Abstract
The existence of macrophages (Mφ) of yolk-sac (YS) origin has been reported in all vertebrate models. However, the nature of their precursors and pathways of differentiation have not been elucidated. Phenotypic and differentiation potential analyses of YS at 7.5 to 10 postcoital days (dpc), performed in CX3CR1GFP embryos, allowed us to discern 3 independent Mφ populations. A first transient wave consisted of mature, maternal-derived Mφpresent as early as 7.5 to 8 dpc. A second wave of committed Mφ precursors arose at 8 dpc (2-4 somite stage) and was followed by a third wave of erythromyeloid precursors (4-6 somite stage). Both types of precursors displayed similar phenotypes and gave rise to CX3CR1/green fluorescent protein (GFP)–positive Mφ, but differed by their differentiation potential, at the clonal level. The combined data of phenotypic, gene-expression, and in situ analyses allowed us to conclude that the previously named “primitive Mφ” corresponded to a mixture of the first transient wave and committed Mφ precursors. Both YS-derived precursors followed a developmental pathway common to adult Mφ and could be qualified as definitive.
Introduction
During mouse embryogenesis, the first hematopoietic cells appear in the yolk-sac (YS) blood islands shortly after the onset of gastrulation. In the past, this site had been considered the source of hematopoietic stem cells (HSCs), which would expand to form a pool of definitive HSCs ultimately responsible for adult hematopoiesis.1 During the past decade, this scheme drastically evolved. Evidence, obtained first in avian then in mouse and human embryos2 (for a review, see Godin and Cumano3 ), indicated that the region around the dorsal aorta contains precursors responsible for the establishment of definitive hematopoiesis. The hematopoietic precursors generated in the extraand intra-embryonic sites differ in their self-renewal and differentiation potentials. Precursors generated in the intra-embryonic compartment provide in vivo long-term multilineage reconstitution of the hematopoietic compartment, whereas those from the YS provide only short-term myeloid reconstitution.4,5
The first hematopoietic cells generated in the YS belong to the erythroid and myeloid lineages. Erythroid cells consist of nucleated erythrocytes, larger than their adult counterparts and expressing a different set of hemoglobins (ζ, βH1, and ∈), that are qualified as primitive.6 Although the presence of macrophages (Mφ) in the YS has been reported since the early 1900s, their in situ production was first documented only approximately 10 years ago, both in the chicken7 and in the mouse embryo.8,9 In the mouse YS, Naito et al9,10 described 2 different Mφ lineages, qualified as “primitive” and “definitive.” Primitive Mφ are morphologically identifiable in the YS at 8 to 8.5 postcoital days (dpc), express the terminal differentiation marker F4/80 soon after, and apparently bypass the promonocyte and monocyte stages for differentiation. The second wave of YS Mφ, detected at the same stage, displays the myeloperoxidase (MPO) activity9,10 typical of promonocyte and monocyte intermediates and thus follows a differentiation pathway similar to that of bone marrow Mφ. These “definitive” YS-Mφ reach full maturation at 10 dpc, a stage at which they express F4/80. It was originally proposed that the 2 lineages derive from a precursor endowed with erythromyeloid differentiation potential.8
Common precursors for erythrocytes, granulocytes, macrophages, and mast cells have indeed been detected in the YS. They have a high proliferative capacity (high proliferative potential colony-forming cells [HPP-CFCs]). They colonize the fetal liver (FL) where they differentiate, thus contributing to fetal hematopoiesis.11 However, HPP-CFCs are detected in the YS at 8.5 dpc, after the 5- to 8-somite stage (5-8S),11 1 day later than the first Mφ-restricted precursors and primitive erythrocytes.12 This observation is, therefore, not consistent with the hypothesis of a hierarchic relationship between HPP-CFCs and primitive Mφ.
We thus readdressed the lineage relationship between early YS macrophages and erythromyeloid precursors by associating cellsurface–marker characterization and differentiation potential analysis with a genetic marker of the monocyte/macrophage lineage. We used embryos from CX3CR1GFP mice, harboring a targeted replacement of the CX3CR1 chemokine receptor gene by a cDNA encoding the enhanced green fluorescence protein (eGFP). In adult mice, CX3CR1 is expressed by monocytes and a subset of peripheral mononuclear phagocytes that includes Mφ and dendritic cells.13 In the monocyte population, the CX3CR1 expression level distinguishes 2 Gr-1– and Gr-1+ monocyte subsets that yield resident and inflammatory Mφ, respectively.14
Here we characterize the successive waves of Mφ and precursors that are present in the YS. We demonstrate the existence of 2 distinct pools of committed Mφ in the early YS, before the emergence of erythromyeloid precursors. The first Mφ population, detected in 0 to 8S embryos (8 dpc) as CD45+Mac-1+F4/80+, does not express CX3CR1 (GFP–). These Mφ are maternally derived and are found inserted in the embryonic mesoderm at a specific location and developmental stage.
The second Mφ population derives from monopotent precursors that appear in the YS shortly before erythromyeloid precursors. Both monopotent Mφ and erythromyeloid precursors share a CD45–c-Kit+ phenotype. Both display high proliferative capacity and differentiate into Mac-1+F4/80+CX3CR1+ macrophages, a phenotype similar to that displayed by recently generated adult Mφ.13 These 2 waves are generated in situ in the early YS, in the absence of lymphomyeloid multipotent precursors.
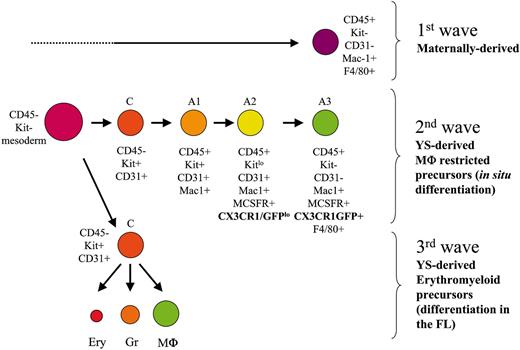
Thus, during a short window of development, the extraembryonic mesoderm contains 3 consecutive types of macrophages, 2 of which are generated in situ, with increasing differentiation potential but following a differentiation pathway similar to that of definitive macrophage precursors through a MPO+ monocyte stage.
Materials and methods
Animals and dissections
Two C57BL/6 congenic lines (H2b haplotype) bearing the CD45.1 and CD45.2 alleles of the pan-hematopoietic marker CD45 and their F1 progeny were used in this study. C57BL/6-CX3CR1-GFP (CX3CR1GFP) knock-in mice were also used. Mice were bred in the animal facilities of the Pasteur Institute (Paris, France).
Embryonic development was estimated considering the day of vaginal plug observation as 0.5 dpc. Embryos between 7.5 and 10.5 dpc were staged by somite counting and, during presomitic stages, according to criteria previously defined.15 Dissections of precirculatory YS (7.5-8.5 dpc) and of 10 to 10.5 dpc YS and AGM and in vitro cultures were performed as described.5,16
Flow cytometry analyses and cell sorting
Flow cytometry analyses were performed in an LSR (Life Sciences Research) flow cytometer with CellQuest software (Becton Dickinson, Mountain View, CA). The following antibodies (biotinylated or coupled with different fluorochromes) recognizing the following surface antigens, all from PharMingen (San Diego, CA), were used: CD45.1 (clone A20), c-Kit (clone 2B8), Gr-1 (clone RB6-8C5), Mac-1 (clone M1/70), CD45R/B220 (clone RA3-6B2), immunoglobulin M (IgM), Ter-119, CD31 (clone MEC 13.3), and CD45 (30-F-11). Macrophage–colony-stimulating factor receptor α (M-CSFRα) (AFS98) was from e-Biosciences (San Diego, CA), and F4/80 (clone CI:A3-1) was from TEBU-Caltag (Burlingame, CA). Biotinylated antibodies were revealed with streptavidin coupled with phycoerythrin (PE)–CY7 or allophycocyanin (APC).
Cell sorting was performed with a MoFlo cell sorter (DakoCytomation, Trappes, France). The single-cell dispenser robot (CyClone software) was used for direct deposition of single cells on culture plates.
Immunostaining and in situ hybridization
Embryos ranging from the LS to the 5S stages were fixed for 2 to 4 hours with 4% paraformaldehyde and processed for whole-mount immunolabeling using F4/80-PE (Caltag; clone CI:A3-1) and CD45-Cychrome (PharMingen; clone 30F11) antibodies. To determine the localization of labeled cells, embryos were further counterstained for 20 minutes at room temperature with the fluorescent vital dye Bodipy-ceramide (D3521; Molecular Probes, Eugene, OR) at a 1/2500 dilution. Embryos, placed in a phosphate-buffered saline (PBS) and 10% fetal calf serum (FCS) solution, were observed with a laser scanning confocal microscope (LSM 510; Zeiss, Oberkochen, Germany). Image stacks were collected using a 20 ×/0.75 NA apochromat plan objective, using LSM 5 image examiner 3.0 SP2 software (Zeiss, Oberkochen, Germany). Excitation wavelengths for Bodipy-ceramide, PE, and Cychrome were 488 nm, 543 nm, and 488 nm, respectively. Images were acquired using BP505-530, BP560-615, and LP650.
Whole-mount in situ hybridizations were performed as previously described.17 Digoxin (DIG)–labeled (Boehringer Mannheim, Mannheim, Germany) βH1 riboprobes were obtained from a 251-bp cDNA fragment subcloned into a pCRII vector, kindly provided by Isabelle Max-Audit (Paris, France).
Fetal liver organ cultures
γ-Irradiated (2.5 Gy) 10.5 dpc fetal liver lobes from CD45.1 × CD45.2 embryos were seeded with either 8 dpc YS cells (0.5-4 YS freshly isolated or after 4-day organ culture) or with 10.5 dpc AGM cells, all explanted from CD45.2 embryos. After 1 day in hanging drop, fetal liver organ cultures (FLOCs) were transferred onto filters, as previously described for fetal thymus organ cultures (FTOCs).18 Ten days later, FLOCs were analyzed by flow cytometry for the presence of CD45.1– myeloid cells (Mac-1, Gr-1) and B cells (B220, IgM).
Differentiation potential assay
Single sorted YS cells were cultured for 14 days on an irradiated layer of OP9 stromal cells (kindly provided by S.-I. Nishikawa and T. Nakano, Kyoto, Japan), as previously described.19 Culture medium was supplemented with interleukin-3 (IL-3), c-Kit-L, granulocyte macrophage–colony-stimulating factor (GM-CSF), and L929-conditioned medium containing M-CSF.
YS precursor kinetic assay
YSs were explanted from wild-type (C57BL/6) or CX3CR1-GFP embryos at different stages from 7.5 dpc (presomitic stages) to 9.5 dpc (25S). Cells obtained from individual YSs were distributed in 48-well (0-8S) or 96-well (10-25S) culture plates on an irradiated OP9 stromal-cell layer, as described under “Differentiation potential assay.” After 2 weeks in culture, wells were scored for the presence of hematopoietic progeny. Only plates showing less than 37% of wells positive for cell growth were scored, ensuring clonality of the system, according to the Poisson equation. Erythromyeloid precursors were detected by flow cytometry through their capacity to generate Ter119+ and Gr-1+Mac1+ cells, whereas Mφ precursors could only give GFP+ macrophage colonies, as observable on a fluorescence microscope (Axiovert 135 TV; Zeiss). Giemsa staining was performed as described. Phagocytosis assay was performed by incubating cells at a ratio of 10:1 with fluorescent (PE-labeled) latex beads for 2 hours at 37°C. Cells were washed, observed under a microscope, and analyzed by flow cytometry.
Gene-expression analysis
Cells were lysed in TRIzol (Gibco BRL, Carlsbad, CA), and total RNA was extracted according to the manufacturer's protocol. Oligo(dT)–primed cDNA was prepared from 6000 to 15 000 cells using AMV Reverse Transcriptase (Gibco BRL) in a reaction volume of 20 μL. cDNA from 15 dpc FL cells, TER119 depleted or CD19+ sorted, were used as standard for the transcript quantification. cDNA from S17 stromal cells was used as negative control.
Quantitative reverse transcription–polymerase chain reaction (RT-PCR) was performed on the GeneAmp 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) in a total volume of 25 μL, using 1 μL cDNA, 1 μL each primer (10 μM), and 12.5 μL SYBR Green PCR Master Mix 2 × (Applied Biosystems). Each sample was tested in triplicate. For each independent experiment, hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression was scored in each population tested. Finally, the signals detected in each population for each transcript tested were normalized by HPRT. Primers used were: HPRT forward, 5′-GAC TGA AAG ACT TGC TCG AG-3′; HPRT reverse, 5′-CCA GCA AGC TTG CAA CCT TAA CCA-3′; AML-1 forward, 5′-CCAGCAAGC TGAGGAGCG GC-3′;AML-1 reverse, 5′-CGGATT TGT AAA GAC GGT GA-3′; TAL-1 forward, 5′-AAC CGG GTG AAG AGG AGG CCC TCC-3′; TAL-1 reverse, 5′-AAG CAC GTC CTG TAG AAG GTC-3′; MPO forward, 5′-CGC TTC TCC TTC TTC ACT GG-3′; MPO reverse, 5′-CTG CCA TTG TCT TGG AAT CG-3′; lysozyme M forward, 5′-ATG AAG ACT CTC CTGACT CTG GGAC-3′; lysozyme-M reverse, 5′-CCACGG TTG TAG TTT GTA GCT CGT G-3′. Other primers were GATA-220 and LMO-2,21 as described.
Results
Characterization of the macrophage lineage in early stages of yolk sac development
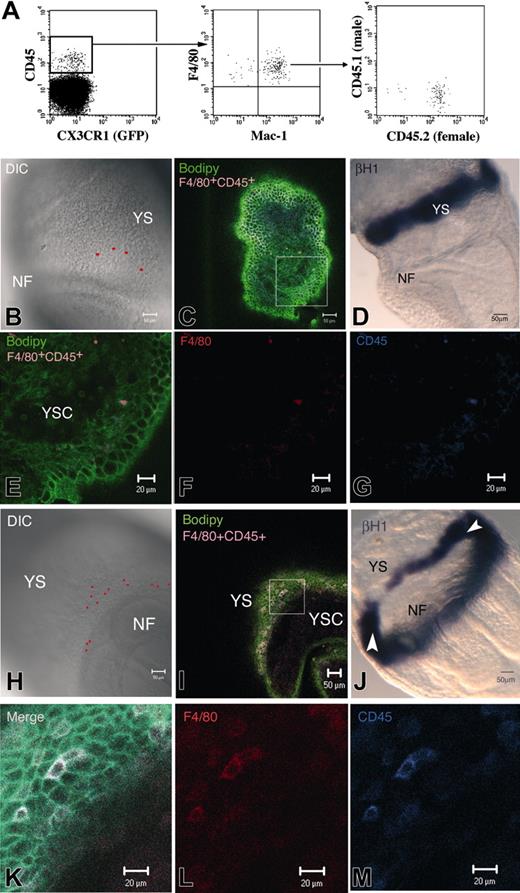
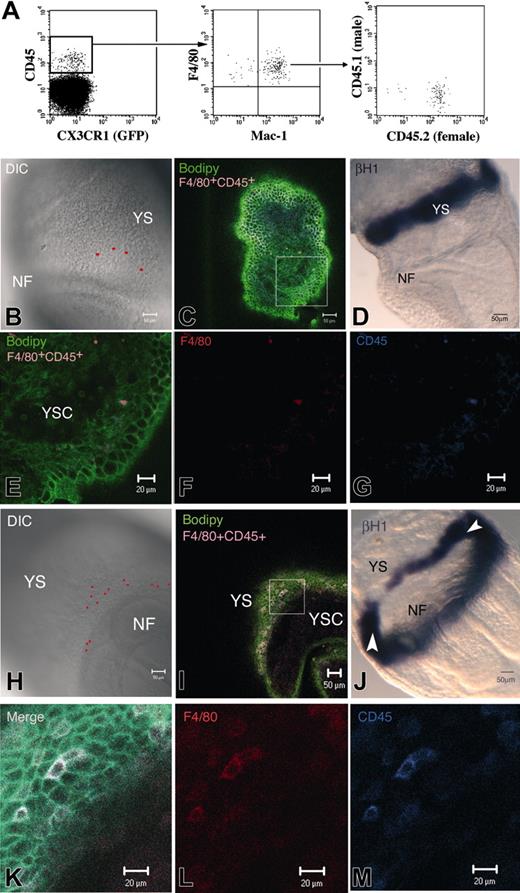
Transient mature macrophages. We recently described that, before the onset of FL hematopoiesis, cells belonging to the macrophage lineage, in the 10.5 dpc YS and AGM, express high levels of CD45, whereas HSCs are CD45–/low.19 To further characterize these Mφ, we analyzed the phenotype of 7.5 to 8 dpc (0-8S) YS cells isolated from CX3CR1GFP/+ embryos. A CD45+cKit– cell subset could already be detected at this stage. These cells express the late macrophage differentiation marker F4/80 but are negative for GFP (Figures 1A, 2A). Most of these cells also express Mac-1. Sorted CD45+c-Kit– cells from 8 dpc YS, cultured in limiting dilution, did not proliferate. Thus, CD45+ cells from 7.5 to 8 dpc YS seem to represent a mature subset of macrophages. We analyzed the origin of this population in embryos resulting from a CD45.1 × CD45.2 cross. Figure 1A shows that the 2 alleles are never coexpressed and that only the maternal allele is detected. These macrophages are, therefore, maternally derived.
To locate these Mφ, we performed whole-mount double immunostaining using the F4/80 and CD45 markers. Embryos were further counterstained with Bodipy-ceramide, a vital dye that labels intercellular spaces.22 Embryos were scanned for the presence of double-stained Mφ through the use of confocal microscopy.
Whereas embryos ranging from LS to LB stages (7 dpc; 8 embryos analyzed) did not display positive cells, most embryos from the LHF-0S (8 of 9) and up to 5S (3 of 7) stages contained F4/80+CD45+ cells. Because of the labeling of the structure by Bodipy-ceramide, we further ascertained that F4/80+CD45+ cells were located within the YS (Figure 1E-G, I, K-M) and did not merely adhere to its surface. Figure 1E, which shows a confocal YS section spanning the YS cavity, mesoderm, and endoderm, clearly indicates that the F4/80+CD45+ Mφ are located within the mesoderm.
F4/80+CD45+ Mφ were mainly found in the extra-embryonic compartment, close to the neural folds (Figure 1B, H) and caudal part of the embryo (data not shown). At the latest examined stages, a few F4/80+CD45+ cells were also found in the intra-embryonic compartment (data not shown). Careful examination of the location of these Mφ within the extra-embryonic compartment (Figure 1B, H), allowed us to conclude that they are mainly positioned below the YS blood islands. Direct visualization of the blood islands was performed with βH1 staining, which labeled their erythrocyte content (Figure 1D, J). Examination of the YS blood islands did not lead to the detection of labeled Mφ in this location (data not shown).
Characterization of maternally derived neural fold (NF) in the early YS. (A) At 8 dpc (0-8 S), a population of CD45+GFP– cells expressed Mac-1 and the terminally differentiated Mφ marker F4/80. In embryos resulting from a CD45.1 × CD45.2 cross, only the maternal allele was detected. These Mφ are thus maternally derived. (B-M) LHF embryo (B-G) and 0S embryo (H-M), stained with F4/80, CD45, and Bodipy-ceramide (C, E-G; I, K-M) or hybridized with a βH1 embryonic globin riboprobe (D, J). The red spots in the DIC picture (B, H) correspond to the confocal software–generated projection of F4/80+CD45+cells, as seen in the merged figure (C, I). YS-F4/80+CD45+cells are mainly located close to the NF and below the YS-BI compared with its position in LHF embryo hybridized with βH1 riboprobe (D, J). White arrowheads in J indicate the YS-BI level anteriorly because its caudal aspect is also visible. F4/80+CD45+cells are located within the YS mesoderm (E-G, higher magnification of the square in panel C; K-M, higher magnification of the square in panel I). NF indicates neural fold; YSC, yolk-sac cavity.
Characterization of maternally derived neural fold (NF) in the early YS. (A) At 8 dpc (0-8 S), a population of CD45+GFP– cells expressed Mac-1 and the terminally differentiated Mφ marker F4/80. In embryos resulting from a CD45.1 × CD45.2 cross, only the maternal allele was detected. These Mφ are thus maternally derived. (B-M) LHF embryo (B-G) and 0S embryo (H-M), stained with F4/80, CD45, and Bodipy-ceramide (C, E-G; I, K-M) or hybridized with a βH1 embryonic globin riboprobe (D, J). The red spots in the DIC picture (B, H) correspond to the confocal software–generated projection of F4/80+CD45+cells, as seen in the merged figure (C, I). YS-F4/80+CD45+cells are mainly located close to the NF and below the YS-BI compared with its position in LHF embryo hybridized with βH1 riboprobe (D, J). White arrowheads in J indicate the YS-BI level anteriorly because its caudal aspect is also visible. F4/80+CD45+cells are located within the YS mesoderm (E-G, higher magnification of the square in panel C; K-M, higher magnification of the square in panel I). NF indicates neural fold; YSC, yolk-sac cavity.
Quantification of these maternally derived Mφ by flow cytometry indicates that CD45+F4/80+ accounts for 0.4% of YS cells (approximately 35-40 cells per YS; Figure 2A) at 7.5 to 8 dpc (0-8S). This number, determined on pooled populations, is consistent with the number of Mφ observed in situ per embryo, where at the LHF-0S stages we detected up to 100 of these cells (Figure 1I). In subsequent developmental stages, the CD45+F4/80+ population regressed to 0.03% (2-5 cells per YS) at 8.5 to 9 dpc (10-15S) (data not shown). This decrease in population size indicated that YS colonization by maternally derived Mφ is a transient event.
Macrophages deriving from either monopotent or erythromyeloid precursors. Given that all the CD45+ cells present in the early developmental stages correspond to maternally derived Mφ, the phenotype of the previously described YS hematopoietic precursors12 remains unclear. We thus sorted cells from early YS (under 8S) based on the expression of CD45 and c-Kit. Figure 2A shows the staining profile of YS cell suspensions. The maternal CD45+ cell population described as F4/80+ does not express c-Kit. Three subsets—CD45+c-Kit–, CD45–c-Kit+, and CD45–c-Kit–—were sorted from 8 dpc YS, and their differentiation potentials were analyzed in a single cell assay (Table 1). In these cultures, 2 types of large colonies (more than 200 cells)—mixed colonies comprising erythrocytes, granulocytes, and macrophages and colonies containing only macrophages—were observed after 10 days in culture. In all instances macrophages were characterized by flow cytometry, GIEMSA staining, and the ability to phagocytose latex beads (data not shown). The macrophages derived from both types of precursors expressed CX3CR1-GFP (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). This analysis allowed us to define 2 types of precursors, a macrophage-restricted precursor (Mp) and a precursor with erythromyeloid potential (EMp), both with high proliferative capacity.
Identification of myeloid precursors in the 8 dpc YS. (A) Fractionation of 8 dpc YS using CD45 and c-Kit markers allows the discrimination of 3 subsets for further in vitro potential analysis. The percentage and absolute number per YS appear near the sorting gates. (B) Kinetics of macrophage-restricted (▪) and erythromyeloid precursors [□] in the YS from 2S to 8S, obtained by limiting-dilution experiments. Regression curves show that Mp (straight line) appear before EMp (dotted line).
Identification of myeloid precursors in the 8 dpc YS. (A) Fractionation of 8 dpc YS using CD45 and c-Kit markers allows the discrimination of 3 subsets for further in vitro potential analysis. The percentage and absolute number per YS appear near the sorting gates. (B) Kinetics of macrophage-restricted (▪) and erythromyeloid precursors [□] in the YS from 2S to 8S, obtained by limiting-dilution experiments. Regression curves show that Mp (straight line) appear before EMp (dotted line).
No hematopoietic cells could be recovered from the CD45–c-Kit– subset. As mentioned, the maternal CD45+ F4/80+ c-Kit– macrophage population did not expand in culture. The CD45–c-Kit+ population contains both EMp and Mp, at frequencies of 1:80 (0.12 per embryo) and 1:7.5 (1-2 per embryo), respectively (Table 1).
It was previously described that the Mp population appears before the EMp population.12 The higher enrichment of Mp compared with EMp in the CD45–c-Kit+ 8 dpc YS fraction tends to correlate with this statement. Nevertheless, EMp differentiation into Mp could not be ruled out. We reinvestigated, in a limitingdilution assay, the kinetics of Mp and EMp emergence in unsorted 8 dpc YS (2-8S). Figure 2B shows that the first restricted Mps are detected at the 2S stage, whereas the first EMps are consistently found from the 4-5S stage. Thus, consistent with previously published data, committed Mps emerge in the YS before the first EMps can be detected.12
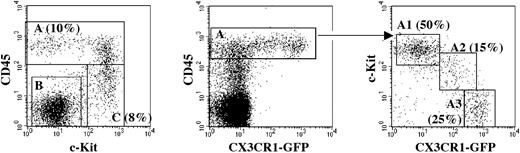
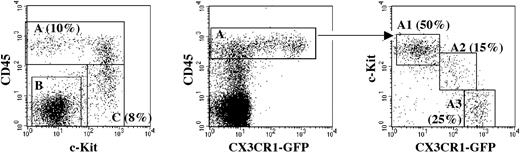
Distribution of myeloid precursors in the 10 dpc YS. Fractionation of CX3CR1GFP YS cells, using antibodies against CD45 and c-Kit, allows discrimination of 3 subsets (left). GFP expression is restricted to the CD45+ cells (middle). The latter can be subdivided into 3 fractions (right) according to the levels of c-Kit and GFP expression, namely A1 (c-Kit+GFP–), A2 (c-KitloGFPlo), and A3 (c-Kit–GFP+). Percentages of the respective populations are shown.
Distribution of myeloid precursors in the 10 dpc YS. Fractionation of CX3CR1GFP YS cells, using antibodies against CD45 and c-Kit, allows discrimination of 3 subsets (left). GFP expression is restricted to the CD45+ cells (middle). The latter can be subdivided into 3 fractions (right) according to the levels of c-Kit and GFP expression, namely A1 (c-Kit+GFP–), A2 (c-KitloGFPlo), and A3 (c-Kit–GFP+). Percentages of the respective populations are shown.
Finally, the higher number of committed Mφ precursors (1-2 per YS at 0-8S) compared with EMp (0.12 per YS) and the delayed appearance of EMp are concordant with the independent generation of both precursor types, as previously stated.12
These experiments allow us to define 3 independent Mφ lineages from the early YS: (1) an early population of maternally derived Mφ displaying a CD45+F4/80+c-Kit– CX3CR1– phenotype; (2) CX3CR1+ Mφ which derives from a CD45–c-Kit+ monopotent precursor (YS-Mp); and (3) CX3CR1+ Mφ which derives from a CD45–c-Kit+ erythromyeloid precursor (YS-EMp).
Characterization of the macrophage lineage in the 10 dpc yolk sac
We next characterized the YS myeloid precursors in the 10 dpc YS from CX3CR1GFP/+ embryos. The fluorescence-activated cell sorter (FACS) profile (Figure 3) shows that CX3CR1 expression is restricted to a CD45+ cell subset. In the CD45+ population, we could further isolate 3 subpopulations according to the expression of c-Kit and CX3CR1 (Figure 3), as follows: A1, CD45+cKit+GFP–; A2, CD45+c-KitloGFPlo; A3, CD45+c-Kit–GFP+. We tested the differentiation potential of these 3 fractions at the single-cell level in conditions that allowed the differentiation of granulocytes, erythrocytes, and Mφ. Culture wells were scored either by flow cytometry analysis (Mac-1, Gr-1, Ter119) or by fluorescence microscopy to visualize GFP+ macrophage colonies.
Table 2 shows the results of the clonal assays. Collectively, the CD45+ population was highly efficient in generating pure macrophage colonies, resembling those derived from YS-Mp identified at earlier stages. It also contained a low frequency of EMps.
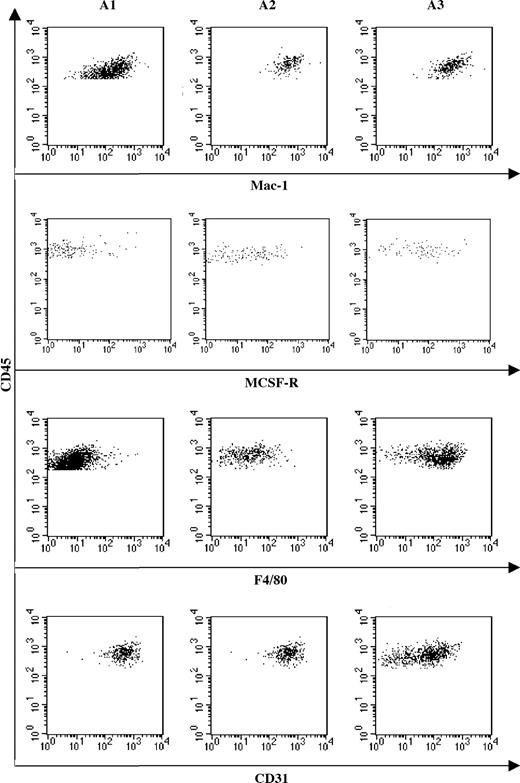
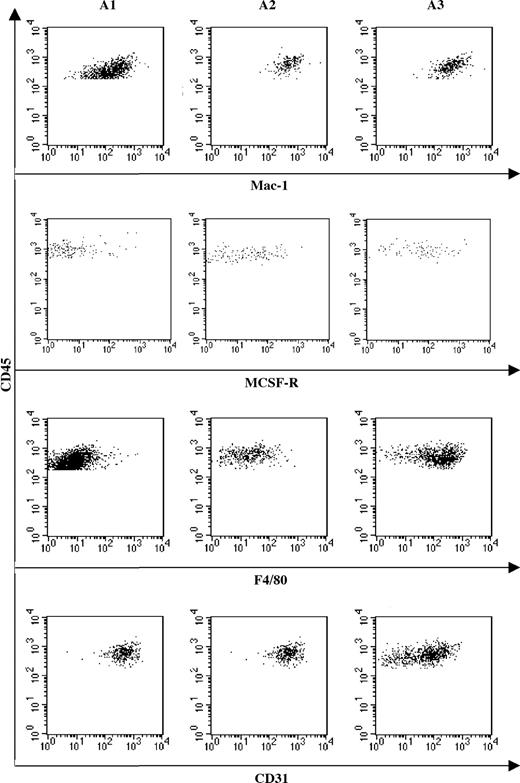
Distribution of macrophage markers suggests that maturation occurs from an A1 to A3 sequence. FACS analysis of Mac-1, M-CSF-R, F4/80, and CD31 expression by A1, A2, and A3 cell subsets. Mac-1 expression remains constantly expressed in the 3 subsets, whereas differentiation marker (M-CSFR and F4/80) expression increases from A1 to A3. Reverse correlation is observed for the immature macrophage marker CD31, whose expression decreases from A1 to A3.
Distribution of macrophage markers suggests that maturation occurs from an A1 to A3 sequence. FACS analysis of Mac-1, M-CSF-R, F4/80, and CD31 expression by A1, A2, and A3 cell subsets. Mac-1 expression remains constantly expressed in the 3 subsets, whereas differentiation marker (M-CSFR and F4/80) expression increases from A1 to A3. Reverse correlation is observed for the immature macrophage marker CD31, whose expression decreases from A1 to A3.
Fraction A3, c-Kit–GFP+, devoid of EMp, appears as a mature Mφ population expressing high GFP levels and displaying low expansion capacity. In contrast, the c-Kit+GFP– fraction (A1), though highly efficient in generating Mφ, also contained EMp. Fraction A2 shows an intermediate pattern of differentiation. Fraction B was devoid of hematopoietic differentiation capacity, whereas fraction C (CD45–/loc-Kit+GFP–) contained EMp and monopotent Mp.
Fractions A1, A2, and A3 were further characterized for the expression of Mac-1, M-CSFR, F4/80, and CD31 (Figure 4). Mac-1 is equally expressed in the 3 CD45+ subpopulations. The expression of M-CSFR, together with that of the mature Mφ marker F4/80, increased from A1 to A3, whereas CD31 expression, which in the Mφ lineage characterizes immature precursors, concomitantly decreased. These data suggest that maturation along the Mφ lineage occurred along an A1 to A3 sequence.
The combination of the differentiation potential analysis (Figure 3) with the phenotypic characterization of the CD45+ subset (Figure 4) suggests that, at 10 dpc, YS monopotent Mps initially express c-Kit only, then progressively acquire, as they mature, CD45 and CX3CR1 and concomitantly lose c-Kit expression and proliferation ability.
Gene-expression analysis of 10 dpc monopotent Mps
To define the differentiation state of the CD45+ subsets, we performed quantitative real-time PCR on fractions A1, A2, and A3 and made comparisons with adult peritoneal Mφ.
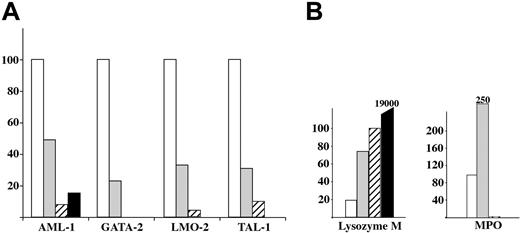
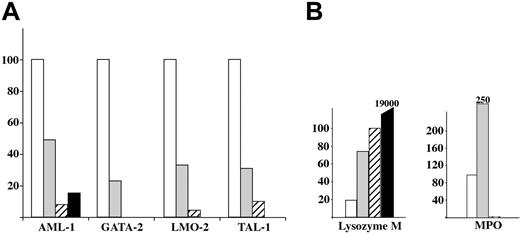
We quantified transcripts from genes known to be involved in mesoderm-to-hematopoiesis transition (LMO2, GATA2, TAL1, AML1; Figure 5A) or genes involved in adult Mφ differentiation (MPO, lysozyme M [lysM]; Figure 5B). LMO2, GATA2, TAL1, and AML1 follow the same kinetics of expression, with a decreasing expression from A1 to A3 fractions. Lysozyme M shows the reverse kinetics, with an increased expression from A1 to A3. Nevertheless, the higher expression levels detected in fraction A3 are still 1 to 2 orders of magnitude lower than those found in adult peritoneal macrophages. Whereas the A1 and A2 subsets expressed significant levels of MPO, this enzyme, expressed in precursors but not in mature macrophages, was no longer detectable in fraction A3. MPO transcripts were detected at significant levels in fractions A1 and A2 but not in fraction A3. This is in agreement with the known expression of MPO in adult Mφ precursors (monocytes) and its extinction on Mφ differentiation.
Analysis of genes involved in Mφ differentiation shows a sequence similar to that observed in bone marrow monocyte/Mφ lineage progression. These results corroborate the conclusion drawn from the phenotype analysis, which is that Mφ differentiation progresses from fraction A1 to fraction A3.
YS-EMp from 8 dpc embryos can contribute to the initial phase of fetal liver hematopoiesis
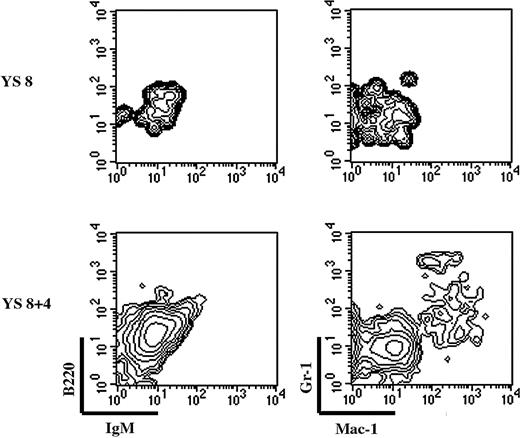
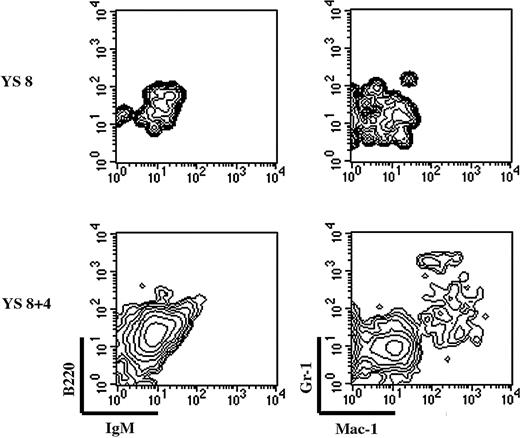
We finally tested the ability of YS precursors to develop in the FL isolated at the time of hematopoietic colonization. We established a fetal liver organ culture (FLOC) in which 10.5 dpc liver lobes were cocultured with 8 dpc YS cells; AGM cells were the positive control. FLOC was analyzed by flow cytometry for the presence of donor-derived B (B220, IgM) and myeloid (Mac-1, Gr-1) cells (Figure 6) because AGM cells can give rise to all lineages in this system (data not shown).
Freshly isolated 8 dpc YS cells could not give rise to a myeloid progeny in the FLOC (Figure 6, upper panel), confirming that YS myeloid precursors are still rare at this stage (Figure 2).12 To allow the number of precursors to increase, we performed organ culture of 8 dpc YS. Precursors generated during this 4-day organ culture could colonize the FLOC and give rise to a myeloid progeny. We thus conclude that YS precursors (monopotent Mφ, EMp, or both) may contribute to the initial phases of FL hematopoiesis.
Quantification of hematopoietic and myeloid-related transcripts in fractions A1 to A3. □ indicates A1; ▦, A2; ▨, A3, and ▪, adult F4/80+ peritoneal Mφ (taken as a control). (A) The histogram shows a dramatic decrease from A1 to A3 in the expression of the AML-1, GATA-2, LMO-2, and TAL-1 transcription factors involved in the transition from mesoderm to hematopoiesis. (B) Quantification of lysozyme M and MPO transcripts. The histogram shows a gradual increase in the transcripts for lysozyme M from A1 to A3, but only transient expression of MPO in fractions A1 and A2. These variations correlated with those observed during adult macrophage maturation.
Quantification of hematopoietic and myeloid-related transcripts in fractions A1 to A3. □ indicates A1; ▦, A2; ▨, A3, and ▪, adult F4/80+ peritoneal Mφ (taken as a control). (A) The histogram shows a dramatic decrease from A1 to A3 in the expression of the AML-1, GATA-2, LMO-2, and TAL-1 transcription factors involved in the transition from mesoderm to hematopoiesis. (B) Quantification of lysozyme M and MPO transcripts. The histogram shows a gradual increase in the transcripts for lysozyme M from A1 to A3, but only transient expression of MPO in fractions A1 and A2. These variations correlated with those observed during adult macrophage maturation.
YS-EMp can contribute to FL hematopoiesis. FL lobes from 10-dpc embryos (CD45.1+) were cocultured with either freshly isolated or 4-day in toto–cultured 8-dpc YS cell suspensions. After 10 days, FL lobes were scored for the presence of donor-derived B cells (B220, IgM) and myeloid cells (Gr-1, Mac-1). Plots are gated on donor-derived cells. YS cells can only give rise to a myeloid progeny in the FL environment, after an organ culture period.
YS-EMp can contribute to FL hematopoiesis. FL lobes from 10-dpc embryos (CD45.1+) were cocultured with either freshly isolated or 4-day in toto–cultured 8-dpc YS cell suspensions. After 10 days, FL lobes were scored for the presence of donor-derived B cells (B220, IgM) and myeloid cells (Gr-1, Mac-1). Plots are gated on donor-derived cells. YS cells can only give rise to a myeloid progeny in the FL environment, after an organ culture period.
Discussion
During mouse ontogeny, the first differentiated hematopoietic cells appear in the YS blood islands as early as the neural plate stage (7.0 dpc).23 The best-characterized population at this stage is the first wave of erythrocytes (nucleated and expressing embryonic hemoglobins), which are called primitive, in contrast to definitive erythrocytes, which are enucleated and express adult forms of hemoglobin. Other primitive lineages have been described in the early YS, namely primitive megakaryocytes prone to an accelerated production of platelets and a reduced ploidy compared with megakaryocytes present in the adult24 and Mφ that bypass the monocyte stage during their maturation.8 So far, these primitive Mφ have been characterized through electron microscopy and immunocytochemistry9,10 or through in vitro differentiation potential analyses.12
Although the presence of Mφ in 8 dpc embryos has been well established,9,10,12 the in situ detection of macrophage lineage markers (Mac-1, F4/80, c-fms) shows the first patent expression in Mφ-like cells between 9 (Mac-1,25 F4/809,26 ) and 9.5 dpc (c-fms27,28 ).
To define the lineage relationship among the different types of previously described macrophages, we analyzed YS macrophage development in CX3CR1 embryos in which monocytes and tissue macrophages express eGFP.13 We describe 3 different pathways leading to the establishment of Mφ populations in the YS through the correlation of precursor phenotypes and differentiation potentials.
The first Mφ population detected in the YS consists of maternally derived cells that display a mature phenotype (expressing Mac-1, CD45, and F4/80). These Mφ are detected in wholemount embryos labeled with their specific markers (CD45, F4/80) from the late head fold stage (7.5 dpc). A possible contribution of maternal hematopoietic cells to the developing embryo has been previously suggested. For instance, maternally derived Mφ have been shown to participate in the clearing of pre-teratogenic cells, because radiationor chemical-induced malformation was significantly reduced when activated macrophages were injected in the treated mother.29 Our observations show that maternally derived Mφ are found deeply inserted in the mesoderm layer and thus cannot be accounted for by dissection contamination; their consistent timing of appearance (late head fold stage) and location (close to the junction between the YS and the intraembryonic compartment) in independent embryos suggests that these cells may play a scavenger (clearing) function during early developmental stages, when Mφ of embryonic origin are not yet available.
The previously described Mφ8-10 population, qualified as primitive, was detected in the YS between 8 and 9 dpc, considered to bypass the monocyte stage of differentiation, and was shown to express F4/80 and exhibit proliferative capacity in vitro. Using embryos obtained from the cross between a CX3CR1-GFP male and a wild-type female, we observed that, at 8.5 to 9 dpc, the population of CD45+F4/80+ comprises a mixture of maternally derived (GFP–) and embryonic (GFP+) Mφ, originated from MPO-expressing monocytes. We suggest that the Mφ population, identified as primitive by Naito et al,8,9 encompasses the first 2 waves described here. F4/80+ cells were accounted for by maternally derived cells, and proliferating Mφ was accounted for by Mp.
The second wave consists of monopotent macrophage precursors (YS-Mp), which display a CD45–c-Kit+ phenotype. These Mφ precursors appear later at 8 dpc (2-4S) and give rise to F4/80+CX3CR1+ Mφ. Precursors with an erythromyeloid potential (YS-EMp), constituting the third wave, arise independently at around the 5-somite stage, also from the CD45–c-Kit+ fraction. These 2 successive populations correspond, by the timing of their appearance and their differentiation potential, respectively, to the 8 dpc Mac-CFC described by Palis et al12 and the HPP-CFC described by Palis et al.30 YS-Mp, initially CD45–c-Kit+, acquire CD45 expression at 9 dpc and further up-regulate CX3CR1 while losing c-Kit expression. From lineage analyses performed at 10 dpc, it appears that Mφ develop through a CD45–c-Kit+CX3CR1– stage (fraction C) to the CD45+c-Kit–CX3CR1+ stage (fraction A3), as ascertained by the down-regulation of CD31, and concomitant up-regulation of F4/80 and M-CSF-R from 9.5 dpc (data not shown), as detected by others.26-28
To characterize the intermediate differentiation stages, the expression of genes involved in myelomonocyte differentiation (MPO, a gene expressed in precursors but not in mature Mφ and lysozyme M, the expression of which persists in mature Mφ) was analyzed. Lysozyme M expression increases from fraction A1 to A3 but remains at a low level compared with that found in mature adult Mφ, a result that correlates with previously published observations.31 MPO is expressed only by cells from fractions A1 and A2. These observations suggest that Mφ derived from c-Kit+CD45– precursors—that is, both YS-Mp and YS-EMp progress through an MPO+ monocyte stage during their differentiation and can thus be correlated with the “definitive” lineage of macrophages described by Naito et al.8,9
Here we further show that YS cells isolated before the 5S stage (comprising maternally derived Mφ and the first appearing YS-Mp and YS-EMp) hardly display hematopoietic potential when transferred to irradiated FL lobes (maternally derived Mφ, scarcely detected after 9.5 dpc, are not likely to contribute to FL hematopoiesis). After an organ culture step during which YS-Mp and YS-EMp numbers increase, YS cells acquire the capacity to give rise to myeloid, but not lymphoid, progeny in the FL environment. This clearly shows that YS hematopoietic progenitors can participate in definitive hematopoiesis in the FL. Palis et al11 also described the presence of multi-myeloid progenitors in the FL, thought to arise in the YS,12 corresponding to the YS-EMp we describe here.
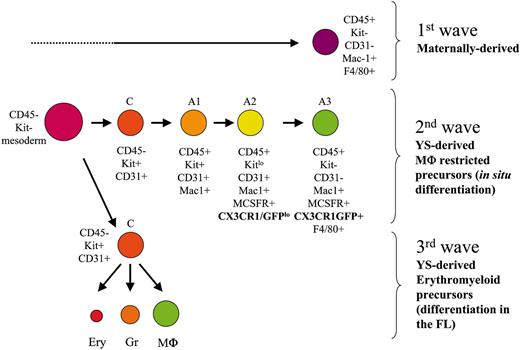
Developmental stages followed by the 3 distinct YS-Mφ populations, characterized by their phenotype, origin, differentiation potential, and lineage relationship. Ery indicates erythrocytes; Gr, granulocytes.
Developmental stages followed by the 3 distinct YS-Mφ populations, characterized by their phenotype, origin, differentiation potential, and lineage relationship. Ery indicates erythrocytes; Gr, granulocytes.
Thus we propose the following 3-step scheme leading to the establishment of macrophage populations in the YS (Figure 7). A first wave of maternally derived Mφ (CD45+F4/80+CX3CR1–) colonize the mesoderm layer from 7.5 dpc on. This contribution is transient because maternally derived Mφ are no longer detected in the YS after 9.5 dpc.
A second wave of monopotent Mφ precursors (YS-Mp) appears in the YS slightly later. These Mφ precursors will develop through a differentiation pathway (CD45+c-Kit+, CD45+c-KitloCX3CR1lo, CD45+c-Kit–CX3CR1+) similar to that found in the adult. Finally, a third wave sharing a similar phenotype (c-Kit+CD45–) and Mφ differentiation pathway arises and differs from the previous one only because, at the clonal level, the precursors (YS-EMp) display erythromyeloid potential.
These YS-derived Mφ may contribute to ongoing developmental events by their ability to clear apoptotic bodies, and they may contribute to the establishment of resident tissue macrophages. It has been shown that early YS-Mφ, considered by the authors as primitive, colonize the developing brain and differentiate into primitive microglia in the zebrafish32,33 and chicken7,34 models. In the mouse embryo, it was shown that c-fms-GFP cells, detected first in the YS, are later found distributed in the brain,28 and a contribution of early somites YS cells to developing microglia has been suggested.35
It appears from our results that, in the mouse, such a contribution cannot be expected from cells corresponding to the maternally derived lineage, which is already undetectable before organogenesis begins. The contribution of the 2 further YS-Mφ populations (and their respective specificities) to resident Mφ populations, including microglia, remains to be evaluated.
In conclusion, hematopoiesis occurring in the YS follows a reverse course of events than that found in FL and bone marrow. Mature hematopoietic cells can be detected before immature multipotent precursors and in the absence of recognizable hematopoietic stem cells. The nature of the immediate precursors of these 3 Mφ populations and the pathway of differentiation they pursue remain open questions.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-02-0461.
Supported by grants from the Pasteur-Weizmann Scientific Council, the Fondation pour la Recherche Médicale (FDT20040700960) (J.Y.B.), Fondation pour la Recherche Médicale (INE200330307040) (I.G.), Ligue Régionale contre le Cancer Comité des Yvelines (GL/CB/0167-02) (I.G.), and Institut Gustave Roussy (CRI-SPS-2003-02) (I.G.).
J.Y.B. designed and performed the research and contributed to manuscript redaction; A.J. and M.K. performed the research; S.J. provided a vital tool for research (knock-in mice, CX3CR1-GFP) and contributed to manuscript redaction; A.C. designed and performed the research and contributed to manuscript redaction; and I.G. designed and performed the research and contributed to manuscript redaction.
A.C. and I.G. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Philippe Herbomel for critical reading of the manuscript and encouragement and A. Louise for cell sorting. S.J. is an Incumbent of the Pauline Recanati Career Development Chair and a Scholar of the Benoziyo Center for Molecular Medicine.

![Figure 2. Identification of myeloid precursors in the 8 dpc YS. (A) Fractionation of 8 dpc YS using CD45 and c-Kit markers allows the discrimination of 3 subsets for further in vitro potential analysis. The percentage and absolute number per YS appear near the sorting gates. (B) Kinetics of macrophage-restricted (▪) and erythromyeloid precursors [□] in the YS from 2S to 8S, obtained by limiting-dilution experiments. Regression curves show that Mp (straight line) appear before EMp (dotted line).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-02-0461/6/m_zh80210585850002.jpeg?Expires=1769310039&Signature=cR5IyshJDtYeXORh0DpZjHhbgmuyhV9drtXBFrCUjU~SCiTPLzsEznwKqmBr65qouoDQF1jW57Ep7x72zB7gGwDzmA-tRGl54Zl0azdUEi7HFvBc5z6OQTveLkcgHnNGc7wltTNFm5W~kkf~t4y~U48T0EVC0d0aGeoz6ZKMbUwGaZrDGIB8SAjKLxYO8kTQXZwxWSwDZmCqA0caN4BJWLxSkMSu~swWAR8Ux35DleK7BqNQjnJ~3ZMLGMyoVwL410F~ij10qm4UPYP~2VHF9nVqNdB2QYrh~O4oivN1GmDc7-xuVM1z85wBHZKS1PI4hkn8fH0JJyXraSsR-nArYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Identification of myeloid precursors in the 8 dpc YS. (A) Fractionation of 8 dpc YS using CD45 and c-Kit markers allows the discrimination of 3 subsets for further in vitro potential analysis. The percentage and absolute number per YS appear near the sorting gates. (B) Kinetics of macrophage-restricted (▪) and erythromyeloid precursors [□] in the YS from 2S to 8S, obtained by limiting-dilution experiments. Regression curves show that Mp (straight line) appear before EMp (dotted line).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-02-0461/6/m_zh80210585850002.jpeg?Expires=1769310040&Signature=MsfdlDxVbvPrwf1V9JHnxUbvnUP9kd4jPD0JBtHPgfbsvP3rZ5lAMk1Vn5rwZu8~TXqx8J3MehwrrBIgFqcFw3S13ia23pizTlMvQNf-Jb4i5YfY754RDZtSPVsvSlauF1RqhZ4kYNEgU-v-4U0WDLF1XijkaZGqdHRo8ouJVNz2wO0KQQf1lCSZuKkS0hBoCV31xzFuGrDFLo~w0p0mQMDkKoOvpp5zhHhkohrRTr6aJ1MlQpuPGVjr3056sM5juNSHxXCNJZ9bT-sRIDc1Iu-o345DM1uO1iUbBPpj7nsayGfLPIP-S~m5dqqxF7y5JqEA1WrH3Ik51AnjRnppzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)