Abstract
The zeta-associated protein of 70 kDa (ZAP-70) is expressed in patients with aggressive chronic lymphocytic leukemia (CLL). We found that ZAP-70+ CLL cells expressed activated heat-shock protein 90 (Hsp90) with high binding affinity for Hsp90 inhibitors, such as 17-allyl-amino-demethoxy-geldanamycin (17-AAG), whereas normal lymphocytes or ZAP-70- CLL cells expressed nonactivated Hsp90. Activated Hsp90 bound and stabilized ZAP-70, which behaved like an Hsp90 client protein only in CLL cells. Treatment with Hsp90 inhibitors such as 17-AAG and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) induced ZAP-70 degradation and apoptosis in CLL cells but not in T cells, and also impaired B-cell receptor signaling in leukemia cells. Transduction of ZAP-70- CLL cells with an adenovirus encoding ZAP-70 activated Hsp90 and specifically rendered the leukemia cells sensitive to 17-AAG. These data indicate that Hsp90 is necessary for ZAP-70 expression and activity; that ZAP-70 is unique among Hsp90 clients, in that its chaperone-dependency is conditional on the cell type in which it is expressed; and also that ZAP-70 is required for cell survival and signaling in CLL. Additionally, ZAP-70 expression in CLL cells confers markedly heightened sensitivity to 17-AAG or 17-DMAG, suggesting that these or other Hsp90 inhibitors could be valuable therapeutically in patients with aggressive CLL. (Blood. 2005;106:2506-2512)
Introduction
The clinical course of patients with B-cell chronic lymphocytic leukemia (CLL), the most common adult leukemia, is heterogeneous. Whereas some patients require treatment relatively soon after diagnosis, others have indolent disease that can persist for years without therapy.1 At least 2 subtypes of CLL can be differentiated by clinical presentation, mutational status of the immunoglobulin heavy-chain variable-region (IgVH) gene, and more recently also by gene expression profiling using DNA microarray technology.2 Several prognostic factors correlate with the clinical progression of patients with CLL, and among those the level of expression of the zeta-associated protein of 70 kDa (ZAP-70) appears the strongest indicator of the need for early treatment.3
We examined the effects of 17-allylaminogeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) on primary leukemia cells from patients with CLL with early-stage disease. 17-AAG and 17-DMAG are heat-shock protein 90 (Hsp90) inhibitors undergoing clinical testing in a variety of cancers.4,5 Hsp90 is a molecular chaperone that catalyzes the conformational maturation of a range of oncogenic signaling proteins collectively referred to as “clients.”6-9 Hsp90 exists in 2 major multichaperone complexes. In the intermediate complex, a client protein is loaded onto Hsp90 with the help of the cochaperones Hsp70, Hsp40, Hop, and Hip. Upon adenosine triphosphate (ATP) binding and hydrolysis, the complex switches to a mature form, in which Cdc37, p23, and immunophilins replace the original cochaperones to assist in conformational maturation of the client, helping it to maintain an active, functional state.10 We recently demonstrated that Hsp90 in advanced tumors is present primarily in multichaperone complexes with high ATPase activity, whereas Hsp90 from normal tissues is in a latent, uncomplexed state.11 17-AAG and 17-DMAG selectively bind to activated Hsp90, competing with ATP and locking the nonproductive intermediate complex, resulting in the release and proteasomal degradation of the client protein.11-14 Because Hsp90 inhibitors have been demonstrated to be active in other tumors, we investigated whether CLL cells were sensitive to apoptosis induced by these agents. In addition, because ZAP-70 represents a potential target for treatment in CLL, we investigated whether inhibitors of the Hsp90 multichaperone complex could modulate the level of expression and function of this kinase in CLL cells.
Materials and methods
Cells and reagents
Peripheral blood mononuclear cells (PBMCs) from patients with CLL were obtained from the CLL Research Consortium (CRC) tissue bank. PBMCs were isolated by density gradient centrifugation over Histopaque 1077 as described.15 These samples had more than 95% CD19+/CD5+ cells by flow cytometry. ZAP-70 expression and IgVH gene mutational status were assessed as previously described.3 Cells were incubated in RPMI media at 37°C with 5% CO2. The MCF-7 breast cancer cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). In some experiments the cells were treated with 2-Fluoro-Ara-A (gift from Drs Reed and Kitada; Burnham Institute, La Jolla, CA), 17-DMAG (InvivoGen, San Diego, CA), 17-AAG, or EC116 (17-AAG analog; Conforma Therapeutics, San Diego, CA). The biotin-geldanamycin (GM) probe was prepared by displacing the 17-methoxy of GM with a biotinyl-linked amine as described.11 Cell samples were incubated also in media with dimethyl sulfoxide (DMSO; 1%) as a control.
Antibodies used were as follows: Hsp90 (SPA-835; recognizes Hsp90α and Hsp90β and immunoprecipitates free and complexed Hsp90), Hsp90* (SPA-830; recognizes Hsp90α and Hsp90β and immunoprecipitates uncomplexed Hsp90; Stressgen Biotechnologies, Victoria, BC, Canada), p23 (804-023-R100; Alexis Biochemicals, San Diego, CA), Hop (a gift from D. Toft), ZAP-70 nonconjugated and Alexa 488 (Caltag Laboratories, Burlingame, CA), p72Syk (clone 4D10.1; Upstate Biotechnology, Lake Placid, NY), 4G10 (Upstate Biotechnology, Lake Placid, NY), Hsp70 (Stressgen Biotechnologies), CD3-PE (BD-Pharmingen, San Diego, CA), β actin (clone AC-15; Sigma Immunochemicals, St Louis, MO), anti-PARP-1 (clone C2-10; BD Pharmingen, La Jolla, CA), IkB-α, and IKK-α (BD-Pharmingen, San Diego, CA).
Hsp90 binding assays
Purified native Hsp90 protein (Stressgen Biotechnologies) or cell lysates in lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.3, 1 mM EDTA [ethylenediaminetetraacetic acid], 5 mM MgCl2, 100 mM KCl) were incubated with or without 17-AAG for 30 minutes at 4°C, and then incubated with biotin-GM linked to BioMag streptavidin magnetic beads (Qiagen, Valencia, CA) for 1 hour at 4°C. Tubes were placed on a magnetic rack, and the unbound supernatant removed. The magnetic beads were washed in lysis buffer and heated for 5 minutes at 95°C in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Samples were analyzed on SDS protein gels, and immunoblots were performed using the indicated antibodies. We assessed the band intensities in the immunoblots using the Bio-rad Fluor-S MultiImager (BioRad, Hercules, CA), and calculated the percentage inhibition of binding of Hsp90 to the biotin-GM. The reported IC50 is the concentration of 17-AAG required to cause half-maximal inhibition of binding.
Immunoblot and coimmunoprecipitation
Lysates were prepared as described in “Hsp90 binding assays.” Protein-A Sepharose beads (Zymed Laboratories, South San Francisco, CA) were preblocked with 5% bovine serum albumin (BSA). The cell lysates were precleared by incubating with 50 μL Protein-A Sepharose beads (50% slurry). To 100 μL of the precleared cell lysate, either no antibody or antibodies to Hsp90, ZAP-70, p23, Hop, or p72Syk were added, and incubated by rotating for 1 hour at 4°C. Precleared beads (50 μL; 50% slurry) were then added and incubated by rotating for 1 hour at 4°C. Bound beads were centrifuged at 3000g and unbound samples were collected. Beads were washed thrice in lysis buffer and then once with 50 μM Tris, pH 6.8. We added SDS-sample buffer and then incubated the samples for 5 minutes at 95°C. Bound and unbound samples were analyzed by SDS-PAGE and immunoblot analyses using the indicated antibodies.
Protein lysates for immunoblot studies were prepared using RIPA buffer with protease inhibitors (10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, and 1 mM phenylmethylsulfonyl fluoride), and in some cases phosphatase inhibitors (1 mM Na-Vanadate and 10 mM β-glycerophosphate). Membranes were probed with antibodies as indicated. Detection was accomplished by chemiluminescence using horseradish peroxidase (HRP)-conjugated antibodies followed by development with ECL Plus (Amersham-Biosciences, Piscataway, NJ) and autoradiography with Super RX film (Fuji, Tokyo, Japan).
Flow cytometry and apoptosis detection
Fluorochrome-conjugated monoclonal antibodies were used for flow cytometry as described.15 The samples were processed using a FACScalibur (Becton Dickinson BD, Franklin Lakes, NJ) and the data were analyzed using Flo-Jo 3.6 software (Stanford University-Tree Star, San Francisco, CA). Apoptotic and viable cells were discriminated via flow cytometry with 3,3′ dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes, Eugene, OR) and propidium iodide (PI; Sigma), as described.16 Using this method, viable cells exclude propidium iodide (PI) and stain brightly positive for DiOC6.
Adenovirus transduction of CLL cells
We cloned the cDNA of ZAP-70 extracted from normal T cells (provided by A. Weiss)17 into the cytomegalovirus (CMV) promoter and polyadenylation signal of pcDNA3. This construct was then subcloned into the shuttle vector MCS(SK)pXCX2 as described before.18 This shuttle vector was cotransfected with pJM17 into 293 cells using the calcium phosphate method. Isolated adenovirus plaques were harvested individually and used to infect 293 cells. High titer adenovirus preparations were obtained, as described.18 CLL cells were infected with either Ad-ZAP-70 or a control adenovirus vector (Ad-LacZ) for 48 hours at 37°C using a multiplicity of infection (MOI) of 1000.
Results
Hsp90 inhibitors induce apoptosis in CLL cells that express adverse prognostic markers
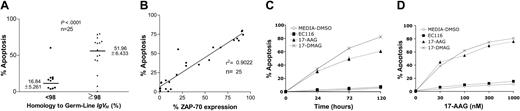
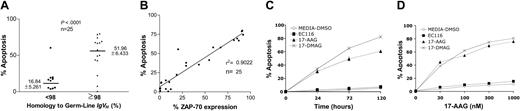
We treated primary leukemia cells (n = 25) with 17-AAG and examined them for drug-induced apoptosis at 48 hours (Table 1). Leukemia cells that used unmutated IgVH genes were significantly more sensitive to 17-AAG than were CLL cells that expressed mutated IgVH genes (Figure 1A; P < .001). Furthermore, there was a significant association between the level of apoptosis induced at 48 hours by 17-AAG and the level of ZAP-70 expressed by each of the leukemia-cell samples (r2 = 0.9022; Figure 1B). 17-DMAG, which is another Hsp90 inhibitor, induced a similar proapoptotic activity in CLL cells. However, a synthetic analog of 17-AAG lacking the capacity to inhibit Hsp90 (EC116) did not induce significant apoptosis (Figure 1C-D).
ZAP-70 physically associates with activated Hsp90 in CLL cells
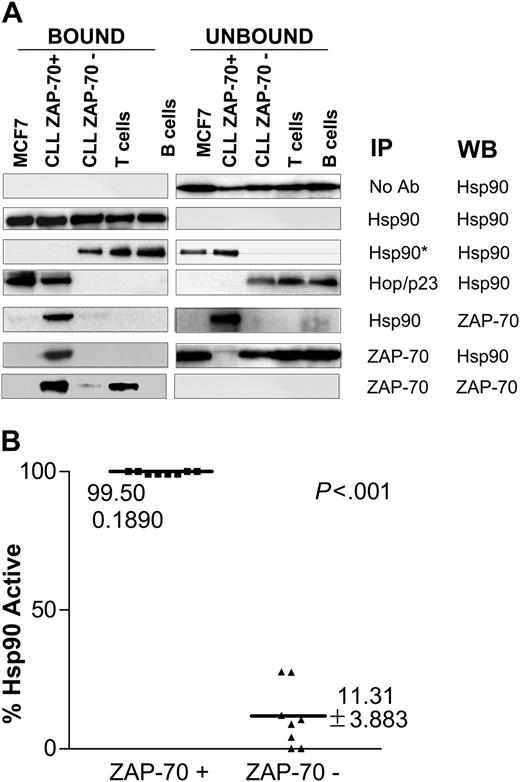
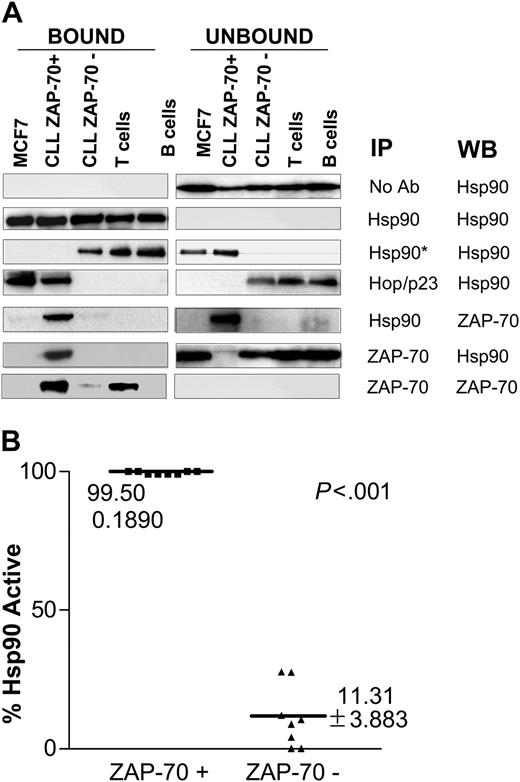
Protein lysates from primary leukemia cells were immunoprecipitated using different specific monoclonal antibodies, as shown in Figure 2A. CLL cells were defined as ZAP-70+ if more than 20% of the stained cells were positive by flow cytometry, as previously described.3,19 Controls included purified human B and T cells from healthy volunteers and MCF-7, a breast cancer cell line that expresses large amounts of activated Hsp90.11 All samples expressed similar levels of Hsp90. ZAP-70+ CLL and MCF-7 cells expressed Hsp90 that was complexed with the cochaperones Hop and p23. Immunoprecipitation with an antibody that recognizes the uncomplexed form of Hsp90 (Hsp90*), however, revealed that ZAP-70- CLL samples, as well as T and B cells of healthy donors, expressed the uncomplexed (nonactive) form of Hsp90. These results were consistently reproduced in larger numbers of CLL samples (n = 16; Figure 2B). Interestingly, the ZAP-70 expressed in CLL cells, but not in normal T cells, coimmunoprecipitated with Hsp90, suggesting that this tyrosine kinase is a conditional Hsp90 client protein, as its client status varies depending on the cell in which it is expressed.
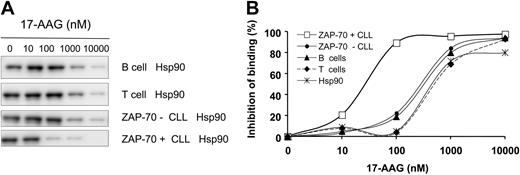
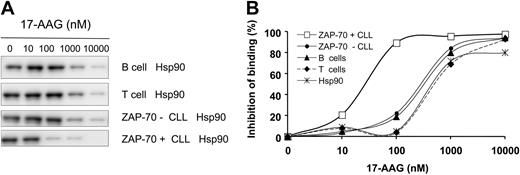
We performed competitive binding assays using a biotinylated GM (biotin-GM) probe to investigate whether the differential expression of active Hsp90 in CLL samples correlated with their binding affinity to 17-AAG (Figure 3A-B). Addition of 17-AAG to cell lysates inhibited the binding of Hsp90 to biotin-GM in a dose-dependent fashion, with ZAP-70+ CLL cell lysates experiencing an IC50 of 31 nM (n = 3; SEM ± 2). In contrast, the IC50 was 300 nM for lysates from ZAP-70- CLL cells or normal T or B cells. Purified native Hsp90 protein binding was inhibited with an IC50 of 600 nM, as reported11 (Figure 3A-B).
Inhibition of Hsp90 induces degradation of ZAP-70 in CLL cells but not in T cells
We monitored the expression levels of ZAP-70 in leukemia cells treated in vitro with 17-AAG, 17-DMAG, and a control synthetic analog (EC116). After 24 hours, 17-AAG and 17-DMAG caused a specific dose-dependent reduction of leukemia-cell ZAP-70 expression (inhibitory concentration of 50% [IC50] = 60 nM). This effect was not observed in the samples treated with EC116 or media containing DMSO (Figure 4A-B). Down-regulation of ZAP-70 after incubation with Hsp90 inhibitors correlated with cleavage of poly ADP-ribose polymerase (PARP-1), indicating leukemia-cell apoptosis. It also correlated with the up-regulation of Hsp70, a chaperone protein that is induced by inhibition of active Hsp9020 (Figure 4B). Of note, treatment with 17-AAG did not affect ZAP-70 expression levels in T cells from patients with CLL or healthy donors, even at high concentrations (300 nM; Figure 4C; and data not shown).
Hsp90 inhibitors induce apoptosis in CLL cells that express adverse prognostic markers. (A) Cell apoptosis was measured in CLL cells (n = 25) after in vitro treatment with 17-AAG at 100 nM during 48 hours. IgVH gene mutation was assessed by gene sequencing. Sequences with less than 98% homology to the corresponding germ-line IgVH sequence were considered mutated. (B) Correlation by linear regression of the level of expression of ZAP-70 and apoptosis induced by 17-AAG (100 nM) after 48 hours of incubation in CLL samples. (C, D) Cell death was assessed in ZAP-70+ CLL cells after treatment with 17-AAG, 17-DMAG, and EC116 using 100 nM at different time points and also during 48 hours using increasing concentrations.
Hsp90 inhibitors induce apoptosis in CLL cells that express adverse prognostic markers. (A) Cell apoptosis was measured in CLL cells (n = 25) after in vitro treatment with 17-AAG at 100 nM during 48 hours. IgVH gene mutation was assessed by gene sequencing. Sequences with less than 98% homology to the corresponding germ-line IgVH sequence were considered mutated. (B) Correlation by linear regression of the level of expression of ZAP-70 and apoptosis induced by 17-AAG (100 nM) after 48 hours of incubation in CLL samples. (C, D) Cell death was assessed in ZAP-70+ CLL cells after treatment with 17-AAG, 17-DMAG, and EC116 using 100 nM at different time points and also during 48 hours using increasing concentrations.
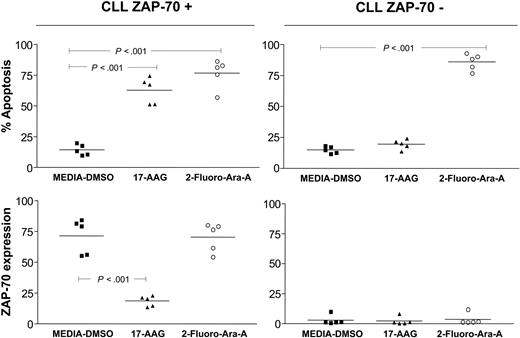
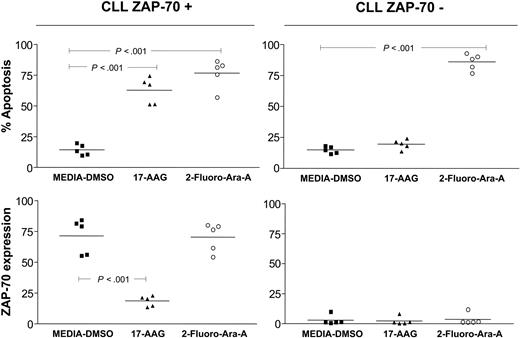
In addition, we observed that treatment of CLL cells with Fludara (2-Fluoro-Ara-A) did not induce down-regulation of ZAP-70 despite promoting apoptosis in leukemia cells. This suggests that down-regulation of ZAP-70 after treatment with Hsp90 inhibitors is specific and not due to apoptosis induced by an antileukemia agent per se (Figure 5).
ZAP-70 physically associates with activated Hsp90 in CLL cells. (A) Protein lysates from different cell types including ZAP-70+ and ZAP-70-CLL cells, normal T and B cells, and the breast cancer cell line MCF-7 were immunoprecipitated with the indicated antibodies, including Hsp90*, which is an antibody that recognizes the uncomplexed form of Hsp90. The bound and unbound immunoprecipitated products were probed by immunoblot using the antibodies indicated in the WB column. (B) Immunoprecipitation was used to assess the level of expression of complexed (activated) and uncomplexed (nonactivated) Hsp90 in different CLL samples (8 ZAP-70+ CLL and 8 ZAP-70-CLL). The figure shows the percentage of activated Hsp90 for each sample. This percentage was calculated after analyzing the digitalized composite ratio of immunoblot signal for Hsp90 uncomplexed and total Hsp90 using the following formula: % Hsp90active = 1-(Hsp90*/Hsp90total) × 100.
ZAP-70 physically associates with activated Hsp90 in CLL cells. (A) Protein lysates from different cell types including ZAP-70+ and ZAP-70-CLL cells, normal T and B cells, and the breast cancer cell line MCF-7 were immunoprecipitated with the indicated antibodies, including Hsp90*, which is an antibody that recognizes the uncomplexed form of Hsp90. The bound and unbound immunoprecipitated products were probed by immunoblot using the antibodies indicated in the WB column. (B) Immunoprecipitation was used to assess the level of expression of complexed (activated) and uncomplexed (nonactivated) Hsp90 in different CLL samples (8 ZAP-70+ CLL and 8 ZAP-70-CLL). The figure shows the percentage of activated Hsp90 for each sample. This percentage was calculated after analyzing the digitalized composite ratio of immunoblot signal for Hsp90 uncomplexed and total Hsp90 using the following formula: % Hsp90active = 1-(Hsp90*/Hsp90total) × 100.
Inhibition of Hsp90 blocks B-cell receptor (BCR) signaling in ZAP-70+ CLL cells
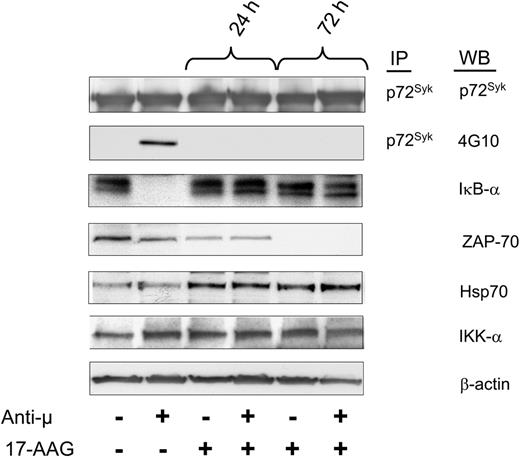
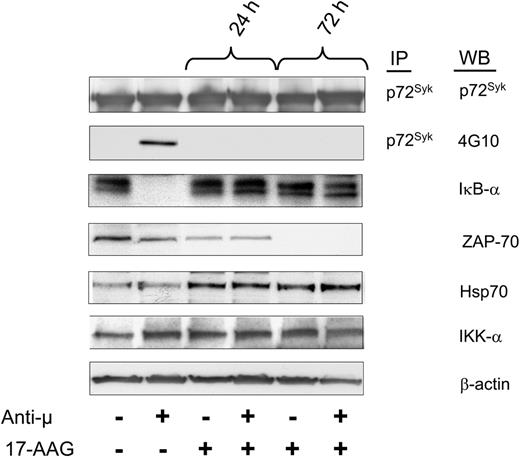
We treated ZAP-70+ CLL cells with 17-AAG and monitored their capacity to respond to ligation of the BCR with F(ab')2 anti-μ monoclonal antibody. We found that 17-AAG did not alter the level of p72Syk, which was detected in all samples. Stimulation of control-treated samples with anti-μ induced both phosphorylation of p72Syk and activation of nuclear factor kappa B (NF-κB), as assessed by degradation of IκB-α (Figure 6). However, prior treatment with 17-AAG for 24 hours rendered such leukemia cells inert to stimulation with anti-μ antibody.
Transduction of CLL cells to express ZAP-70 activates Hsp90 and induces sensitivity to 17-AAG-mediated apoptosis
We transduced ZAP-70- CLL cells with an adenovirus vector encoding ZAP-70 (Ad-ZAP-70) or β-galactosidase (Ad-LacZ) as a control. After 48 hours, Ad-ZAP-70-transduced CLL cells expressed ZAP-70 at levels that were comparable to that of ZAP-70+ CLL cells (Figure 7A). Transduction of CLL cells with Ad-ZAP-70, but not Ad-LacZ, induced formation of Hop and p23 multichaperone complexes, indicating a change in the conformation of Hsp90 from the latent state to the activated complexed form (Figure 7B). In addition, the de novo expression of ZAP-70 sensitized the cells to 17-AAG, which now could induce apoptosis in ZAP-70-transduced CLL cells even at low drug concentrations (Figure 7C).
Discussion
In the present study we show that ZAP-70 is an Hsp90 client protein in CLL cells but not in T cells and that ZAP-70 degradation via inhibition of Hsp90 leads to impaired signaling after BCR ligation and leukemia-cell apoptosis.
We found that CLL cells from different patients vary in their susceptibility to apoptosis induced by treatment with Hsp90 inhibitors. We investigated the basis for this and found a statistically significant correlation between the relative sensitivity to 17-AAG and expression of unmutated IgVH genes and ZAP-70. Moreover, there was a significant association between the level of apoptosis induced by 17-AAG and the level of ZAP-70 expression in the leukemia-cell samples.
Hsp90 expressed in ZAP-70+ CLL has increased binding affinity for 17-AAG. (A) Protein lysates from different samples were evaluated for their binding affinity to Hsp90 inhibitors in a competitive binding assay using a biotinylated geldanamycin (biotin-GM) probe and increasing concentrations of 17-AAG. (B) Hsp90 derived from ZAP-70+ CLL cells showed a higher binding affinity for 17-AAG with an IC50 of 31 nM (SEM ± 2), whereas Hsp90 from ZAP-70-CLL cells, and normal B and T cells had an IC50 of 300 nM. Purified recombinant Hsp90 had an IC50 of 600 nM. This experiment was reproduced 3 times.
Hsp90 expressed in ZAP-70+ CLL has increased binding affinity for 17-AAG. (A) Protein lysates from different samples were evaluated for their binding affinity to Hsp90 inhibitors in a competitive binding assay using a biotinylated geldanamycin (biotin-GM) probe and increasing concentrations of 17-AAG. (B) Hsp90 derived from ZAP-70+ CLL cells showed a higher binding affinity for 17-AAG with an IC50 of 31 nM (SEM ± 2), whereas Hsp90 from ZAP-70-CLL cells, and normal B and T cells had an IC50 of 300 nM. Purified recombinant Hsp90 had an IC50 of 600 nM. This experiment was reproduced 3 times.
Consistent with this notion, we found that ZAP-70+ CLL cells expressed Hsp90 in multichaperone complexes with high binding affinity for 17-AAG. Conversely, ZAP-70- CLL cells as well as normal T and B lymphocytes expressed the inactive, uncomplexed form of Hsp90. This is in accordance with our previous data showing that the molecular basis for the selective antitumor activity of 17-AAG and other ansamycins is related to the presence of an increased binding affinity to these compounds in tumor tissues compared with normal cells.11 The increased affinity appears to be due to cochaperone-induced changes in the ATP binding site of Hsp90, because tumor Hsp90 is present entirely in multichaperone complexes with high ATPase activity, whereas Hsp90 from normal tissues is in a latent, apparently uncomplexed state.11 We have also observed that the degree of Hsp90 activation correlated with expression of certain client kinases associated with poor prognosis, most notably HER-2 in breast cancer.11 Here, we provide evidence indicating that Hsp90 activation and sensitivity to 17-AAG in early-stage CLL cells correlate with poor prognostic factors such as unmutated IgVH genes and expression of ZAP-70 kinase.
Inhibition of Hsp90 induces degradation of ZAP-70 in CLL cells but not in T cells. (A) ZAP-70+ CLL cells were treated in vitro with increasing concentrations of 17-AAG (▴), 17-DMAG (×) and the analog EC116 (▪) for 48 hours. Cells incubated in media with DMSO 1% final concentration were used as a control (⋄). ZAP-70 expression was evaluated by intracellular staining. (B) ZAP-70 expression in CLL cells after treatment with 17-AAG (100 nM) was evaluated by immunoblot at different time points. In addition, immunoblots were probed with anti-Hsp70 and anti-poly ADP-ribose polymerase (PARP-1) monoclonal antibodies.β-actin was used as a protein loading control. Apoptosis after treatment with 17-AAG was 30% and 50% at 24 and 72 hours, respectively. (C) Peripheral blood mononuclear cells from ZAP-70+ patients were treated for 48 hours with 17-AAG (300 nM). The cells were stained with specific antibodies and analyzed by flow cytometry. The panel shows a density plot of cells labeled with anti-CD3 and anti-ZAP-70 antibodies. In each quadrant the percentage of cells is shown. The ZAP-70 mean florescence intensity (MFI) prior to treatment with 17-AAG in CLL cells was 110 and in T cells was 210. The posttreatment MFI value for ZAP-70 was 25 for CLL cells and 190 for T cells.
Inhibition of Hsp90 induces degradation of ZAP-70 in CLL cells but not in T cells. (A) ZAP-70+ CLL cells were treated in vitro with increasing concentrations of 17-AAG (▴), 17-DMAG (×) and the analog EC116 (▪) for 48 hours. Cells incubated in media with DMSO 1% final concentration were used as a control (⋄). ZAP-70 expression was evaluated by intracellular staining. (B) ZAP-70 expression in CLL cells after treatment with 17-AAG (100 nM) was evaluated by immunoblot at different time points. In addition, immunoblots were probed with anti-Hsp70 and anti-poly ADP-ribose polymerase (PARP-1) monoclonal antibodies.β-actin was used as a protein loading control. Apoptosis after treatment with 17-AAG was 30% and 50% at 24 and 72 hours, respectively. (C) Peripheral blood mononuclear cells from ZAP-70+ patients were treated for 48 hours with 17-AAG (300 nM). The cells were stained with specific antibodies and analyzed by flow cytometry. The panel shows a density plot of cells labeled with anti-CD3 and anti-ZAP-70 antibodies. In each quadrant the percentage of cells is shown. The ZAP-70 mean florescence intensity (MFI) prior to treatment with 17-AAG in CLL cells was 110 and in T cells was 210. The posttreatment MFI value for ZAP-70 was 25 for CLL cells and 190 for T cells.
Our studies demonstrated that ZAP-70 associates with activated Hsp90 in CLL cells and that treatment with Hsp90 inhibitors, such as 17-AAG or 17-DMAG, induced specific degradation of this kinase. Consistent with this, we did not observe degradation of p72Syk in 17-AAG-treated CLL cells, despite the high degree of similarity of this protein kinase to ZAP-70. Also, treatment with 2-Fluoro-Ara-A did not induce changes in ZAP-70 expression despite inducing apoptosis in CLL cells. This indicates that down-regulation of ZAP-70 mediated by Hsp90 inhibitors is a specific effect that is not due to proteolysis induced by apoptosis per se.
Together, these data indicate that ZAP-70 is itself an Hsp90 client protein susceptible to specific degradation induced by Hsp90 inhibitors. To our knowledge, this is the first time that such association has been reported. Interestingly, the requirement for Hsp90 chaperoning support by ZAP-70 was limited to CLL cells and was not observed in T cells where this kinase is normally expressed.17 In this particular sense, ZAP-70 is unique among identified Hsp90 clients as its chaperone dependency is conditional on the type of cell in which it is expressed.
The transduction experiments that we performed in ZAP-70- CLL cells employed a T-cell-derived ZAP-70 construct,17 suggesting that sequence or splicing variation of the ZAP-70 gene in CLL cells or T cells is not responsible for these discrepancies. Posttranslational modifications (eg, phosphorylation, SUMOylation, or acetylation) or changes in tertiary structure required to accommodate different substrates may underlie the differential chaperoning support required by ZAP-70 in CLL cells versus T cells. Further studies are under way to address this.
Degradation of ZAP-70 mediated by Hsp90 inhibitors is specific and is not induced by cytotoxic chemotherapy in vitro. ZAP-70+ and ZAP-70-CLL cells (n = 10) were treated with 17-AAG (100 nM) and 2-Fluoro-Ara-A (2.5 μM) for 48 hours. Flow cytometry was used to assess apoptosis (top panels) and ZAP-70 expression (bottom panels) on each sample. Control samples were incubated with media-DMSO as indicated. Error bars indicate the mean value for each group. P values were calculated using a one-way analysis of variance (ANOVA) with Bonferroni posttest analysis.
Degradation of ZAP-70 mediated by Hsp90 inhibitors is specific and is not induced by cytotoxic chemotherapy in vitro. ZAP-70+ and ZAP-70-CLL cells (n = 10) were treated with 17-AAG (100 nM) and 2-Fluoro-Ara-A (2.5 μM) for 48 hours. Flow cytometry was used to assess apoptosis (top panels) and ZAP-70 expression (bottom panels) on each sample. Control samples were incubated with media-DMSO as indicated. Error bars indicate the mean value for each group. P values were calculated using a one-way analysis of variance (ANOVA) with Bonferroni posttest analysis.
Inhibition of Hsp90 impairs B-cell receptor (BCR) signaling in ZAP-70+ CLL cells. ZAP-70+ CLL cells were preincubated with 17-AAG for 24 and 72 hours and then treated for 10 minutes with F(ab')2 anti-μ antibodies to induce cellular activation. Protein lysates were immunoprecipitated (IP) with specific anti-p72Syk antibody and assessed by Western blot (WB) for tyrosine phosphorylation using 4G10 antibody and p72Syk expression. Protein lysates from the same samples were evaluated by Western blot using the indicated antibodies. Activation of NF-κB was assessed by degradation of IκB-α. These results were reproduced more than 3 times.
Inhibition of Hsp90 impairs B-cell receptor (BCR) signaling in ZAP-70+ CLL cells. ZAP-70+ CLL cells were preincubated with 17-AAG for 24 and 72 hours and then treated for 10 minutes with F(ab')2 anti-μ antibodies to induce cellular activation. Protein lysates were immunoprecipitated (IP) with specific anti-p72Syk antibody and assessed by Western blot (WB) for tyrosine phosphorylation using 4G10 antibody and p72Syk expression. Protein lysates from the same samples were evaluated by Western blot using the indicated antibodies. Activation of NF-κB was assessed by degradation of IκB-α. These results were reproduced more than 3 times.
It is conceivable that the expression of nonmutated oncogenic kinases or proteins in nonphysiologic cell types or intracellular compartments may render them chaperone-dependent. Although we are unaware of other examples of proteins acquiring Hsp90 dependence due to inappropriate expression in a different mammalian cell, there are parallels in other systems. Viruses must translate their proteins in the foreign environment of the host cell, and several essential proteins of such human pathogens are Hsp90 clients, including the hepatitis B virus (HBV) reverse transcriptase and hepatitis C virus (HCV) protease.21,22 Similarly, the BSA protein attains client status when it is inappropriately expressed in the HeLa-cell cytosol, presumably because it adopts an aberrant conformation.23
Transduction of CLL cells to express ZAP-70 activates Hsp90 and induces sensitivity to 17-AAG-mediated apoptosis. (A) ZAP-70-leukemia cells were transduced with adenovirus expressing ZAP-70 (Ad-ZAP-70; solid histogram) and β-galactosidase (Ad-LacZ; bold line histogram). Cells were kept in media as a control (dashed line histogram). After 48 hours in culture Ad-ZAP-70- but not Ad-LacZ-transduced cells expressed the ZAP-70 transgene (46% expression with a mean fluorescence intensity ratio [MFIR] of 2.8). (B) ZAP-70-CLL cells were transduced with adenovirus and after 48 hours they were harvested and lysed. Immunoprecipitations and immunoblots were performed with the antibodies indicated. The bound fractions represent the immunoprecipitated proteins and the unbound samples represent the soluble protein lysate after immunoprecipitation. (C) After 48 hours of transduction with adenovirus, the leukemia cells were treated in vitro with 17-AAG (100 nM) for additional 48 hours and then assessed for apoptosis by flow cytometry using DiOC6 and PI. After treatment with 17-AAG, CLL cells transduced with Ad-ZAP-70 had a significantly higher level of apoptosis compared with nontransduced or Ad-LacZ-transduced cells; P = .001.  indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.
indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.
Transduction of CLL cells to express ZAP-70 activates Hsp90 and induces sensitivity to 17-AAG-mediated apoptosis. (A) ZAP-70-leukemia cells were transduced with adenovirus expressing ZAP-70 (Ad-ZAP-70; solid histogram) and β-galactosidase (Ad-LacZ; bold line histogram). Cells were kept in media as a control (dashed line histogram). After 48 hours in culture Ad-ZAP-70- but not Ad-LacZ-transduced cells expressed the ZAP-70 transgene (46% expression with a mean fluorescence intensity ratio [MFIR] of 2.8). (B) ZAP-70-CLL cells were transduced with adenovirus and after 48 hours they were harvested and lysed. Immunoprecipitations and immunoblots were performed with the antibodies indicated. The bound fractions represent the immunoprecipitated proteins and the unbound samples represent the soluble protein lysate after immunoprecipitation. (C) After 48 hours of transduction with adenovirus, the leukemia cells were treated in vitro with 17-AAG (100 nM) for additional 48 hours and then assessed for apoptosis by flow cytometry using DiOC6 and PI. After treatment with 17-AAG, CLL cells transduced with Ad-ZAP-70 had a significantly higher level of apoptosis compared with nontransduced or Ad-LacZ-transduced cells; P = .001.  indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.
indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.
We did not observe activated Hsp90, 17-AAG-mediated ZAP-70 degradation, or apoptosis in normal T cells or T cells derived from patients with CLL, even when higher concentrations of 17-AAG were used. In addition, Hsp90 from T cells had a 10-fold-lower binding affinity for 17-AAG than did Hsp90 from ZAP-70+ CLL cells. As such, 17-AAG and its derivatives may selectively target CLL cells at concentrations that do not affect T cells. These results are similar to those recently reported for geldanamycin,24 which also appears selectively nontoxic for T cells of patients with CLL. In contrast to the results reported here, this prior study did not discern a significant difference in the toxicity of geldanamycin for ZAP-70+ versus ZAP-70- CLL cells, as several ZAP-70- CLL samples were highly sensitive.24 This may be because such leukemia-cell samples were from patients with advanced-staged disease, in which CLL cells may have incurred secondary genetic changes that could increase their sensitivity to geldanamycin, as has been noted for other leukemias.25
Treatment of ZAP-70+ CLL cells with 17-AAG also resulted in loss of BCR signaling. We have reported previously that expression of ZAP-70 in CLL allows for more effective IgM signaling in CLL cells, a feature that could contribute to the relatively aggressive clinical behavior associated with CLL cells that express unmutated IgVH genes.26 This enhanced signaling is associated with higher levels of phosphorylated p72Syk, B-cell linker protein (BLNK), and phospholipase-Cγ, and greater Ca2+ flux.26 Therefore, our findings lend further support to the emerging physiologic role of ZAP-70 in CLL cells. The signaling events after BCR ligation might not be specifically due to inhibition of ZAP-70, as other kinases that participate in BCR signaling also may be influenced by Hsp90 inhibition.27-29 Nevertheless, we found that 17-AAG did not induce degradation of p72Syk or IKK-α, suggesting that the loss of BCR signaling most likely is due to degradation of ZAP-70 resulting from inhibition of Hsp90. Reconstitution of the ZAP-70/Hsp90/cochaperone complex in vitro or the use of RNA interference techniques targeting ZAP-70 might provide additional support to this notion.
ZAP-70+ CLL cells underwent apoptosis upon treatment with low concentrations of 17-AAG or 17-DMAG. This effect was time- and dose-dependent and correlated closely with the level of ZAP-70 expression. Conceivably, other proteins, including some that support leukemia-cell survival, are also degraded upon inhibition of Hsp90, thereby contributing to the effects seen in treated CLL cells. Indeed, some proteins identified as Hsp90 client proteins are known survival-signaling kinases, such as IGF-1R, Akt, Raf-1, and IKK.6-8,11,30 However, transduction of ZAP-70- CLL cells with an adenovirus encoding ZAP-70, but not with a control adenovirus vector, activated Hsp90 and specifically rendered the leukemia cells sensitive to apoptosis induced by 17-AAG. This indicates that the expression of wild-type ZAP-70 in CLL cells was sufficient to induce activation of Hsp90. Also, our experiments suggest that ZAP-70+ CLL cells are dependent on ZAP-70 for their survival and that degradation of this kinase, as observed after treatment with Hsp90 inhibitors, effectively impairs not only BCR signaling, but also cell survival.
Taken together, our data suggest that differences in clinical prognosis and cancer progression in CLL may be linked to the activity of Hsp90. Inappropriate ZAP-70 expression in malignant cells of the B lineage increases their malignant potential, indicating that ZAP-70 may represent an adaptive change in the somatic evolution of CLL in vivo. Provocative recent findings indicate that Hsp90 may play a buffering role in Darwinian evolution rescuing potentially misfolded mutant or aberrantly expressed proteins such that they do not become lethal to the cell.31,32 By analogy, activation of Hsp90 might protect aggressive CLL cells by stabilizing ZAP-70, thus allowing this kinase to support tumor-cell survival and/or proliferation. By the same token, our data indicate that ZAP-70 expression in CLL cells confers heightened sensitivity to 17-AAG or 17-DMAG, indicating that Hsp90 inhibitors might have substantial therapeutic activity, particularly in patients with aggressive, ZAP-70+ disease.
Prepublished online as Blood First Edition Paper, June 21, 2005; DOI 10.1182/blood-2005-03-1099.
Supported by a National Institutes of Health K08 grant CA106 605-01 (J.E.C.) and PO1 grant CA 81534 for the CLL Research Consortium (T.J.K.).
A.K. and F.J.B. are employed by Conforma Therapeutics Corporation, whose product was studied in the present work.
J.E.C. and C.E.P. contributed equally to this work.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are thankful for the collaboration provided by Dr Laura Rassenti, Chronic Lymphocytic Leukemia Research Consortium (CRC), and the technical assistance of Dr Frank Dicker (Laboratory for Leukemia Diagnostics, University Hospital Grosshadern, Germany).


![Figure 7. Transduction of CLL cells to express ZAP-70 activates Hsp90 and induces sensitivity to 17-AAG-mediated apoptosis. (A) ZAP-70-leukemia cells were transduced with adenovirus expressing ZAP-70 (Ad-ZAP-70; solid histogram) and β-galactosidase (Ad-LacZ; bold line histogram). Cells were kept in media as a control (dashed line histogram). After 48 hours in culture Ad-ZAP-70- but not Ad-LacZ-transduced cells expressed the ZAP-70 transgene (46% expression with a mean fluorescence intensity ratio [MFIR] of 2.8). (B) ZAP-70-CLL cells were transduced with adenovirus and after 48 hours they were harvested and lysed. Immunoprecipitations and immunoblots were performed with the antibodies indicated. The bound fractions represent the immunoprecipitated proteins and the unbound samples represent the soluble protein lysate after immunoprecipitation. (C) After 48 hours of transduction with adenovirus, the leukemia cells were treated in vitro with 17-AAG (100 nM) for additional 48 hours and then assessed for apoptosis by flow cytometry using DiOC6 and PI. After treatment with 17-AAG, CLL cells transduced with Ad-ZAP-70 had a significantly higher level of apoptosis compared with nontransduced or Ad-LacZ-transduced cells; P = .001. indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-03-1099/6/m_zh80190584790007.jpeg?Expires=1769744087&Signature=v9ppzKjCq4hqLZyKS-uAkTRDSNLdCHwr3lBC2-b8~8HndB6mga1FUnUurIwF0hHHr5jME~8XpgQvYIHPLtMfi5y~ag2WR8tpRaSZ8nibEJcJgtASqg3tstTi3kKtxL9QMekk9TYqV0V2T75JB9NTx2KRz2BL1MXmQGHx5ByIRWcE4zRo78RrnUA-dEgHpnBqWbkEUdvMFEDMj1-nfAFFucjaQzSuz0~a~Tg~7rL9rxIccqmcBkztUMyOuz8sozZd79X3f7kHCmtd3m~K~eH~XiC2L6N90wvTl8mCOnEso7Eg54T5b2McfPc~O8egxwEaAhYOfE9hsY99ej~LdYxaGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 7. Transduction of CLL cells to express ZAP-70 activates Hsp90 and induces sensitivity to 17-AAG-mediated apoptosis. (A) ZAP-70-leukemia cells were transduced with adenovirus expressing ZAP-70 (Ad-ZAP-70; solid histogram) and β-galactosidase (Ad-LacZ; bold line histogram). Cells were kept in media as a control (dashed line histogram). After 48 hours in culture Ad-ZAP-70- but not Ad-LacZ-transduced cells expressed the ZAP-70 transgene (46% expression with a mean fluorescence intensity ratio [MFIR] of 2.8). (B) ZAP-70-CLL cells were transduced with adenovirus and after 48 hours they were harvested and lysed. Immunoprecipitations and immunoblots were performed with the antibodies indicated. The bound fractions represent the immunoprecipitated proteins and the unbound samples represent the soluble protein lysate after immunoprecipitation. (C) After 48 hours of transduction with adenovirus, the leukemia cells were treated in vitro with 17-AAG (100 nM) for additional 48 hours and then assessed for apoptosis by flow cytometry using DiOC6 and PI. After treatment with 17-AAG, CLL cells transduced with Ad-ZAP-70 had a significantly higher level of apoptosis compared with nontransduced or Ad-LacZ-transduced cells; P = .001. indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-03-1099/6/m_zh80190584790007.jpeg?Expires=1769744088&Signature=f-nwhAKtVsSeasJgOe7OGsongiLtB-BGtWI5VmfvhEGN8cg4p5MEYVb6~ao-4AmiFqKBD85Gwp2Pf8rVORP5YRal-Y9m9l3dSwgFs9Q3fKCVp7ERrrRsq3lJkV6cqeB~0IlbX~nv5jScO1hk4vDMVXf5faeyBWp8CeRiQYmathCgI8q47ARJGqk2kmfzgAO4zUJVesOOjCwiHHYpmW3BwBYNDzdUD3Z6pghzWwfGcYDOuzMkqSgiFVHWgYUw3XrdQQkfULFKiSrdNgf-6lFQcu2JIR1dB~7oxqi4U42Mhk6JF5q6kMqVMGSG62GmkqdSZ8isOqB2vxvcUWGbXzgpXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.
indicates control, untreated with 17-AAG; ▪, treated with 17-AAG.