Dysregulated tyrosine kinase activity by the Fip1-like1 (FIP1L1)–platelet-derived growth factor receptor alpha (PDGFRA) (F/P) fusion gene has been identified as a cause of clonal hypereosinophilic syndrome (HES), called F/P-positive chronic eosinophilic leukemia (CEL) in humans. However, transplantation of F/P-transduced hematopoietic stem cells/progenitors (F/P+ HSCs/Ps) into mice results in a chronic myelogenous leukemia–like disease, which does not resemble HES. Because a subgroup of patients with HES show T-cell–dependent interleukin-5 (IL-5) overexpression, we determined if expression of the F/P fusion gene in the presence of transgenic T-cell IL-5 overexpression in mice induces HES-like disease. Mice that received a transplant of CD2-IL-5–transgenic F/P+ HSC/Ps (IL-5Tg-F/P) developed intense leukocytosis, strikingly high eosinophilia, and eosinophilic infiltration of nonhematopoietic as well as hematopoietic tissues, a phenotype resembling human HES. The disease phenotype was transferable to secondary transplant recipients of a high cell dose, suggesting involvement of a short-term repopulating stem cell or an early myeloid progenitor. Induction of significant eosinophilia was specific for F/P since expression of another fusion oncogene, p210-BCR/ABL, in the presence of IL-5 overexpression was characterized by a significantly lower eosinophilia than IL-5Tg-F/P recipients. These results suggest that F/P is not sufficient to induce a HES/CEL-like disease but requires a second event associated with IL-5 overexpression.
Introduction
The hypereosinophilic syndrome (HES) was described by Hardy and Anderson.1 The diagnostic criteria of this hematologic disorder as proposed by Chusid et al2 include unexplained severe peripheral blood eosinophilia (higher than 5 × 109 eosinophils/L [1500 eosinophils/mm3]) sustained for more than 6 months and accompanied by end-organ damage resulting from direct organ infiltration by eosinophils. Although the etiology of HES remains unclear, a subset of patients with HES has been shown to have an interstitial deletion in chromosome 4q12, which results in the generation of a fusion protein between the platelet-derived growth factor receptor alpha (PDGFRα) gene and a previously uncharacterized gene, Fip1-like1 (FIP1L1). The fusion gene product acts as a constitutively active tyrosine kinase.3,4 The FIP1L1-PDGFRA (F/P) fusion gene has been identified in 14% to 60% of patients with HES.3,5-8 This subgroup of HES patients is now diagnosed as chronic eosinophilic leukemia (CEL) according to World Health Organization disease classification criteria. Those patients with F/P+ HES/CEL appear to have a more severe disease phenotype involving extensive end-organ pathology.6,7,9,10 Notably, the F/P tyrosine kinase is susceptible to imatinib inhibition, and this drug is thus effective in some HES patients.11-13
A murine study of retroviral transduction of hematopoietic stem cells/progenitors (HSCs/Ps) with the F/P fusion gene was previously shown to be associated with the development of a myeloproliferative disorder similar to that of the murine model of BCR/ABL leukemogenesis.14,15 Affected mice demonstrated neutrophilia and a modest eosinophilia (5%-20% of all cells in the peripheral blood). In addition, in this model, tissue infiltration by F/P-expressing cells was dominated by granulocytic cells mainly composed of noneosinophils. Thus, this murine model did not entirely mimic the characteristics of human HES/CEL.15,16
A subgroup of patients with HES displays aberrant, sometimes clonal, Th2 lymphocytes secreting large amounts of interleukin-5 (IL-5), a well-known eosinophil maturation- and function-activating cytokine.17-19 We hypothesized that the F/P fusion protein may cooperate with hematopoietins, such as IL-5, to induce abnormal eosinophil responses typical of HES. The present study indicates that the transplantation of F/P-expressing IL-5–transgenic (Tg) HSCs/Ps into syngeneic recipients induces a murine model of tissue-infiltrating hypereosinophilia similar to human HES/CEL. These results establish that F/P is necessary but not sufficient to induce HES/CEL in mice and cooperates with IL-5–dependent signaling in driving abnormal eosinophil development, which mimics human HES/CEL.
Materials and methods
Mice
Age- and sex-matched wild-type and CD2-IL-5Tg BALB/c mice20 were used as bone marrow (BM) donors. These mice and other T-cell–dependent IL-5–transgenic mice have shown high IL-5 plasma levels in either primary mice or mice that underwent BM transplantation.21,22 BALB/c (wild type) female mice (7-12 weeks old) were obtained from the National Cancer Institute (Frederick, MD) and Taconic Farms (Germantown, NY) and used as HSC/P transplant recipients. All mice were maintained under specific pathogen-free conditions in Cincinnati Children's Hospital Animal Facility. Animal protocols were approved by the Animal Care Committee of Cincinnati Children's Hospital Medical Center.
Retroviral constructs and viral supernatants
Retroviral constructs were murine stem cell virus (MSCV)–based23 bicistronic vectors called MSCV-F/P-IRES-EGFP, MSCV-BCR/ABL (p210)-IRES-EGFP, and MSCV-IRES-EGFP (mock vector) (kindly provided by Drs Gary Gilliland and Jan Cools, Harvard Medical School, Boston, MA). Efficient expression of F/P and p210-BCR/ABL by these retroviral vectors has been previously shown.15,24 Retrovirus supernatant was generated in the Phoenix-gp cells25 as previously described.26
Retroviral transduction into hematopoietic progenitor cells
Mice were treated with 150 mg/kg 5-fluorouracil administered intraperitoneally beginning 6 days prior to BM harvest. Femora, tibiae, and iliac crests were harvested and their BM content was isolated by bone crunching. Low-density BM (LDBM) cells were separated by density gradient fractionation according to the manufacturer's instructions (Histopaque 1083; Sigma-Aldrich, St Louis, MO), prestimulated in the presence of recombinant mouse (rm) IL-3 (6 ng/mL; PeproTech, Rocky Hill, NJ), recombinant rat (rr) stem cell factor (SCF, 10 ng/mL; Amgen, Thousand Oaks, CA), and rm IL-6 (10 ng/mL; PeproTech), and transduced during 2 days in a protocol that included spinoculation (1800g for 90 minutes) followed by 2 cycles of incubation (for 24 and 6 hours, respectively) with transient retroviral supernatants expressing MSCV-based retroviral bicistronic vectors containing F/P or BCR/ABL (p210) fusion genes and the enhanced green fluorescent protein (EGFP) as previously described.15,27 An aliquot of transduced cells was cultured for 2 extra days for transduction efficiency analysis of EGFP-expressing BM cells. Transduction efficiency of all experiments was 10% ± 8.9%, without any significant difference among the different groups.
HSC/P transplantation
Retrovirally transduced LDBM cells were transplanted 6 hours after the second round of spinoculation/transduction. A total of 2.5 to 6.5 × 106 cells/mouse was injected into the lateral tail vein of previously lethally irradiated (4.5 Gy for 2 doses, 3 hours apart; 137Cs source: dose rate, 60-65 cGy/min) recipient mice (BALB/c, wild type).15 For serial transplantations, splenocytes from diseased mice or controls were injected into lethally irradiated syngeneic secondary recipients. Myeloproliferation was defined according to the established NIH-Bethesda criteria for myeloproliferative disorder–like myeloid leukemia,28 although this classification does not include any specific hypereosinophilia criteria.
Treatment with imatinib mesylate
The tyrosine kinase inhibitor, imatinib (formally known as CGP57148B or STI571), was kindly provided by Novartis Pharmaceuticals (Basel, Switzerland). A stock solution of imatinib was prepared by dissolving it in water and, prior to administration, diluting the solution with PBS. Concurrent PBS-injected mice were used as controls. Imatinib was administered intraperitoneally at 50 mg/kg per dose, twice a day29 from day 10 after transplantation until one week after all the control mice had died or were killed due to progressive disease, as described previously.15
Peripheral blood counts
Peripheral blood samples were collected in EDTA microtainer tubes (Becton Dickinson, Franklin Lakes, NJ) by retro-orbital bleeding. Manual and automated total cell counts and differential counts using smears stained with Diff-Quick according to manufacturer's instructions (Fisher Diagnostics, Middletown, VA) were performed. In addition, blood eosinophil levels were determined by counting cells after staining whole blood with Discombe solution.30
Histopathology
Cytospins of BM and spleen specimens were stained with Diff-Quick as indicated in “Peripheral blood counts.” For tissue histology, relevant organs were fixed in 10% buffered formalin and embedded in paraffin. The tissue sections were stained with hematoxylin/eosin and an antibody directed against the major basic protein (MBP) of eosinophils as previously described.31,32 In brief, endogenous peroxidase in the tissues was quenched with 0.3% hydrogen peroxide in methanol and specimens were treated with pepsin for antigen retrieval, followed by nonspecific protein blocking with normal goat serum. Tissue sections were then incubated with rabbit anti-MBP (1:10 000, kindly provided by Dr James Lee, Mayo Clinic, Scottsdale, AZ) overnight at 4°C, followed by a 1:200 dilution of biotinylated goat anti–rabbit IgG secondary antibody and avidin-peroxidase complex (Vector Laboratories, Burlingame, CA) for 30 minutes each. These slides were further developed with nickel diaminobenzidine-cobalt chloride solution to form a black precipitate and counterstained with nuclear fast red. As a negative control, preimmune rabbit serum was used to replace the primary antibody and revealed no immunoreactivity. The tissue samples stained by anti-MBP were used for eosinophil quantification in the heart and small intestine. At least 3 random sections per mouse were analyzed. Slides were read using an Olympus BX51 upright microscope equipped with 10 ×/0.40 and 40 ×/0.85 UPlanApo objective lenses (Olympus, Melville, NY). Images were captured with an Olympus U-MCAD-2 digital camera, and were acquired with Magnafire 2.1C software (Optronics, Goleta, CA). Adobe Photoshop Elements 2.0 software was used to process images (Adobe Systems, San Jose, CA). Quantification of stained cells per square millimeter of myocardium or intestinal lamina propria was performed by blind morphometric analysis (ImagePro Plus version 4.1; Media Cybernetics, Silver Spring, MD).
Flow cytometric analysis
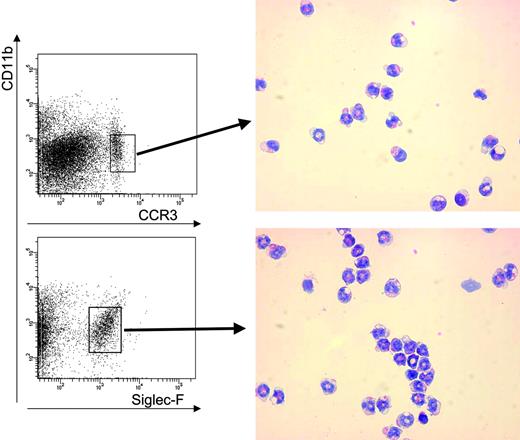
Single-cell suspensions from the mouse peripheral blood, spleen, and BM cells were lysed with NH4Cl red blood cell (RBC) lysis buffer (pH 7.4) and stained according to manufacturer's instructions with the following monoclonal antibodies: PE-conjugated anti-CD3ϵ (clone 145-2C11; BD Biosciences, San Jose, CA), APC-conjugated anti-B220 (clone RA3-6B2; BD Biosciences), APC-conjugated anti-CD11b (clone M1/70; BD Biosciences), biotin-conjugated anti-CD49d (clone R1-2; BD Biosciences), PE-conjugated anti-CCR3 (clone 83101; R&D, Minneapolis, MN), PE-conjugated anti–Siglec-F (clone E50-2440; BD Biosciences), and biotin-conjugated anti–IL-5 receptor alpha (clone T21, kindly provided by Dr Kiyoshi Takatsu, University of Tokyo, Tokyo, Japan), or isotype-matched control antibodies. PerCP- or APC-conjugated streptavidin (BD Biosciences) was used as a secondary reagent to detect the binding of the biotinylated primary antibody (BD Biosciences). 7-Aminoactinomycin D (7-AAD; Molecular Probes, Eugene, OR) was added to exclude dead cells, except for samples labeled with antibodies bound to PerCP. Multicolor flow cytometric analysis was performed with a FACScalibur flow cytometer, cells were sorted with a FACS-Vantage Diva, and the data were analyzed using CellQuest Pro 5.2 software (BD Biosciences).
Plasma IL-5 concentration analysis
The plasma level of IL-5 was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Mouse IL-5 OptEIA ELISA set; BD Biosciences). Samples were diluted 1:4 and 1:10. The sensitivity of the assay was 16 pg/mL.
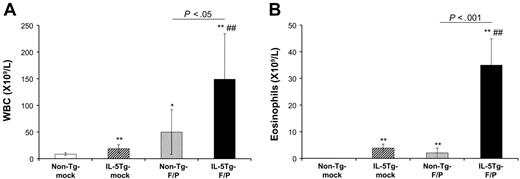
Blood cell counts in the murine eosinophilic disease model induced by the F/P fusion gene. Mice that received a transplant of nontransgenic (Tg), mock-transduced (non–Tg-mock); IL-5Tg, mock-transduced (IL-5Tg-mock); non-Tg, F/P-transduced (non–Tg-F/P); and IL-5Tg, F/P-transduced (IL-5Tg-F/P) HSCs/Ps were analyzed at 4 weeks after transplantation. Automated total cell leukocyte (A) and eosinophil (B) counts assessed by Discombe staining were performed. Data are shown as mean ± SD and represent 10 to 12 mice per group, pooled from 3 independent experiments. *P < .05 and **P < .01, compared with non–Tg-mock; ##P < .01, compared with IL-5Tg-mock.
Blood cell counts in the murine eosinophilic disease model induced by the F/P fusion gene. Mice that received a transplant of nontransgenic (Tg), mock-transduced (non–Tg-mock); IL-5Tg, mock-transduced (IL-5Tg-mock); non-Tg, F/P-transduced (non–Tg-F/P); and IL-5Tg, F/P-transduced (IL-5Tg-F/P) HSCs/Ps were analyzed at 4 weeks after transplantation. Automated total cell leukocyte (A) and eosinophil (B) counts assessed by Discombe staining were performed. Data are shown as mean ± SD and represent 10 to 12 mice per group, pooled from 3 independent experiments. *P < .05 and **P < .01, compared with non–Tg-mock; ##P < .01, compared with IL-5Tg-mock.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) except when otherwise stated. Statistical analysis of data was performed by Student t test when comparing 2 groups and one-way analysis of variance (ANOVA) followed by Bonferroni t test for 3 or more groups. For ELISA tests and survival analysis, data are presented as medians and 25% and 75% interquartiles. Statistical comparisons were performed with either Mann-Whitney U test or log P rank test. P values less than .05 were considered significant.
Results
F/P cooperates with IL-5 overexpression to induce murine eosinophilic disease
We investigated whether the F/P fusion gene collaborates with IL-5 signaling in eosinophilic leukemogenesis. To test this, lethally irradiated wild-type mice received a transplant of the F/P-transduced HSCs/Ps derived from CD2-IL-5 Tg mouse BM (IL-5Tg-F/P recipients). These mice overexpress IL-5 in a T-cell–dependent fashion.22 In order to confirm that the level of IL-5 was elevated in recipients of transplanted CD2-IL-5Tg HSCs/Ps, plasma IL-5 levels were measured (at the end point, n = 6-7 pooled from two independent experiments). IL-5 plasma levels were 162 pg/mL (interquartile range, 94.4-252.4 pg/mL) in IL-5Tg-mock vector and 266 pg/mL (interquartile range, 130.3-442.4 pg/mL) in IL-5Tg-F/P recipients (P = .31). In contrast, IL-5 was undetectable in 7 non–Tg-mock vector and 6 non–Tg-F/P recipients. In addition to plasma IL-5, there were no significant differences in T-cell engraftment between IL-5Tg-mock vector and IL-5Tg-F/P recipients (frequency of EGFP+/CD3+ cells was 4.9 ± 2.52% vs 4.4 ± 2.50%, respectively; n = 8, pooled from two independent experiments).
Once transplanted, F/P-expressing cells engrafted and rapidly proliferated in vivo. All IL-5Tg-F/P recipients developed a myeloproliferative disease with a latency of 4 weeks, characterized by leukocytosis with significant eosinophilia in peripheral blood, splenomegaly, and eosinophilic infiltrate of multiple organs. IL-5Tg-F/P recipients developed an intense leukocytosis (149.0 ± 85.50 × 109 leukocytes/mm3) (Figure 1A) and a significant eosinophilia (35.0 ± 9.90 × 103 eosinophils/mm3) that was 9-fold higher (P < .001) than that observed in mock vector–transduced CD2-IL-5Tg HSC/P recipient (IL-5Tg-mock vector recipient) mice (Figure 1B). In contrast, F/P-transduced wild-type HSC/P recipients (non–Tg-F/P recipients) showed a myeloproliferative disorder with predominant granulocytic involvement as previously reported,15 with similar survival as IL-5Tg-F/P recipients (28 days [interquartile range, 27-33 days] vs 28 days [interquartile range, 27-30 days]; P = .61). In order to further characterize the eosinophilia induced by the F/P fusion gene in the presence of IL-5 overexpression, we performed flow cytometric analysis. Flow cytometry identification of eosinophils was based on the pattern of expression of the antigens CD11b, CCR3, and Siglec-F33-35 . Eosinophil populations were identified as CCR3+/CD11b+/low and Siglec-F+/CD11b+/low and were analyzed on the gated EGFP+. For comparison, the gated EGFP– populations were also analyzed. Indeed, sorted CCR3+/CD11b+/low and Siglec-F+/CD11b+/low populations contained more than 90% eosinophils, and accounted for 88% and 98% of all eosinophils, respectively (Figure 2). We analyzed the percentage of CCR3+/CD11b+/Low cells in circulating EGFP+ cells. The percentage of circulating EGFP-expressing, CCR3+/CD11b+/Low cells of IL-5Tg-F/P recipients was 1.6-fold and 4.7-fold higher than that of recipients of IL-5Tg-mock vector and of non–Tg-F/P recipients, respectively (Table 1), confirming that the F/P fusion gene and IL-5 overexpression had additive effects in promoting eosinophil proliferation and/or differentiation in vivo.
Spleen and bone marrow eosinophilia
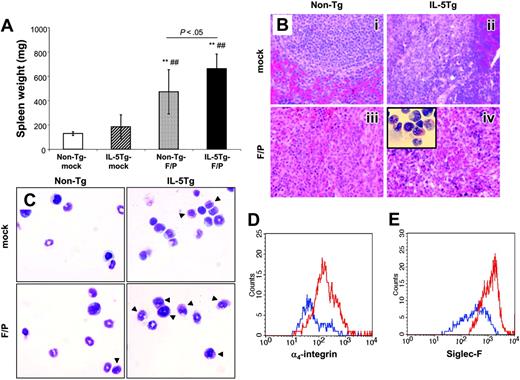
Spleen weight was significantly higher in the IL-5Tg-F/P recipients compared with other groups including non–Tg-F/P recipients (Figure 3A). Upon histopathologic analysis, IL-5Tg-F/P recipients demonstrated a diffuse eosinophilic infiltrate with disruption of follicular architecture of the spleen. Non–Tg-F/P recipients showed mostly neutrophil infiltration with reduced eosinophils (Figure 3B). In addition, the BM in IL-5Tg-F/P recipients showed myeloid hyperplasia dominated by eosinophils (Figure 3C). Similarly to peripheral blood, we observed that the percentages of EGFP+ eosinophils as determined by CCR3+/CD11b+/Low or Siglec-F+/CD11b+/Low staining in the BM and spleen of IL-5Tg-F/P recipients were higher than in IL-5Tg-mock recipient mice (Table 1). The non–Tg-F/P recipients also showed a higher percentage of EGFP+/CCR3+/CD11b+/Low cells and EGFP+/Siglec-F+/CD11b+/Low cells in the spleen and BM compared with mock vector–transduced wild-type HSC/P recipient (non–Tg-mock vector recipient) mice (Table 1), suggesting that the expression of the fusion gene alone promoted eosinophil lineage cell proliferation. Taken together, these observations suggested that the F/P fusion gene induced a significant eosinophilic infiltrate in the spleen and BM of IL-5Tg-F/P recipients. Expression of F/P alone led to a modest increase of eosinophilic infiltrates in these organs.
CD11b, Siglec-F, and CCR3 coexpression defines a highly specific murine eosinophil population by flow cytometry. Peripheral blood from CD2-IL-5–transgenic BALB/c mice was stained with anti–CCR3-PE, anti–Siglec-F-PE, and CD11b-APC antibodies followed by 7-AAD and sorted for CD11b+/CCR3+/low and CD11b+/Siglec-F+/low populations in 7-AAD– cells. Cytospins were prepared from sorted cells and were stained with Diff-Quick (optical magnification × 500).
CD11b, Siglec-F, and CCR3 coexpression defines a highly specific murine eosinophil population by flow cytometry. Peripheral blood from CD2-IL-5–transgenic BALB/c mice was stained with anti–CCR3-PE, anti–Siglec-F-PE, and CD11b-APC antibodies followed by 7-AAD and sorted for CD11b+/CCR3+/low and CD11b+/Siglec-F+/low populations in 7-AAD– cells. Cytospins were prepared from sorted cells and were stained with Diff-Quick (optical magnification × 500).
Eosinophil infiltration of nonhematopoietic organs
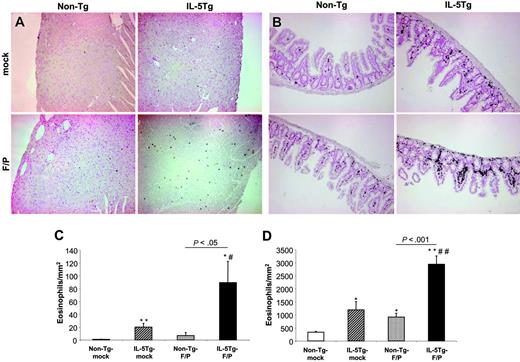
Human HES is characterized by persistent eosinophilia and organ involvement associated with eosinophilic infiltration. IL-5Tg-F/P recipients showed an up-regulation of the α4-integrin in the spleen and BM (Figure 3D) and up-regulation of Siglec-F expression in the BM (Figure 3E). This was especially relevant since α4-integrin and Siglec-F have been associated with eosinophil adhesion and migration,33,36,37 suggesting the presence of activation in the F/P+ eosinophils. In order to analyze whether the expression of activation markers in IL-5Tg-F/P mice correlated with nonhematopoietic organ infiltration mimicking HES/CEL, we analyzed the eosinophil content of the liver, kidneys, intestine, lungs, and heart of all the experimental groups. In the IL-5Tg-F/P recipients, eosinophils dominated infiltration in perivascular regions of the liver (Figure 4A), whereas in non–Tg-F/P recipients most infiltrating cells were neutrophils (data not shown). In addition, the IL-5Tg-F/P recipients showed eosinophilic infiltrates in peritubular regions of the kidney (Figure 4B) and lung (Figure 4C). IL-5Tg-F/P recipients showed 4.5-fold and 2.5-fold higher eosinophil content in the myocardium and lamina propria of the small intestine, respectively, than in IL-5Tg-mock vector recipients (Figure 5). Representative examples of heart and small intestine histology are shown in Figure 5A and 5B, respectively.
Analysis of spleen and bone marrow in a murine eosinophilic disease model induced by the F/P fusion gene. (A) Spleen weight of the same groups of mice that underwent transplantation as shown in Figure 1. Data are shown as mean ± SD and represent 10 to 12 mice per group, pooled from 3 independent experiments. **P < .01, compared with non–Tg-mock; ##P < .01, compared with IL-5Tg-mock. Histopathologic analysis of spleen (B) and bone marrow (C) was performed at 4 weeks by hematoxylin/eosin staining for sectioned tissue and Diff-Quick staining for cytospins. Representative examples (of 5-12 mice per group, pooled from 3 independent experiments) are shown in panels B-C (i: non–Tg-mock; ii: non–Tg-F/P; iii: IL-5Tg-mock; iv: IL-5Tg-F/P; optical magnification × 500). Arrows in panel C indicate eosinophils. Inset of panel B shows cytospin of IL-5Tg-F/P recipient spleen (optical magnification × 500). CD49d (α4-integrin) expression on CD49d+ cells in CCR3+/EGFP+/7-AAD– BM cells (D) and Siglec-F expression on Siglec-F+ cells in EGFP+/7-AAD– BM cells (E) in IL-5Tg-mock (blue lines) and IL-5Tg-F/P (red lines) were analyzed by flow cytometry. Representative examples (3-12 mice per group, pooled from 2 or 3 independent experiments) are shown in panels D and E.
Analysis of spleen and bone marrow in a murine eosinophilic disease model induced by the F/P fusion gene. (A) Spleen weight of the same groups of mice that underwent transplantation as shown in Figure 1. Data are shown as mean ± SD and represent 10 to 12 mice per group, pooled from 3 independent experiments. **P < .01, compared with non–Tg-mock; ##P < .01, compared with IL-5Tg-mock. Histopathologic analysis of spleen (B) and bone marrow (C) was performed at 4 weeks by hematoxylin/eosin staining for sectioned tissue and Diff-Quick staining for cytospins. Representative examples (of 5-12 mice per group, pooled from 3 independent experiments) are shown in panels B-C (i: non–Tg-mock; ii: non–Tg-F/P; iii: IL-5Tg-mock; iv: IL-5Tg-F/P; optical magnification × 500). Arrows in panel C indicate eosinophils. Inset of panel B shows cytospin of IL-5Tg-F/P recipient spleen (optical magnification × 500). CD49d (α4-integrin) expression on CD49d+ cells in CCR3+/EGFP+/7-AAD– BM cells (D) and Siglec-F expression on Siglec-F+ cells in EGFP+/7-AAD– BM cells (E) in IL-5Tg-mock (blue lines) and IL-5Tg-F/P (red lines) were analyzed by flow cytometry. Representative examples (3-12 mice per group, pooled from 2 or 3 independent experiments) are shown in panels D and E.
Murine eosinophilic disease was transplantable into secondary recipients
To examine whether the eosinophilic disease that developed in primary transplant recipients was transferable to secondary recipients, we transplanted increasing numbers of splenocytes from diseased primary IL-5Tg-F/P recipients into lethally irradiated recipients. IL-5Tg-F/P secondary recipients showed an eosinophilic disease (9 of 9 mice, pooled from two independent experiments) when transplanted with one fifth or one tenth of the spleen cell content from IL-5Tg-F/P primary recipients at 4 weeks after transplantation. However, when secondary recipients received a transplant of fewer cells (1/100 or 1/1000 of total splenocytes), it took 7 and 10 weeks, respectively, to develop the murine eosinophilic disease in a fraction of mice (approximately 30%). We analyzed the frequency of EGFP+ cells in the peripheral blood of recipient mice at 4 weeks after transplantation until death. The percentage of EGFP+ cells in IL-5Tg-F/P secondary recipients gradually decreased during this period of time; however, EGFP+ cells were identified at 10 weeks after secondary transplantation (minimum of 2.5%; Table 2). Detectable EGFP+ B and T lymphocytes were observed in F/P-transduced BM recipients at 10 weeks after transplantation (data not shown). Collectively, these results suggest that either short-term stem cells or early myeloid progenitors with prolonged survival and/or extended proliferation are involved in the development of this phenotype.
Eosinophil infiltration in the liver, kidney, and lung of murine eosinophilic disease model induced by F/P fusion gene and IL-5 overexpression. Histopathologic analysis of liver (A), kidney (B), and lung (C) in IL-5Tg-F/P recipient mice was performed by hematoxylin and eosin (A-C) and anti-MBP staining (C [inset]). Representative examples (of 3-7 mice per group, pooled from three independent experiments) are shown. Optical magnifications × 125. Insets represent blow-ups of original pictures with overall magnifications of × 2000 in panels A-B and × 500 in C. Arrow is pointing to an eosinophil infiltrate in the (A) liver and (B) kidney.
Eosinophil infiltration in the liver, kidney, and lung of murine eosinophilic disease model induced by F/P fusion gene and IL-5 overexpression. Histopathologic analysis of liver (A), kidney (B), and lung (C) in IL-5Tg-F/P recipient mice was performed by hematoxylin and eosin (A-C) and anti-MBP staining (C [inset]). Representative examples (of 3-7 mice per group, pooled from three independent experiments) are shown. Optical magnifications × 125. Insets represent blow-ups of original pictures with overall magnifications of × 2000 in panels A-B and × 500 in C. Arrow is pointing to an eosinophil infiltrate in the (A) liver and (B) kidney.
Quantitative histopathologic analysis of the heart and small intestine in the murine eosinophilic disease model induced by F/P fusion gene and IL-5 overexpression. Representative examples (of 3-4 mice per group) of eosinophil content of heart (A) and small intestine (B) of non–Tg-mock, non–Tg-F/P, IL-5Tg-mock, and IL-5Tg-F/P mice are shown in panels A-B. Optical magnifications × 125. Quantitative analysis of the content of MBP+ eosinophils in heart (C) and small intestine (D) of mice depicted in panels A-B was performed. Data are shown as mean ± SD. *P < .05 and **P < .01, compared with non–Tg-mock; #P < .05 and ##P < .01, compared with IL-5Tg-mock.
Quantitative histopathologic analysis of the heart and small intestine in the murine eosinophilic disease model induced by F/P fusion gene and IL-5 overexpression. Representative examples (of 3-4 mice per group) of eosinophil content of heart (A) and small intestine (B) of non–Tg-mock, non–Tg-F/P, IL-5Tg-mock, and IL-5Tg-F/P mice are shown in panels A-B. Optical magnifications × 125. Quantitative analysis of the content of MBP+ eosinophils in heart (C) and small intestine (D) of mice depicted in panels A-B was performed. Data are shown as mean ± SD. *P < .05 and **P < .01, compared with non–Tg-mock; #P < .05 and ##P < .01, compared with IL-5Tg-mock.
F/P expression is specifically required to induce murine eosinophilic disease and is associated with increased expression of IL-5Rα
To determine whether the development of the murine eosinophilic disease requires the expression of F/P in combination with overexpression of IL-5 in hematopoietic cells, we performed two additional groups of experiments. In the first experiment, IL-5Tg HSCs/Ps were transduced with the p210-BCR/ABL fusion gene (IL-5Tg-BCR/ABL) and transplanted into irradiated recipient mice. The p210-BCR/ABL fusion gene was chosen because a myeloproliferative disease induced by this transgene in mice shows abnormalities of all myeloid lineages, including eosinophils,14 and human chronic myelogenous leukemia (CML) occasionally involves hypereosinophilia (for example, the eosinophilic variant of CML).38-40 Although the efficiencies of F/P and BCR/ABL transductions were not significantly different (7% ± 5.2% vs 3% ± 1.9%, respectively, P = .20), IL-5Tg-BCR/ABL recipients developed myeloproliferative disease in the third week after transplantation, while IL-5Tg-F/P recipient mice established full disease by 4 weeks. IL-5Tg-BCR/ABL recipients showed similar eosinophil counts to IL-5Tg-mock vector mice (Figure 6A) at the study end point. IL-5Tg-BCR/ABL–induced myeloproliferative disorder was characterized by leukocytosis (143.90 ± 129.61 × 109 leukocytes/L [143 900 ± 129 610 leukocytes/mm3]) with neutrophilia and splenomegaly and was similar to that observed in BCR/ABL-transduced wild-type HSC/P (non–Tg-BCR/ABL) recipients. IL-5Tg-BCR/ABL–transduced BM recipients, at 3 weeks after transplantation, had low levels of circulating IL-5 (undetectable, n = 5) compared with the levels of IL-5Tg-mock vector BM recipients (356 and 381 pg/mL, n = 2). Curiously, the levels of eosinophilia of both groups were similar by day 21 after transplantation (5.620 ± 2.970 × 109 eosinophils/L and 3.680 ± 1.165 eosinophils/L [5620 ± 2970 eosinophils/mm3 and 3680 ± 1165 eosinophils/mm3], respectively), suggesting that the plasma IL-5 level, by itself alone, does not correlate with the severity of eosinophilia. In addition, IL-5Tg-BCR/ABL recipients showed an eosinophilic peak by 18 days after transplantation (15.880 ± 10.432 × 109 eosinophils/L [15 880 ± 10 432 eosinophils/mm3]), which significantly dropped by 21 days after transplantation (5.620 ± 2.970 × 109 eosinophils/L [5620 ± 2970 eosinophils/mm3], P < .03 derived from the same mice), while the leukocyte count increased (83.480 ± 30.133 × 109 leukocytes/L [83 480 ± 30 133 leukocytes/mm3] 18 days after transplantation vs 96.733 ± 42.985 × 109 leukocytes/L [96 733 ± 42 985 leukocytes/mm3] 21 days after transplantation derived from the same mice). Of importance, this eosinophil peak was higher than non–Tg-BCR/ABL control mice (0.076 ± 0.059 × 109 eosinophils/L [76 ± 59 eosinophils/mm3]), indicating that indeed BCR/ABL had the potential ability to develop HES/CEL under these conditions. This makes it unlikely that the lower eosinophilia found in IL-5Tg-BCR/ABL mice was due to mouse death before IL-5 exerted its full effect.
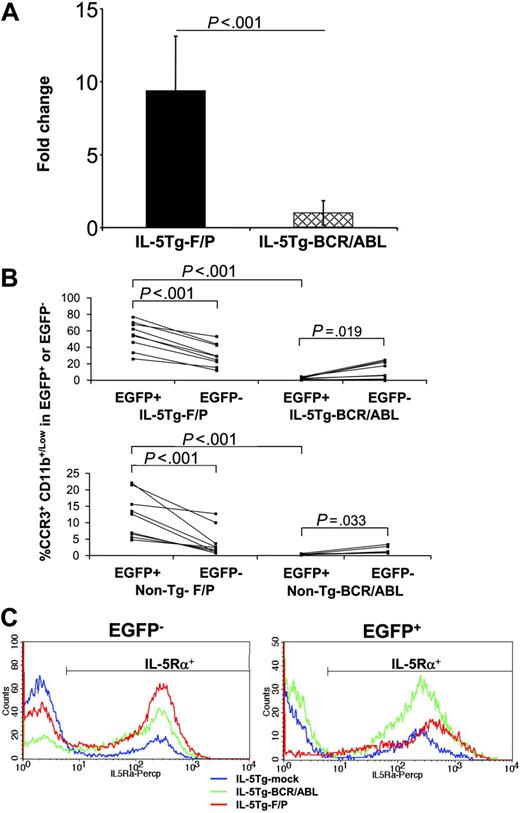
IL-5Tg-F/P recipients showed 10-fold higher eosinophilia than IL-5Tg-BCR/ABL recipients. In addition, non–Tg-F/P and IL-5Tg-F/P recipients demonstrated approximately 23-fold and approximately 32-fold higher percentages, respectively, of EGFP+/CCR3+/CD11b+/Low cells than those of non–Tg-BCR/ABL and IL-5Tg-BCR/ABL recipients in peripheral blood (Figure 6B). We compared the percentages of EGFP+/CCR3+/CD11b+/Low and EGFP–/CCR3+/CD11b+/Low in the F/P and BCR/ABL groups. F/P recipients showed eosinophilia due to a specific expansion of EGFP-expressing cells. In fact, in non–Tg-F/P and IL-5Tg-F/P recipients, the percentages of EGFP+/CCR3+/CD11b+/Low cells were 3.0- and 1.8-fold higher than those of EGFP–/CCR3+/CD11b+/Low cells, respectively. In contrast to F/P recipients, the eosinophil population observed in IL-5Tg-BCR/ABL recipients was due mostly to non–EGFP-expressing cell expansion (Figure 6B).
We next aimed to determine if F/P-associated disease in mice was imatinib sensitive. Accordingly, we administered PBS (vehicle) or imatinib to IL-5Tg-F/P recipient mice beginning 10 days after transplantation until 1 week after all control mice had developed severe disease 37 days after transplantation. Imatinib-treated IL-5Tg-F/P mice had an approximately 8-fold decrease in eosinophils compared with control-treated mice (eosinophil counts in peripheral blood were 25.720 ± 11.268 × 109 eosinophils/L [25 720 ± 11 268 eosinophils/mm3] vs 3.260 × 1.261 × 109 eosinophils/L [3260 ± 1261 eosinophils/mm3], respectively, with a percentage of EGFP+ leukocytes of 49.0 ± 16.65% vs 4.7 ± 1.97%, respectively). Imatinib-treated IL-5Tg-F/P recipients did not show leukocytosis or splenomegaly, and had only moderate eosinophilia (similar to basal IL-5Tg-mock vector recipients), indicating no disease development. All imatinib-treated IL-5Tg-F/P recipients survived until the study end point, whereas control-treated IL-5Tg-F/P recipients had died due to severe disease by 26 days (interquartile range, 24-29 days) after transplantation (P = .002).
Comparison between the murine eosinophilic disease model induced by the F/P fusion gene and the BCR/ABL-induced disorder under conditions of IL-5 overexpression. Mice that received a transplant of non-Tg, F/P-transduced (non–Tg-F/P); IL-5 Tg, F/P-transduced (IL-5Tg-F/P); non-Tg, p210-BCR/ABL–transduced (non–Tg-BCR/ABL); and IL-5 Tg, p210-BCR/ABL–transduced (IL-5Tg-BCR/ABL) HSCs/Ps were analyzed just after disease development. Total eosinophil counts using Discombe staining (A) and frequency of CCR3+CD11b+/low cells in EGFP+/7AAD– or EGFP–/7AAD– by flow cytometer (B) were measured when the mice fully developed diseases. The eosinophil count in all samples was divided by the average of those of IL-5Tg-mock recipients in each experiment for standardization. Data represent 6 to 10 mice per group, pooled from 2 or 3 independent experiments. Data in panel A are shown as mean ± SD. (C) Representative example (of 3-5 mice per group, pooled from 1 or 2 independent experiments) of expression of IL-5 receptor α on EGFP– and EGFP+ splenocytes in IL-5Tg-mock (blue line), IL-5Tg-F/P (red line), and IL-5Tg-BCR-ABL (green line) as analyzed by flow cytometry is shown.
Comparison between the murine eosinophilic disease model induced by the F/P fusion gene and the BCR/ABL-induced disorder under conditions of IL-5 overexpression. Mice that received a transplant of non-Tg, F/P-transduced (non–Tg-F/P); IL-5 Tg, F/P-transduced (IL-5Tg-F/P); non-Tg, p210-BCR/ABL–transduced (non–Tg-BCR/ABL); and IL-5 Tg, p210-BCR/ABL–transduced (IL-5Tg-BCR/ABL) HSCs/Ps were analyzed just after disease development. Total eosinophil counts using Discombe staining (A) and frequency of CCR3+CD11b+/low cells in EGFP+/7AAD– or EGFP–/7AAD– by flow cytometer (B) were measured when the mice fully developed diseases. The eosinophil count in all samples was divided by the average of those of IL-5Tg-mock recipients in each experiment for standardization. Data represent 6 to 10 mice per group, pooled from 2 or 3 independent experiments. Data in panel A are shown as mean ± SD. (C) Representative example (of 3-5 mice per group, pooled from 1 or 2 independent experiments) of expression of IL-5 receptor α on EGFP– and EGFP+ splenocytes in IL-5Tg-mock (blue line), IL-5Tg-F/P (red line), and IL-5Tg-BCR-ABL (green line) as analyzed by flow cytometry is shown.
To investigate the mechanism of cooperation between F/P and IL-5, we further analyzed the expression of IL-5 receptor α chain (IL-5Rα). IL-5Tg-F/P recipients showed a 1.9-fold higher expression of IL-5Rα on EGFP+ splenocytes than IL-5Tg-mock vector and IL-5Tg-BCR/ABL recipients (mean fluorescence intensity of EGFP+/IL-5Rα+: 494.7 ± 31.39 vs 368.3 ± 57.57 [P < .01] and 304.5 ± 48.29 [P < .01]; respectively). EGFP+ cells from IL-5Tg-F/P recipients had higher IL-5Rα expression compared with F/P– cells derived from the same mice (P = .013), suggesting that F/P may specifically up-regulate IL-5Rα expression. In contrast to EGFP+ cells, the mean fluorescence intensity of IL-5Rα expression on EGFP– cells was not different among the recipient groups (P = .44). A representative set of histograms is shown in Figure 6C.
Discussion
In this study, a murine model for HES/CEL, which uses the coexpression of F/P with transgenic IL-5 overexpression by T cells, has been described. The introduction of F/P together with T-cell overexpression of IL-5 induced a striking eosinophilia in the peripheral blood and tissue eosinophil infiltration of the heart, lungs, kidneys, small intestine, liver, and spleen. Prior to this study, it has been an enigma why CD2-IL-5 Tg mice develop blood eosinophilia but not tissue eosinophilia as seen in HES.22 Our study shows that neither IL-5 nor F/P overexpression alone induces substantial tissue eosinophilia, but together these two events result in the development of eosinophil-associated end-organ infiltration. It is interesting to note that patients with persistent eosinophilia due to parasitic infection41 or various malignancies42 remain at risk for developing end-organ damage. Perhaps Th2-associated eosinophilia transforms into HES-like disease when accompanied by the de novo activation of the appropriate tyrosine kinase, in this case F/P. In HES patients, the cardiovascular system is the most common organ system involved, and cardiovascular system abnormalities are the primary cause of morbidity and mortality.43 The HES/CEL model described here showed a rapidly progressive increase of eosinophil infiltration in the myocardium. The absence of myocardial mural thrombosis and fibrosis-related endomyocardial thickening, characteristic of HES/CEL patients, may be explained by the short life expectancy of mice coexpressing the F/P fusion gene in the presence of IL-5 overexpression. Extrahematologic manifestations in HES/CEL patients also include pulmonary infiltration in approximately 50% of patients,43,44 and spleen and liver involvement in approximately 40% and approximately 30% of patients, respectively.43,44 The model of HES/CEL-like disease described here also demonstrated eosinophil infiltration of other nonhematopoietic organs, as well as blood and hematopoietic tissue hypereosinophilia. In humans, F/P+ HES/CEL has been associated with a poor prognosis compared with F/P– HES. These patients specifically exhibit cardiac complications and poor response to corticosteroids.6,7,9,10,45 In this HES/CEL-like disease model, mice developed a severe and rapidly progressive disease featuring hepatosplenomegaly and tissue eosinophilia in most organs that may be similar to poor-prognosis HES phenotype.
IL-5 has been shown to be the most relevant cytokine in the pathogenesis of HES/CEL.46 Indeed, anti–IL-5 therapy appears to be an effective therapy even in patients with undetectable or low levels of IL-5.47,48 In addition to circulating IL-5, paracrine effects of IL-5 locally produced by T cells18 and/or eosinophils49 may have critical roles in HES. In fact, a fraction of HES/CEL patients have T-cell–dependent IL-5 overexpression.44 The relationship between the F/P mutation and high levels of IL-5 still remains unclear.44 Of interest, serum IL-5 levels have been reported to be elevated in imatinib-responder HES patients,50 including F/P+ patients,8 and the existence of anti–IL-5 responder F/P+ patients has been observed (Klion et al47 ; and Y.Y. and M.E.R., unpublished data, July 2004). In our study, HES/CEL-like mice generally showed high circulating IL-5 levels. IL-5 likely synergizes with F/P on the development of hypereosinophilia in this murine HES/CEL-like model; this may occur through systemic or local (paracrine or autocrine) effects and indirectly through a putative up-regulation effect on IL-5Rα in F/P+ cells, which would increase the sensitivity of IL-5R–expressing myeloid progenitors and precursors.
In agreement with Cools et al,15 we have shown that F/P fusion gene expression without IL-5 overexpression induced the development of a myeloproliferative disorder resembling p210-BCR/ABL–induced myeloproliferative disease. However, in non–Tg-F/P recipients, the frequencies of F/P+/CCR3+/CD11b+/Low and F/P+/Siglec-F+/CD11b+/Low cells in BM and spleen were increased, suggesting that F/P promoted differentiation into eosinophils, at least as defined by these markers. In addition to these markers, the frequency of IL-5Rα+ cells on the F/P+ population was increased in both non–Tg-F/P and IL-5Tg-F/P recipient spleens (Figure 6C and data not shown). These results suggest that the introduction of F/P induces myeloid proliferation and primes eosinophil differentiation but that development of the full HES/CEL picture requires additional cytokines or molecular events. While neither the F/P fusion gene nor IL-5 overexpression alone, as indicated by IL-5Tg-mock vector recipients, is sufficient to cause HES-like disease, F/P expression and dysregulated IL-5 overexpression appear to cooperate to induce HES/CEL.
Expression of the F/P fusion gene or deletion of the surrogate marker CHIC2 has been detected in noneosinophilic myeloid cells and in lymphoid cells, suggesting that the F/P mutation may occur in early HSC.9,51,52 In order to test whether the phenotypic expression of hypereosinophilia seen in this model of HES/CEL-like disease derived from a primitive hematopoietic cell, we performed serial transplantation of splenocytes from F/P recipients after overt disease development. F/P fusion gene–induced disease was consistently transplantable into secondary recipients but only when large numbers of cells were used, suggesting that the transformed stem/progenitor cell expressing the combination of F/P and IL-5 overexpression was infrequent in the spleen of diseased animals. Alternatively, the disease may not be associated with long-term repopulating stem cells. These results suggest that either short-term hematopoietic repopulating cells or myeloid progenitors with prolonged survival and/or extended proliferation are responsible for the F/P induction of the HES/CEL-like disorder in IL-5Tg-F/P recipient mice.
In addition to F/P-positive HES/CEL, an eosinophilic variant of CML (eoCML) identified by the BCR/ABL rearrangement has been reported.38,40 Indeed CML patients with more than 5% of eosinophils are at risk for an eosinophilic blast crisis.39 Accordingly, we investigated whether the BCR/ABL fusion gene could cooperate with IL-5 overexpression in the development of HES/CEL-like disease in mice. When compared with IL-5Tg-F/P recipients, we observed that the eosinophil count of IL-5Tg-BCR/ABL recipients was significantly lower at the time of disease development. These results suggest that expression of the F/P fusion gene specifically induces eosinophil differentiation. The molecular event that transforms BCR/ABL-associated CML into CEL remains to be determined.
In conclusion, a murine HES/CEL model has been established in this study. The pathogenic mechanism of the HES/CEL-like disease might be explained by the expansion of a short-term stem cell–associated myeloid proliferation or of an early myeloid progenitor with prolonged survival and/or extended proliferation. Either would have an enhanced sensitivity to IL-5, which evokes eosinophil-precursor proliferation and terminal differentiation. Moreover, tissue eosinophil infiltrations were induced by the combination of overexpression of IL-5 and the introduction of the F/P fusion gene, suggesting additional specific activation and migratory activity of IL-5–stimulated, F/P-transduced eosinophils.
F/P+ cells have shown exquisite sensitivity to imatinib inhibition in vitro and in vivo,3,9,15,53 and imatinib has become a first-line treatment for F/P+ disease. However, the presence of a subgroup of imatinib-resistant F/P+ HES/CEL patients and the longer life expectancy of F/P+ HES/CEL patients compared with CML patients highlight the need to develop additional therapy for HES/CEL. As such, this model may aid in the development of drugs that specifically target F/P+ HES/CEL.
Prepublished online as Blood First Edition Paper, January 17, 2006; DOI 10.1182/blood-2005-08-3153.
Supported by the Akita Medical School Fund for International Cooperation and Exchange (Y.Y.), the Japan Allergy Foundation Fund for International Cooperation and Exchange (Y.Y.), the American Heart Association Ohio Valley Affiliate Postdoctoral Fellowship (Y.Y.), the Campaign Urging Research for Eosinophilic Disease (CURED; M.E.R.), the Leukemia & Lymphoma Society Translational Research Grant (D.A.W., J.A.C.), the National Blood Foundation (J.A.C.), and National Institutes of Health (NIH) grants 1P01HL69974 and 1R01DK062757 (D.A.W.).
Y.Y. designed and performed the research, analyzed data, and wrote the paper; M.E.R. designed and supervised the study and assisted in writing the paper; A.W.L. assisted in performing experiments; H.S.A. and E.B.B. participated in performing experiments and analyzing the data; D.A.W. supervised the study and assisted in writing the paper; J.A.C. designed, performed, and supervised the study, assisted in analyzing the data, and wrote the paper. All authors checked the final version of the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are thankful to Gary Gilliland for his critical comments and suggestions; Jan Cools for his advice in the experiment using imatinib and his suggestions; Elizabeth Stover for her advice in the experiment using imatinib; Shuichi Abe for technical assistance and suggestions in histologic analysis; Patricia Fulkerson and Susan Wert for their advice in the histologic analysis of the lung; Jeff Bailey for technical assistance in bone marrow transplantation; Melissa McBride for preparing IL-5 (CD2) Tg mice; Victoria Summey-Harner, Chad Harris, Kathy Szczur, and Tracy Hopkins for their assistance in the experiment using imatinib; Toru Oka for his advice in heart histology; Andrea Lippelman for her editorial assistance; and the flow cytometry core facility at the Division of Experimental Hematology for providing support in our FACS analysis.

![Figure 4. Eosinophil infiltration in the liver, kidney, and lung of murine eosinophilic disease model induced by F/P fusion gene and IL-5 overexpression. Histopathologic analysis of liver (A), kidney (B), and lung (C) in IL-5Tg-F/P recipient mice was performed by hematoxylin and eosin (A-C) and anti-MBP staining (C [inset]). Representative examples (of 3-7 mice per group, pooled from three independent experiments) are shown. Optical magnifications × 125. Insets represent blow-ups of original pictures with overall magnifications of × 2000 in panels A-B and × 500 in C. Arrow is pointing to an eosinophil infiltrate in the (A) liver and (B) kidney.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/10/10.1182_blood-2005-08-3153/2/m_zh80100695600004.jpeg?Expires=1763635372&Signature=F2TolzD71OiomnkinKwaZ72V2RQrqiX9Ndz~Ubeg4FhOYQwJgWn0Iz5dldLjDEYp3p86gRmB60MF3Q5nss8A-hzvbVhjkL5BxElvPIfE6EdQP7ikCCVYxRRZUlGwqE8Jv0KpUV0q23gQqDfqp5KEGAVGlZ6f8D-t-gmkXpOLPe0aGmUkY0-EkBucbpuMPg-G9F35dzf7EYMmV7ZTUNa4-1wVogCwlkMSLeEkDVnENYbhh8gx42RZKGmRSu1s~188RtcfiudrbVIg6zBxajJ9n9x96kTux6sqH4T6Lj3WLOH-F3Tg7zV2m7iKBC~4MI-A7vJtwzFZGMtB5dKvuPCPZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)