed in our coculture system, because Wnt/β-catenin signaling regulates alkaline phosphatase expression.42 In line with this hypothesis, we observed that alkaline phosphatase expression, but not osteocalcin or collagen I, was inhibited in the same entity both in the presence and absence of a transwell insert. However, our data cannot exclude the role of DKK1/Wnt signaling in myeloma-induced osteoblast inhibition as demonstrated by Tian et al7 but indicate that Runx2/Cbfa1 transcription factor is involved in this process mainly through the cell-to-cell contact between myeloma and osteoblast progenitor cells.
Recent data indicate that IL-7 may act as an uncoupled factor able either to stimulate RANKL production and bone resorption44,45 or to inhibit bone formation.13 In particular, it has been demonstrated that IL-7 decreases the promoter activity of Runx2/Cbfa1 in osteoblastic cells13 and consistently the expression of osteoblast markers and OPG secretion.13 Moreover, IL-7 may inhibit bone formation in vivo in mice.13 The presence of the IL-7 receptor has recently been shown in human BM stromal cells46 ; our previous evidence has demonstrated that BM IL-7 plasma levels are significantly higher in patients with MM compared with healthy subjects14 and that IL-7 is involved in RANKL-dependent osteoclast formation.14 Here we have demonstrated that IL-7 inhibits both CFU-F and CFU-OB formation in human BM culture and reduced Runx2/Cbfa1 activity in human PreOBs in line with the results obtained in the murine system.13 Moreover, blocking anti-IL-7 Ab partially blunted the inhibitory effect of both HMCLs and BM plasma of patients with MM. This evidence indicates that IL-7 contributes to the inhibitory effect of MM cells on osteoblastogenesis and, together with the previously published data, suggests that this cytokine may act as an uncoupled factor in MM.
Our in vitro evidence was supported by the immunohistochemical data obtained in patients with MM. We found that osteolytic patients in comparison with patients without bone lesions had a reduced number of osteoblastic cells with a reduction of Runx2/Cbfa1 stromal/osteoblastic-positive cells and a low percentage of Runx2/Cbfa1-positive osteoblasts. This evidence suggests that the presence of MM cells in the BM, blocking the RUNX2/CBFA1 activity and the osteoblast differentiation from osteoprogenitor cells, may lead to a reduction of the number of osteoblastic cells and to the formation of nonactive stromal/osteoblastic cells, in line with the results obtained by the histomorphometric studies.
Prepublished online as Blood First Edition Paper, June 2, 2005; DOI 10.1182/blood-2004-12-4986.
Supported by grants from the Ministero dell 'Istruzione dell 'Universitàe della Ricerca (MIUR).
N.G. and S. Colla contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





![Figure 7. Effect of IL-7 and DKK1 on osteoblast formation and Runx2 activity. (A) Human BMMNCs were incubated in osteoblast differentiation medium as described in “Patients, materials, and methods” in the presence or absence of rhIL-7 (20 pg/mL-20 ng/mL) and DKK1 (20-100 ng/mL). CFU-F and CFU-OB formation was evaluated after 14 days with alkaline phosphatase staining and 21 days with alizarin red, respectively. Figures are representative of 3 independent experiments (original magnifications, × 1 [top] and × 5 [bottom]). Graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate. (*P < .05; **P < .01). (B) Confluent human PreOBs were stimulated with or without IL-7 (20 pg/mL-20 ng/mL) or DKK1 (100 ng/mL) for 24 hours. After the culture period, nuclear extracts were obtained and Runx2/Cbfa1 binding activity and protein expression were evaluated by EMSA and Western blot, respectively, as described in “Patients, materials, and methods.” (C) Human BM cells were cocultured with XG-6 (on the top) in the presence or absence of blocking anti-IL-7 Ab (0.5 μg/mL) or anti-DKK1 Ab (5 μg/mL). The CFU-Fs were stained using alkaline phosphatase kit after 14 days as described in “Patients, materials, and methods.” Figures are representative of 3 independent experiments and graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate (*P < .05). (D) BM cells were incubated with BM plasma (dilution = 1:2) of patients with MM (MM1, MM2, and MM3) in the presence or absence of a blocking anti-IL-7 Ab and CFU-F colonies were stained after 14 days. On the left is a representative experiment, with MM1 sample quantified by densitometry as total pixel intensity. Graphs on the right represent the mean number of CFU-Fs/well ± SD of an experiment performed in triplicate with 3 different MM samples (*P < .05). Images were obtained as described for Figure 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2004-12-4986/6/m_zh80190584840007.jpeg?Expires=1765107890&Signature=JMOabg8iyGEk4-fH96JGN47Rp30pGtXtq84pMkjba8l3ABk3zrEdzKDo9A5i59OrTyYI4x5kWt5EkSbRJRgXGocybQP4ac9t~Ld5mMyC52J5GWYCPAQUtLZdVyopIJZfAq7tmtkHGJMmKTQRFNw6UOKmJYSeDtr2k4yna7T9-7GedamCOnuGHAwQVkL3i8d1HzdmPKGHlpZO734b6-Jmkt0kV8MGa3O1H36MHOKgvorUznO5SLoYDdURB~i8Mn6rFnXaR9v8Bcvmt0UB9WxT3fbmdgp-kV1aO~TLOahgkYE5LGvegdP6NY~DnQbQcqUAxG0bWsCBob7J9UvLly1U2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 7. Effect of IL-7 and DKK1 on osteoblast formation and Runx2 activity. (A) Human BMMNCs were incubated in osteoblast differentiation medium as described in “Patients, materials, and methods” in the presence or absence of rhIL-7 (20 pg/mL-20 ng/mL) and DKK1 (20-100 ng/mL). CFU-F and CFU-OB formation was evaluated after 14 days with alkaline phosphatase staining and 21 days with alizarin red, respectively. Figures are representative of 3 independent experiments (original magnifications, × 1 [top] and × 5 [bottom]). Graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate. (*P < .05; **P < .01). (B) Confluent human PreOBs were stimulated with or without IL-7 (20 pg/mL-20 ng/mL) or DKK1 (100 ng/mL) for 24 hours. After the culture period, nuclear extracts were obtained and Runx2/Cbfa1 binding activity and protein expression were evaluated by EMSA and Western blot, respectively, as described in “Patients, materials, and methods.” (C) Human BM cells were cocultured with XG-6 (on the top) in the presence or absence of blocking anti-IL-7 Ab (0.5 μg/mL) or anti-DKK1 Ab (5 μg/mL). The CFU-Fs were stained using alkaline phosphatase kit after 14 days as described in “Patients, materials, and methods.” Figures are representative of 3 independent experiments and graphs represent the mean number of CFU-Fs and CFU-OBs/well ± SD of 3 independent experiments performed in triplicate (*P < .05). (D) BM cells were incubated with BM plasma (dilution = 1:2) of patients with MM (MM1, MM2, and MM3) in the presence or absence of a blocking anti-IL-7 Ab and CFU-F colonies were stained after 14 days. On the left is a representative experiment, with MM1 sample quantified by densitometry as total pixel intensity. Graphs on the right represent the mean number of CFU-Fs/well ± SD of an experiment performed in triplicate with 3 different MM samples (*P < .05). Images were obtained as described for Figure 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2004-12-4986/6/m_zh80190584840007.jpeg?Expires=1765107891&Signature=imHPiy95dc1c6dOkfq3ZGUa~6bmHEPRilZCY55-WpmHcD9feR0sdqcsf~2zGI0zGtQto-od-UdQGCOUPdwm-t97thWLjsODbR5m6R9PljYPQOaq0O0AVq0QdZPqI07n-YJW8hqYdnEuigk6RrLTcGDDIyF31QfOuNYNz4A8sF0Bf5v3ab9j59bok0XzHWuTDf36hlFzjPRO6ZzJIRDd2AHEgPquXB4WPJiLU2lVTa4KdUFW6JrOlFFRlVBNxSBB~NA5BDdIBUVLVPtN7KlviqT16qMMyBhpiWH9HY1CoCB7Vtf9gETNH9GkZcDkLMRxMCuZSIU8jufXnzgJw7jiESw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
