The presence of persistent circulating leukemia cells, or engrafted into extramedullary tissues, is a bad prognostic factor for patients with acute leukemia. However, little is known about the mechanisms that regulate the exit of leukemia cells from the bone marrow (BM) microenvironment. We reveal that vascular endothelial growth factor receptor 1 (FLT-1) modulates acute leukemia distribution within the BM, along VEGF and PlGF gradients, regulating leukemia survival and exit into the peripheral circulation. FLT-1 activation on acute lymphoblastic leukemia (ALL) cells results in cell migration and proliferation in vitro, whereas in vivo FLT-1-overexpressing cells accumulate in the BM epiphysis of nonobese diabetic-severe combined immunodeficient (NOD-SCID) recipients and are detected in circulation 2 weeks after inoculation. In turn, FLT-1 neutralization affects leukemia localization (now in the BM diaphysis), increases leukemia apoptosis, and impedes the exit of ALL cells, prolonging the survival of inoculated mice. We demonstrate further that FLT-1-induced cell migration involves actin polymerization and lipid raft formation. Taken together, we show that FLT-1 regulates the BM localization of ALL cells, determining their survival and exit into the circulation and ultimately the survival of inoculated recipients. FLT-1 targeting on subsets of acute leukemias may delay the onset of extramedullary disease, which may be advantageous in combinatorial therapeutic settings.
Introduction
The early detection of acute leukemia cells outside the bone marrow (BM) microenvironment is considered an unfavorable prognostic factor, both in lymphoblastic as well as in myeloid leukemias.1-5 Moreover, leukemia infiltration into extramedullary sites may also reduce leukemia responsiveness to induction chemotherapy,6,7 whereas persisting blasts correlate with decreased overall survival and confer poor prognosis in patients with acute lymphoblastic leukemia (ALL).8,9 Thus, there is great interest in determining the molecular mechanisms that regulate acute leukemia exit from the BM microenvironment into the peripheral circulation and into extramedullary organs such as the spleen, among others.
The importance of angiogenesis and angiogenesis-related signaling pathways for the growth and expansion of acute leukemias has been amply demonstrated (Aguayo et al10 and Podar and Anderson11 provide updated references on the subject). For instance, we and others have shown that subsets of acute leukemia cells express vascular endothelial growth factor and its receptors (namely VEGFR-2/KDR and VEGFR-3/FLT-4), resulting in autocrine loops that modulate leukemia survival, proliferation, and migration.12,13 Thus, acute leukemia growth involves autocrine and paracrine VEGF/VEGF receptor loops that regulate expansion of the leukemia clones within the BM microenvironment. This has generated great interest in designing strategies to block such VEGF/VEGF receptor loops for the treatment of subsets of acute leukemias (see Giles et al14 as an example).
Regarding the actions of VEGF, several studies suggested it modulates cell proliferation and survival, via KDR, whereas FLT-1 activation has been linked mostly with cell migration.15,16 In the case of BM diseases, such as acute leukemias, there is little information concerning a putative function for FLT-1, although a previous report suggested it may induce proliferation of myeloid leukemia cell lines17 and may also regulate the localization of immature malignant precursors within the BM, in myelodysplastic syndromes.18 More recently, work done in multiple myeloma reinforced the idea that FLT-1 may regulate the migration and possibly the proliferation of the malignant plasma cells.19 In acute leukemias, it remains to be demonstrated whether FLT-1 conveys similar signals and its importance during disease onset and progression.
In the present report, we used acute lymphoblastic leukemia cells as a model to study the role of FLT-1 in leukemia biology. We demonstrate in vitro and in vivo that FLT-1 activation results mainly in leukemia-cell migration, while having a modest effect upon cell proliferation. Strikingly, FLT-1 activation on leukemia cells upon vivo results in distinct cell localizations within the BM; FLT-1-overexpressing cells localize predominantly close to articulations (epiphysis) of long bones, whereas FLT-1-nonexpressing cells (or those where the activation of the receptor was blocked) localize in the central portions (diaphysis) of the bone marrows. This selective localization of leukemia cells conditions their survival, the exit of blasts into the peripheral circulation and ultimately the survival of inoculated recipients (in vivo).
We demonstrate that, taken together, the intra-BM localization of acute leukemia cells is modulated by FLT-1 activation and determines the rate of leukemia exit from the BM microenvironment and ultimately the survival of inoculated recipients.
Materials and methods
Primary leukemia samples
All the primary samples analyzed consisted of BM biopsies and were collected at diagnosis with the informed consent of the patients, according to institutional guidelines. Study protocols were approved by the Institutional Review Board of the Portugese Institute of Oncology, Lisbon.
Leukemia cells were enriched by density gradient centrifugation prior to further analysis. All the patient samples studied were obtained from BM biopsies with a minimum of 75% leukemia blasts (as determined by the Pathology Department at Instituto Português de Oncologia Francisco Gentil [IPOFG], following institutional protocols).
Cell culture
The leukemia cell lines studied included acute myeloid leukemias (HL-60, HEL, kasumi, MV4;11), chronic myelogenous leukemia (CML) (K562, KCL-22), and ALL (TOM-1, RS4;11, 697, REH, BV-173). The ALL cell lines studied in detail were RS4;11, BV-173, REH, and 697. These were cultured in complete RPMI medium (10% FBS, l-glutamine 1X, antimycotic-antibiotic 1X) (Gibco-Invitrogen, Grand Island, NY).
RNA extraction, cDNA synthesis, and RT-PCR
Leukemia cells were first analyzed for VEGF receptors, VEGF, and PlGF expression by RT-PCR (reverse transcriptase-polymerase chain reaction). Total cellular RNA was extracted, and cDNA was synthesized following conventional protocols. PCR was performed using a PCR thermal cycler (Uno II; Biometra, Goettingen, Germany). The PCR program used to amplify VEGF, VEGFR-1 (FLT-1), VEGFR-3 (FLT-4), and P1GF consisted of a denaturation step of 4 minutes at 94°C, followed by 35 cycles of 1 minute at 94°C, 45 seconds at 60°C, and 1 minute at 72°C and concluded with a final extension of 7 minutes at 72°C. For VEGFR-2 (KDR) amplification the program was the same, preceded by a precycle of 4 minutes at 94°C, 45 seconds at 55°C, and 1 minute at 72°C. The primer sequences were as follows: FLT-1 forward primer, CAC CAA GAG CGA CGT GTG; FLT-1 reverse primer, TTT TGG GTC TCT GTG CCA G (FLT-1 PCR product:,196 bp); FLT-4 forward primer, CTG CTG GAG GAA AAG TCT GG; FLT-4 reverse primer, CTT GCA GAA CTC CAC GAT CA (FLT-4 PCR product, 550 bp); KDR forward primer, CTG GCA TGG TCT TCT GTG AAG CA; KDR reverse primer, AAT ACC AGT GGA TGT GAT GCG G (KDR PCR product, 660 bp); VEGF forward primer, AGC TTC CTA CAG CAC AAC AAA TGT; VEGF reverse primer, CGC CTC GGC TTG TCA CA (VEGF PCR product, 212 bp); PlGF forward primer, CTC CTA AAG ATC CGT TCT GG; PlGF reverse primer, GGT AAT AAA TAC ACG AGC CG (PlGF PCR product, 249 bp). The PCR program used to amplify human CD19 expression from circulating mononuclear cells from inoculated nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice was as follows: denaturing step of 95°C for 4 minutes, followed by 30 cycles of 1 minute at 95°C, 2 minutes at 65°C, 2 minutes at 72°C, and finally by an elongation step of 10 minutes at 72°C. The human CD19 primer sequence was as follows: CD19 forward primer, TCACCGTGGCAACCTGACCATG; CD19 reverse primer, GAGACAGCACGTTCCCGTACTG. CD19 PCR product size was 267 bp.
FLT-1 quantification by real-time PCR (RQ-PCR)
FLT-1 mRNA quantification was performed using the ABI Prism 7700 Sequence Detection System and the SYBR Green Master Mix kit (both from Applied Biosystems, Foster City, CA). BCR gene was used as standard reference (normalizer). The relative expression of FLT-1 was calculated by using the comparative threshold cycle (CT) method. The primer sequences were as follows: FLT-1 sense, 5′-CACCAAGAGCGACGTGTG-3′; antisense, 5′-TTTTGGGTCTCTGTGCCAG-3′; BCR sense, 5′-GAGCGTGCAGAGTGGAGGGAGAACA-3′; antisense, 5′-CACAGTATCCTCAGGGTCTGGGA-3′.
VEGF ELISA
Human VEGF production by the leukemia cell lines used in the present study was determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 1 × 106 leukemia cells were placed in 1 mL serum-free RPMI for 24 hours. After this period, the supernatant was collected, and the VEGF production was quantified by ELISA (Calbiochem, Dalmstadt, Germany) following the manufacturer's protocol.
FLT-1 protein expression
Total protein extracts from ALL cell lines were obtained by lysing the cells in cold RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-110, and 0.1% SDS), in the presence of protease and phosphatase inhibitors. After 30 minutes on ice, lysates were centrifuged for 15 minutes at 4°C and 28 770g. Equal protein amounts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% gels) under reducing conditions (in the presence of β-mercaptoethanol) and transferred to nitrocellulose membranes. Blots were blocked in PBS/1% BSA/0.1% Tween-20 for 1 hour at room temperature followed by incubation with primary (overnight at 4°C) and secondary antibodies (1 hour at room temperature). Rabbit polyclonal anti-FLT-1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibody was used at a concentration of 0.5 μg/mL, and secondary anti-rabbit IgG-HRP (Santa Cruz Biotechnology) was used at 1:6000. The electrochemiluminescence (ECL) chemiluminescence detection system (Amersham Biosciences, Buckinghamshire, United Kingdom) was used to visualize the presence of specific proteins on the nitrocellulose blots.
For immunofluorescent detection of FLT-1, the different leukemia cell lines were spun (2 minutes at 27 238g) onto glass microscope slides. The cells were fixed in 4% (vol/vol) formaldehyde/phosphate-buffered saline (PBS) for 10 minutes and washed in PBS. After blocking with 5% goat serum in PBS, the cells were incubated with a mouse anti-human FLT-1 antibody overnight at 4°C (FB6; gift from ImClone Systems, New York, NY). Then, the cells were washed and incubated with a goat anti-mouse Alexa Fluor 488 antibody (Molecular Probes, Eugene, OR) for 1 hour at room temperature. The samples were mounted in Vectashield (Vector Laboratories; Burlingame, CA) and analyzed by fluorescence microscopy (Axioplan Microscope; Zeiss, Oberkochen, Germany).
Transfection
RS4;11 cells were transfected with human full-length FLT-1, inserted in a pcDNA3.1 vector (Invitrogen, Barcelona, Spain) using the reagent Lipofectamine 2000 (Invitrogen). The protocol followed was performed according to the conditions of the transfection reagent used. Transfection efficiency was checked by RQ-PCR and functional assays (described below).
Proliferation assay
For proliferation experiments, leukemia cells were cultured in serum-free RPMI at a cell density of 5 × 105 cells/mL. Cells were treated (10-30 ng/mL VEGF or 10 ng/mL P1GF; Sigma Aldrich, Madrid, Spain) or untreated (RPMI alone) and cultured in the presence or absence of 1 μg/mL 6.12 monoclonal Ab (a mouse monoclonal specific anti-human FLT-1-neutralizing Ab; ImClone Systems). To each condition 5 U/mL heparin (Sigma Aldrich) was added. After 24 and 48 hours, viable cells (as determined by trypan blue exclusion) were counted using a hemocytometer. The FLT-1-neutralizing Ab, VEGF, and PlGF were re-added every 24 hours. Each experimental condition was done in triplicate.
Migration assay
Cells (1 × 106/mL) were placed in serum-free medium for 1 hour in the presence or absence of the FLT-1-neutralizing Ab (6.12 mAb, 1 μg/mL). Cell aliquots (100 μL) were subsequently added to 5-μm pore transwell (Corning-Costar, Cambridge, MA) inserts and plated into the wells of a 24-well plate. The lower compartment contained 600 μL serum-free medium with or without VEGF (20 ng/mL) or PlGF (10 ng/mL) and heparin (5 U/mL). Migration was determined after 4 hours by counting the number of migrated cells in 6 high-power fields (× 400 magnification) in an optical microscope.
Leukemia-cell survival assay
Cells (5 × 105/mL) were cultured in serum-free medium for 60 hours in the presence of an anti-VEGF Ab (1 μg/mL) (clone 4.6.1; gift from Genentech, South San Francisco, CA) or the FLT-1-neutralizing mAb (clone 6.12, 1 μg/mL). All antibodies were re-added every 24 hours. Cell viability was determined at 24 hours and 48 hours by trypan blue exclusion and cell counts (with the aid of a hemocytometer). Each experiment was done in triplicate.
In vivo experiments
RS4;11, RS4;11 FLT-1 transfected and 697 cells were xenotransplanted into sublethally irradiated (2.5 Gy [250 rad]) NOD/SCID mice. Leukemia cells (1 × 107/mouse) were injected intravenously 24 hours after irradiation. For each experiment, 3 groups were established: the control group (vehicle alone), the FLT-1-neutralizing Ab-treated group (200-500 ng/injection of 6.12 mAb), and the KDR-neutralizing Ab-treated group (1 μg/injection IMC1-C11 Ab, mouse anti-human monoclonal neutralizing antibody) (ImClone Systems). The neutralizing Abs were injected 3 days after leukemia-cell inoculation and were administered every other day for the remaining of the experiment. When presenting signs of disease (such as decreased locomotion, loss of weight, etc), the mice were killed, and tissues and blood were collected for further analysis.
Apoptosis analysis
To assess leukemia-cell apoptosis in vivo, we performed analysis of DNA fragmentation by transferase-mediated dUTP nick-end labeling (TUNEL) in paraffin-embedded BM sections. We used the In Situ Cell Death Detection Kit, POD and DAB Substrate Kits (Roche, Brandeburg, NJ) and followed the manufacturer's instructions. The percentage of apoptotic cells was determined by counting a total of 200 cells/area (epiphysis or diaphysis, × 400 magnification) and calculating the proportion of stained (TUNEL positive) nuclei. These quantifications were done in triplicate (3 independent counts/bone marrow section).
In vivo evidence for cell migration
To determine whether FLT-1 neutralization in vivo resulted in the impairment of leukemia-cell migration within the BM microenvironment, BM paraffin sections were stained with Phalloidin (Sigma Aldrich) to detect polymerized actin. The slides were previously deparaffinized and dehydrated using conventional methods. Next, slides were blocked for 1 hour with PBS1X/0.1% BSA/5% goat serum/0.1% TX-100 and incubated for 30 minutes with Phalloidin (0.5 μg/mL in PBS 1X). Slides were washed with PBS, mounted in Vectashield, and analyzed by fluorescence microscopy (Axioplan Microscope; Zeiss).
Immunohistochemistry
To assess the distribution of the leukemia cells in the bone marrow we studied the long bones of the killed animals. The bone was fixed in 10% buffered formalin for a minimum of 24 hours and decalcified in a rapid bone decalcifier (Perudo00-008; Eurobio, Les Ulis, France) for 3 hours and paraffin embedded. For the immunostaining 2-μm sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol.
Slides for VEGF and PlGF staining were blocked with the biotin-blocking solution SP2001 from Vector Laboratories (Burlingame, CA) for 30 minutes. Slides for TdT and Ki67 were blocked with a hydrogen peroxide solution (Dako Cytomation, Glostrup, Denmark).
Antigen retrieval was performed as follows: 20 minutes in 0.01 M pH 6.0 citrate buffer in a Phillips Whirlpool Jet1000 microwave oven (Phillips, Lisbon, Portugal) set at 750 W for VEGF and PlGF; 2 minutes in EDTA for TdT and 6 minutes in citrate buffer for Ki67 in a pressure cooker.
VEGF (Santa Cruz Biotechnology) was used diluted at 1:100 and PlGF (Santa Cruz Biotechnology) at 1:50. Both were incubated overnight at 4°C and detected with a peroxidase method. TdT and Ki67 Abs were obtained from Dako Cytomation. Staining was performed in a Dako Thechmate500 Plus (Dako Cytomation) at room temperature. TdT was used at 1:30 and Ki67 at 1:200.
Statistical analysis
Results are expressed as mean plus or minus SD. Data were analyzed using the unpaired 2-tailed Student t test or the one-way ANOVA Tukey test. P values of less than .05 were considered significant.
Results
FLT-1 is the most common VEGF receptor expressed by leukemia cells
We started our study by analyzing the global VEGF/PlGF and VEGF receptor expression pattern in primary (patient) acute and chronic leukemia samples and also in different cell lines. In total, we evaluated VEGF receptor expression, by RT-PCR, in 11 cell lines (myeloid and lymphoid leukemias) and on 83 patient BM samples. As shown in Table 1, FLT-1 is expressed by 80% of the total samples analyzed (namely all the cell lines), whereas only 18% and 24% of the samples express KDR and FLT-4, respectively. Regardless of the leukemia subtype, FLT-1 was most abundantly expressed and particularly frequent in CML and AML (Table 1). In representative samples, cell sorting was performed to enrich for leukemia blasts; further analysis confirmed the expression of VEGF receptors could be attributed to the leukemia population (data not shown).
With regard to the ligands, VEGF was expressed by all samples studied, at different levels, whereas PlGF was expressed by only one third of all samples studied.
FLT-1 modulates leukemia proliferation and migration
Given the high percentage of FLT-1-expressing leukemias, we reasoned FLT-1 might have a significant role in regulating leukemia biology, such as proliferation, survival, and/or migration. We focused our analysis on acute lymphoblastic leukemias, because a putative role for FLT-1 in these leukemias has not been described.
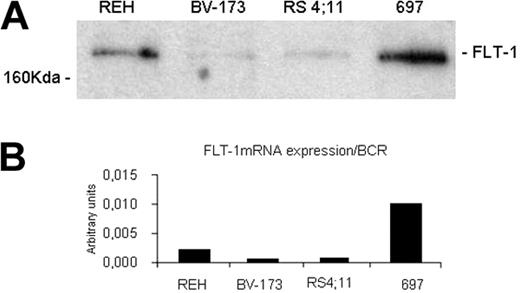
We performed proliferation and migration assays with 4 ALL cell lines corresponding to different B-cell stages and expressing VEGF and VEGF receptors (Table 2). In these cell lines, we quantified FLT-1 expression by Western blotting, immunofluorescent staining, and RQ-PCR (Figure 1A-B). Of the 4 ALL cell lines used in this study, 697 and REH appeared to express more FLT-1, whereas RS4;11 expressed the least. With regard to the ligands, VEGF and PlGF, all 4 cell lines produce VEGF, in varying (but modest; see Table 2) amounts, whereas PlGF is not expressed by these cell lines.
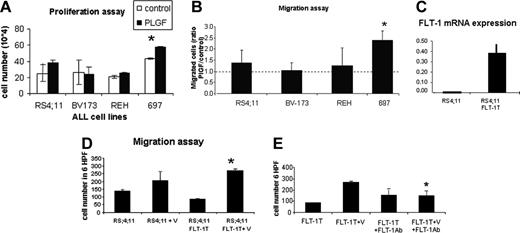
For the proliferation assay, the cells were placed in serum-free medium in the presence or absence of PlGF (FLT-1-specific ligand). As shown in Figure 2A, after 48 hours several cell lines proliferated more in response to PlGF, but significance was obtained only with the 697 cell line (P < .05).
With regard to cell migration, PlGF-induced cell migration was analyzed in transwell migration assays. As shown in Figure 2B, the 697 cell line shows the most robust chemotactic response to PlGF (with a 2.5-fold ratio of PlGF-migrated cells/control; P < .01), with the remaining 3 cell lines showing comparable migration capacity. Taken together, these data show PlGF induces proliferation and migration of subsets of ALL cells in vitro.
FLT-1 overexpression confers a more invasive phenotype to leukemia cells in vivo
To test the putative function of FLT-1 in modulating leukemia-cell migration and BM localization in vivo, we used 2 distinct approaches: 1) gain of function experiment, where an ALL cell line that expresses modest FLT-1 levels (RS 4;11) was transfected with full-length FLT-1, and changes in its in vitro and in vivo phenotype were determined; 2) in vivo inoculation of the ALL cell line that expresses more FLT-1 and shows the strongest responses to VEGF/PlGF in vitro (697 cells), subsequent treatment of inoculated mice with FLT-1 monoclonal neutralizing antibodies and observation of its in vivo phenotype. The results obtained were as follows.
(1) Starting with the gain of function experiments, we demonstrated by RQ-PCR that transfected cells express significantly more FLT-1 than their untransfected counterparts (Figure 2C). In these transfectants, we observed a significant increase in VEGF-induced cell migration (compared with untransfected cells, P < .05) but not proliferation (not shown) in vitro; importantly, this effect was blocked by the FLT-1 monoclonal neutralizing Ab (P < .05 compared with cells exposed to VEGF alone), proving the transfected receptor is functional and proving FLT-1 is the receptor that mediates VEGF-induced cell migration. Similar results were obtained by using PlGF as the chemoattractant (not shown).
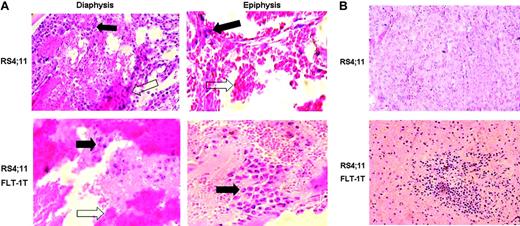
Inoculation of the transfected or untransfected cells in vivo also resulted in distinct phenotypes. FLT--overexpressing RS 4;11 cells localized mostly close to the articulations (epiphysis) of the BM in long bones (Figure 3A) and could also be found in extramedullary organs such as the spleen (Figure 3B), whereas untransfected cells were detected predominantly in the central part of the BM (diaphysis), and, at the same time points, no extramedullary engraftment was observed. Interestingly, inoculation of this cell line was always associated with significant edema in the BM cavity (Figure 3, white arrows), which may result from VEGF production by the leukemia cells.
These data suggested the in vitro phenotype conferred by FLT-1 overexpression (enhanced migration in response to the ligands) is also observed in vivo and may regulate also the exit of leukemia blasts into the peripheral circulation and into extramedullary sites such as the spleen.
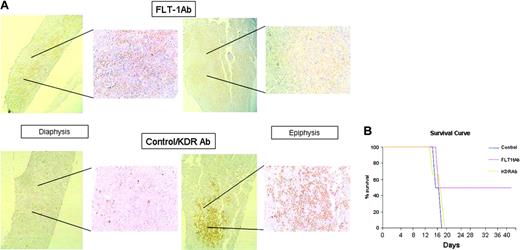
(2) As shown in Figure 1, the 697 cell line is the one that expresses more FLT-1, which is reflected in the ability of these cells to migrate and proliferate in response to VEGF or PlGF. Inoculation of 697 cells in vivo largely reproduced the phenotype observed with the RS4;11 FLT-1-overexpressing cell line. Shortly after inoculation, 697 cells accumulate mostly in the BM epiphysis of inoculated recipients (Figure 4A). As disease develops (after 15-20 days), 697 cells are detected in the circulation of inoculated mice (as determined by RT-PCR; Table 3), and we could also detect leukemia blasts engrafted into extramedullary sites such as the spleen and lungs (not shown). This phenotype is reversed on treatment of inoculated mice with the neutralizing monoclonal Ab to FLT-1 (6.12 Ab) but not with an Ab against KDR (as a control). In 6.12-treated mice, 697 cells are detected in the BM diaphysis of inoculated mice (Figure 4A), and the presence of leukemia cells in the peripheral circulation is impeded up to day 20 following leukemia inoculation (Table 3). As a consequence of FLT-1 neutralization, the group of mice that received the 6.12 Ab therapy survived significantly longer than control or KDR Ab-treated mice (Figure 4B), and at day 20 after inoculation have reduced circulating human VEGF levels, which again suggests reduced leukemia-cell exit from the BM into the peripheral circulation (Figure 5A). Notably, at day 40 after inoculation, 6.12 Ab-treated mice still had reduced (below 20 pg/mL) Human VEGF levels (not shown), demonstrating the efficacy of the treatment in delaying the onset of extramedullary disease.
These data suggest that one function of FLT-1 may be to determine leukemia-cell localization within the BM microenvironment. The selective localization of leukemia cells within the BM seems to regulate their exit into the peripheral circulation and eventual engraftment into extramedullary tissues such as the spleen.
FLT-1 neutralization results in increased leukemia-cell apoptosis in the BM
Next, we sought to determine the mechanisms whereby FLT-1 neutralization and redistribution of leukemia cells within the BM resulted in increased survival of inoculated mice and a decrease in circulating blasts. For this, we stained BM sections at day 18 after inoculation (we chose areas packed with blasts, greater than 80% of the total cells) with Ki67 (proliferation marker) and TUNEL (to detect apoptosis). FLT-1 neutralization resulted in a significant increase in the proportion of apoptotic leukemia cells in the BM diaphysis and epiphysis of inoculated mice (Table 4), and there was no difference in the proportion of proliferating (Ki67 positive) cells, and these were evenly distributed throughout the BM of control and 6.12 Ab-treated mice (not shown). In parallel, we treated 697 cells with the Ab 6.12 in vitro, but surprisingly there was very little effect in cell apoptosis (data not shown). Thus, the intramedullary localization of leukemia cells, which is modulated by FLT-1 activation, regulates leukemia survival in vivo. As a consequence of FLT-1 neutralization, the presence of leukemia cells in the peripheral blood of inoculated mice was significantly delayed (Table 3).
VEGFR-1 (FLT-1) expression level of the different ALL cell lines used in this study. The expression level was determined by Western blotting (A) and real-time quantitative PCR (B). The 697 cell line expresses the highest levels of FLT-1. All procedures are described in “Materials and methods.”
VEGFR-1 (FLT-1) expression level of the different ALL cell lines used in this study. The expression level was determined by Western blotting (A) and real-time quantitative PCR (B). The 697 cell line expresses the highest levels of FLT-1. All procedures are described in “Materials and methods.”
FLT-1 function in ALL cells. (A) Proliferation assays to test the mitogenic effects of PlGF stimulation of the 4 ALL cell lines used in this study. Results show cells that were stimulated for 24 hours with PlGF (10 ng/mL) in the presence of heparin and counted in triplicate experiments (using the trypan blue exclusion test). *PlGF induces a significant increase in cell proliferation in the 697 cell line (P < .05). (B) Transwell migration assay to test the chemotactic effects of PlGF on the 4 ALL cell lines used in this study. Results show the ratio of PlGF-induced cell migration/control cells (calculated from the mean cell number in 6 high power fields; HPF, × 400 magnification) after 4 hours of PlGF (10 ng/mL) stimulation. *697 migrated significantly more in the presence of PlGF (3 independent experiments; P < .01 for 697 cells). (C) RS4;11 cells were transfected with full-length FLT-1 to determine the result of FLT-1 overexpression in vitro and in vivo. FLT-1 mRNA expression, as determined by real-time PCR. Note the increased expression of FLT-1 mRNA in FLT-1-transfected cells (RS4;11 FLT-1T). (D) Transwell migration assay was performed with nontransfected RS4;11 and those transfected with full-length FLT-1, in the presence of VEGF (30 ng/mL) for 4 hours. Results are shown as the mean cell number in 6 HPF (× 400 magnification) and demonstrate that RS4;11 FLT-1T cells migrate significantly more in the presence of VEGF than their untransfected counterparts (*3 independent experiments; P < .05). (E) Migration assay to demonstrate the transfected full-length FLT-1 modulates cell migration. Results show RS4;11 FLT-1-transfected cells exposed to VEGF (30 ng/mL) for 4 hours, alone or in the presence of the FLT-1-neutralizing Ab, 6.12 (1 μg/mL). *VEGF-induced RS4;11 FLT-1T migration is significantly reduced in the presence of the 6.12 Ab (3 independent experiments, P < .05), demonstrating the transfected receptor is functional and modulates cell migration. Error bars depict the standard error of the mean.
FLT-1 function in ALL cells. (A) Proliferation assays to test the mitogenic effects of PlGF stimulation of the 4 ALL cell lines used in this study. Results show cells that were stimulated for 24 hours with PlGF (10 ng/mL) in the presence of heparin and counted in triplicate experiments (using the trypan blue exclusion test). *PlGF induces a significant increase in cell proliferation in the 697 cell line (P < .05). (B) Transwell migration assay to test the chemotactic effects of PlGF on the 4 ALL cell lines used in this study. Results show the ratio of PlGF-induced cell migration/control cells (calculated from the mean cell number in 6 high power fields; HPF, × 400 magnification) after 4 hours of PlGF (10 ng/mL) stimulation. *697 migrated significantly more in the presence of PlGF (3 independent experiments; P < .01 for 697 cells). (C) RS4;11 cells were transfected with full-length FLT-1 to determine the result of FLT-1 overexpression in vitro and in vivo. FLT-1 mRNA expression, as determined by real-time PCR. Note the increased expression of FLT-1 mRNA in FLT-1-transfected cells (RS4;11 FLT-1T). (D) Transwell migration assay was performed with nontransfected RS4;11 and those transfected with full-length FLT-1, in the presence of VEGF (30 ng/mL) for 4 hours. Results are shown as the mean cell number in 6 HPF (× 400 magnification) and demonstrate that RS4;11 FLT-1T cells migrate significantly more in the presence of VEGF than their untransfected counterparts (*3 independent experiments; P < .05). (E) Migration assay to demonstrate the transfected full-length FLT-1 modulates cell migration. Results show RS4;11 FLT-1-transfected cells exposed to VEGF (30 ng/mL) for 4 hours, alone or in the presence of the FLT-1-neutralizing Ab, 6.12 (1 μg/mL). *VEGF-induced RS4;11 FLT-1T migration is significantly reduced in the presence of the 6.12 Ab (3 independent experiments, P < .05), demonstrating the transfected receptor is functional and modulates cell migration. Error bars depict the standard error of the mean.
PlGF and VEGF gradients explain the BM distribution of leukemia cells
To define the mechanisms whereby FLT-1 modulated the localization of leukemia cells within the BM, we started by determining the normal distribution of its ligands, PlGF and VEGF. As shown in Figure 5B, PlGF and VEGF BM staining patterns suggest the existence of a defined gradient, from weak or diffused staining in the diaphysis to a clear accumulation in the epiphysis (Figure 5B shows sections from a littermate that was not inoculated with leukemia cells).
FLT-1-transfected ALL cells show different in vivo phenotype. Native RS4;11 cells or those transfected with full-length FLT-1 were inoculated into sublethally irradiated NOD-SCID mice, as described in “Materials and methods.” (A) Results show the transfected cells (bottom) formed masses of leukemia cells in the epiphysis (here shown at × 400 magnification) of the long bones, 3 to 5 days after inoculation, whereas the untransfected cell line localized predominantly in the diaphysis (top, × 200 magnification). Black arrows indicate leukemia cells in the different areas of the BM of long bones; white arrows, evidence for blood leakage in the bones of inoculated recipients. Results shown are representative of 3 mice per condition. (B) Evidence for the earlier appearance of extramedullary disease in mice inoculated with FLT-1-transfected RS4;11 cells. Bottom panel shows a spleen section (× 200 magnification) of a NOD-SCID recipient 10 days after RS4;11 FLT-1T cells inoculation, clearly evidencing the engraftment of leukemia cells. The results are representative of 3 independent animals. Images were visualized through a 40 ×/0.65 PLAN objective lens, captured with a Sony Cybershot DSC-560 digital camera (Sony, Lisbon, Portugal), and processed with Adobe Photoshop software (Adobe Systems, San Jose, CA).
FLT-1-transfected ALL cells show different in vivo phenotype. Native RS4;11 cells or those transfected with full-length FLT-1 were inoculated into sublethally irradiated NOD-SCID mice, as described in “Materials and methods.” (A) Results show the transfected cells (bottom) formed masses of leukemia cells in the epiphysis (here shown at × 400 magnification) of the long bones, 3 to 5 days after inoculation, whereas the untransfected cell line localized predominantly in the diaphysis (top, × 200 magnification). Black arrows indicate leukemia cells in the different areas of the BM of long bones; white arrows, evidence for blood leakage in the bones of inoculated recipients. Results shown are representative of 3 mice per condition. (B) Evidence for the earlier appearance of extramedullary disease in mice inoculated with FLT-1-transfected RS4;11 cells. Bottom panel shows a spleen section (× 200 magnification) of a NOD-SCID recipient 10 days after RS4;11 FLT-1T cells inoculation, clearly evidencing the engraftment of leukemia cells. The results are representative of 3 independent animals. Images were visualized through a 40 ×/0.65 PLAN objective lens, captured with a Sony Cybershot DSC-560 digital camera (Sony, Lisbon, Portugal), and processed with Adobe Photoshop software (Adobe Systems, San Jose, CA).
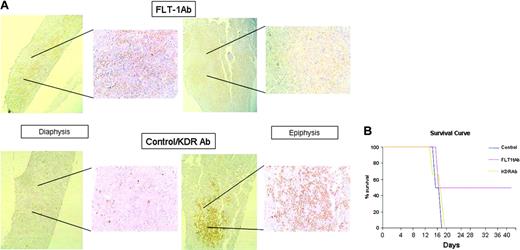
FLT-1 blockade modulates leukemia localization and survival of inoculated recipients. (A) Evidence for distinct BM localization of 697 cells, in untreated recipients or those treated with the neutralizing monoclonal Ab to FLT-1, 6.12 (FLT-1 Ab, administered every 2 days at 500 ng/injection). Bottom panels show evidence for the preferential localization of inoculated 697 cells in the epiphysis of the long bones of NOD-SCID mice (right, × 150 magnification; inset, × 200 magnification), visualized by human TdT staining as described in “Materials and methods.” Note the almost complete absence of TdT staining in the diaphysis of control or KDR Ab-treated mice (bottom left panel, inset). Top panels show BM of long bones of NOD-SCID recipients treated every 2 days with the FLT-1-neutralizing Ab 6.12. Results show a strong accumulation of TdT-positive leukemia cells in the diaphysis of the bone (top left panel, inset), whereas the epiphysis shows no evidence for the presence of 697 cells (top right panel, inset). The results shown are representative of 12 recipients for each condition, as determined in 3 independent experiments. (B) FLT-1 neutralization prolongs the survival of NOD-SCID mice inoculated with ALL cells. The results show a significant increase (P < .05, Kaplan-Meyer curve) in the survival of NOD-SCID mice inoculated with 697 cells and treated every 2 days with the FLT-1-neutralizing Ab 6.12 (FLT-1 Ab, 500 ng/injection). The results shown were obtained from 3 independent experiments (4 mice per condition in all experiments). Image acquisition performed as for Figure 3, except that a PLAN 10 ×/0.25 objective lens was used. Error bars indicate standard deviation.
FLT-1 blockade modulates leukemia localization and survival of inoculated recipients. (A) Evidence for distinct BM localization of 697 cells, in untreated recipients or those treated with the neutralizing monoclonal Ab to FLT-1, 6.12 (FLT-1 Ab, administered every 2 days at 500 ng/injection). Bottom panels show evidence for the preferential localization of inoculated 697 cells in the epiphysis of the long bones of NOD-SCID mice (right, × 150 magnification; inset, × 200 magnification), visualized by human TdT staining as described in “Materials and methods.” Note the almost complete absence of TdT staining in the diaphysis of control or KDR Ab-treated mice (bottom left panel, inset). Top panels show BM of long bones of NOD-SCID recipients treated every 2 days with the FLT-1-neutralizing Ab 6.12. Results show a strong accumulation of TdT-positive leukemia cells in the diaphysis of the bone (top left panel, inset), whereas the epiphysis shows no evidence for the presence of 697 cells (top right panel, inset). The results shown are representative of 12 recipients for each condition, as determined in 3 independent experiments. (B) FLT-1 neutralization prolongs the survival of NOD-SCID mice inoculated with ALL cells. The results show a significant increase (P < .05, Kaplan-Meyer curve) in the survival of NOD-SCID mice inoculated with 697 cells and treated every 2 days with the FLT-1-neutralizing Ab 6.12 (FLT-1 Ab, 500 ng/injection). The results shown were obtained from 3 independent experiments (4 mice per condition in all experiments). Image acquisition performed as for Figure 3, except that a PLAN 10 ×/0.25 objective lens was used. Error bars indicate standard deviation.
Next we sought to visualize leukemia-cell movement within the BM, by looking for evidence of “polarized” leukemia cells. As shown in Figure 6A, phalloidin staining (to detect polymerized actin) demonstrated the existence of polarized cells in the epiphysis of control mice, whereas those treated with the neutralizing Ab 6.12 had little evidence of actin polarization (an almost complete absence of phalloidin staining, Figure 6A).
These results fit with the notion proposed above, and with the in vitro results: inoculated leukemia cells respond to paracrine VEGF/PlGF gradients within the BM microenvironment, migrate, and localize to those areas where VEGF and PLGF production is most abundant. FLT-1 neutralization, in turn, impedes cell movement within the BM microenvironment, thereby conditioning cell survival and resulting in leukemia apoptosis.
PlGF-mediated leukemia migration involves lipid raft formation
Next, we investigated some of the molecular mechanisms whereby PlGF induced leukemia-cell migration. Besides demonstrating VEGF increases matrix metalloproteinase secretion by leukemia cells (not shown), we also sought to define the biochemical mechanisms whereby FLT-1 activation resulted in distinct cell distribution patterns in vivo. As shown in Figure 6B, PlGF-induced leukemia migration was blocked by pretreating the leukemia cell line 697 with nystatin, a cholesterol-sequestering agent that blocks lipid raft formation. These results suggest PlGF-induced leukemia-cell migration involves polarized cell movement requiring lipid raft formation and actin polymerization.
Discussion
Similarly to solid tumor growth, leukemia expansion within the BM microenvironment has been correlated with an increase in vascular content (angiogenesis). This has been held true in acute as well as chronic leukemias, as shown in both laboratory and clinical studies.20-24 Several facts stand out from most studies published to date: leukemia cells release significant amounts of VEGF, and a proportion of leukemia blasts coexpress its receptors VEGFR-1 (FLT-1), VEGFR-2 (KDR), and VEGFR-3 (FLT-4). In the case of KDR, we and others have demonstrated the existence of functional autocrine loops that support acute myeloid leukemia migration and survival both in vitro and in vivo.12,13,25 Interestingly, in the case of FLT-4, its functions appear to be exerted in a paracrine manner, mediating the proliferation and resistance to chemotherapy in subsets of acute myeloid leukemias.13 However, and also reflecting the lack of knowledge in other cell types, much less is known concerning a role for FLT-1 in leukemia biology. In the present report, we sought to define a role for FLT-1 in acute lymphoblastic leukemia, independent of the expression or activation of the other VEGF receptors.
Circulating and local VEGF/P1GF levels correlate with ALL progression. (A) FLT-1 neutralization reduces the levels of circulating human VEGF plasma levels in NOD-SCID mice inoculated with 697 ALL cells. Results show a significant decrease (P < .05) in human VEGF levels (ELISA) in the plasma of NOD-SCID mice inoculated with the 697 cell line (after 10 days) and treated every other day with the FLT-1-neutralizing Ab 6.12 (FLT-1 Ab, 500 ng/injection). These results were obtained in 3 independent experiments. (B) VEGF and PlGF accumulate in the epiphysis of long bones of normal (nonleukemic) NOD-SCID mice, littermate controls of mice used in all the other experiments described in this paper. Images (at × 200 magnification) show BM sections, localized to the diaphysis or epiphysis, stained with mVEGF- and mPlGF-specific Abs. Note the clear accumulation of VEGF and PlGF (brown staining) close to the epiphyseal regions of the bone, whereas the diaphysis shows little evidence for VEGF or PlGF staining. Results shown were obtained from 2 independent determinations, done in animals of different ages. Error bars indicate standard deviation. Image acquisition performed as for Figure 3.
Circulating and local VEGF/P1GF levels correlate with ALL progression. (A) FLT-1 neutralization reduces the levels of circulating human VEGF plasma levels in NOD-SCID mice inoculated with 697 ALL cells. Results show a significant decrease (P < .05) in human VEGF levels (ELISA) in the plasma of NOD-SCID mice inoculated with the 697 cell line (after 10 days) and treated every other day with the FLT-1-neutralizing Ab 6.12 (FLT-1 Ab, 500 ng/injection). These results were obtained in 3 independent experiments. (B) VEGF and PlGF accumulate in the epiphysis of long bones of normal (nonleukemic) NOD-SCID mice, littermate controls of mice used in all the other experiments described in this paper. Images (at × 200 magnification) show BM sections, localized to the diaphysis or epiphysis, stained with mVEGF- and mPlGF-specific Abs. Note the clear accumulation of VEGF and PlGF (brown staining) close to the epiphyseal regions of the bone, whereas the diaphysis shows little evidence for VEGF or PlGF staining. Results shown were obtained from 2 independent determinations, done in animals of different ages. Error bars indicate standard deviation. Image acquisition performed as for Figure 3.
We demonstrate that FLT-1 activation on ALL cells has little effect in proliferation but mediates cell migration in vitro, in agreement with previous studies in other cell types (malignant T cells,26 smooth muscle cells,27 monocytes28 ). We add on to these findings and demonstrate in vivo that FLT-1 activation, or its neutralization, changes the behavior of ALL cells. Using a NOD-SCID mouse model, we observed that FLT-1-overexpressing ALL cells engraft and localize close to articulations (epiphysis) of long bones of inoculated recipients, whereas those expressing less FLT-1 (or where FLT-1 activation was blocked by specific neutralizing monoclonal Abs) localize predominantly to the central portions (diaphysis) of the BM of long bones. This phenotype correlates with a delay in the presence of ALL cells in extramedullary organs or the peripheral circulation and a consequent increase in the survival of inoculated recipients. As a consequence of FLT-1 neutralization, treated mice survive significantly longer than their untreated counterparts, and there is significant reduction in circulating ALL cells.
In human leukemia, the presence of extramedullary disease is considered an unfavorable prognostic factor,1-5 whereas the persistence of circulating blasts after induction regimens indicates patients at higher risk of relapse.6-8 This is particularly relevant in pediatric patients with ALL who may develop extramedullary disease, especially in the CNS.9 Thus, there is great interest in determining the mechanisms that may explain the exit of acute leukemia cells from the BM microenvironment. In the present report we reveal a crucial role for FLT-1 in regulating the distribution of leukemia cells within the BM microenvironment, their survival, and consequently their exit into the peripheral circulation.
The data obtained from our in vivo studies should be interpreted in the context of the vascularization of long bones. Previous studies have revealed that the BM of long bones such as femurs, is vascularized by the afferent (arterial) and the efferent (venous) vascular systems. The principal components of the afferent vascular system of a long bone have been suggested to “enter” the BM in the diaphysis, supplying nutrients and oxygen to the hematopoietic elements, whereas those of the efferent system leave the BM, draining the hematopoietic elements predominantly in the epiphysis.29
In our mouse models, we observed that ALL cells expressing FLT-1 localize predominantly in the epiphysis, supporting the concept that such a subset of acute leukemia cells leave the BM via the efferent circulatory system, en route to establish extramedullary growth. Thus, ALL cells are attracted to these areas of the BM via FLT-1 activation. In agreement, we demonstrate that normal (nonleukemic) BM sections exhibit a clear gradient of murine VEGF and PlGF expression, increasing from the diaphysis to the epiphysis. Conversely, the 697 cell line used in our in vivo models produces very modest VEGF levels (at the assay detection limit, Table 2) and does not express PlGF, suggesting the phenotype seen in vivo results from paracrine stimulation by endogenous murine VEGF/PlGF. The cells that express these ligands in abundance within the BM, and the regulation of such expression, are still undisclosed, although previous reports have shown VEGF is abundantly expressed by osteoclasts and osteoblasts in the epiphysial areas of long bones, where it was shown to be essential for fracture healing.30,31
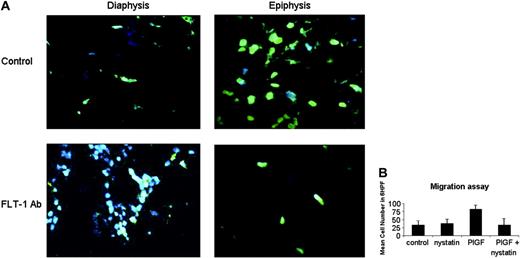
In vivo evidence and molecular basis for leukemia movement within bone marrow. (A) FLT-1 neutralization reduces actin polymerization in leukemia cells within the BM of inoculated NOD-SCID mice. Top panels (× 630 magnification) show BM sections of control (untreated) NOD-SCID mice 10 days after leukemia cells inoculation, stained and visualized in a fluorescence microscope as described in “Materials and methods.” Green staining shows leukemia cells with evidence for polarization/movement (Phalloidin, stains polymerized actin). Note the almost complete absence of Phalloidin staining in the epiphyseal regions of BM of mice treated with the 6.12 FLT-1-neutralizing Ab (FLT-1Ab, bottom panels, × 630 magnification). These results were obtained from 3 independent experiments. (B) Nystatin, a cholesterol-sequestering agent that impedes lipid raft formation, blocks P1GF-induced leukemia-cell migration. Results show transwell migration assay data, demonstrating that 697 cell migration (mean cell number in 6 HPF, × 400) in response to P1GF (10 ng/mL) is significantly (P < .05) impeded by cotreatment with nystatin, used as described in “Materials and methods.” Results shown are representative of 2 independent experiments. Error bars depict the standard error of the mean.
In vivo evidence and molecular basis for leukemia movement within bone marrow. (A) FLT-1 neutralization reduces actin polymerization in leukemia cells within the BM of inoculated NOD-SCID mice. Top panels (× 630 magnification) show BM sections of control (untreated) NOD-SCID mice 10 days after leukemia cells inoculation, stained and visualized in a fluorescence microscope as described in “Materials and methods.” Green staining shows leukemia cells with evidence for polarization/movement (Phalloidin, stains polymerized actin). Note the almost complete absence of Phalloidin staining in the epiphyseal regions of BM of mice treated with the 6.12 FLT-1-neutralizing Ab (FLT-1Ab, bottom panels, × 630 magnification). These results were obtained from 3 independent experiments. (B) Nystatin, a cholesterol-sequestering agent that impedes lipid raft formation, blocks P1GF-induced leukemia-cell migration. Results show transwell migration assay data, demonstrating that 697 cell migration (mean cell number in 6 HPF, × 400) in response to P1GF (10 ng/mL) is significantly (P < .05) impeded by cotreatment with nystatin, used as described in “Materials and methods.” Results shown are representative of 2 independent experiments. Error bars depict the standard error of the mean.
The effects of FLT-1 neutralization extend beyond the blockade of ALL cell movement and appear also to result in leukemia apoptosis. Our results in this regard support the notion that the attraction/tropism of subsets of leukemia cells for particular areas (niches) within the BM microenvironment may in fact condition their survival and expansion. FLT-1 activation on ALL cells could be exerted in a paracrine (external) or autocrine (internal) VEGF/P1GF production. In the case of the ALL cell lines we studied, none expressed PlGF, although VEGF was produced in modest amounts by all of them. However, in vitro neutralization of VEGF or FLT-1 resulted only in a minor delay in leukemia growth and a modest increase in cell apoptosis (results were not significant, data not shown), supporting the notion that autocrine VEGF may not promote relevant FLT-1 activation, at least on the ALL cells studied.
In vivo, the neutralizing Ab used in our studies should block both paracrine and autocrine FLT-1 activation on the leukemia blasts, thus producing a more evident phenotype. There are also examples of heterotypic engagement of adhesion molecules by malignant cells resulting in increased expression and activation of angiogenic molecules such as VEGF (see Podar and Anderson11 as an extensive review of such mechanisms in multiple myeloma). If we extrapolate the studies in multiple myeloma to our model, the interaction of ALL cells with the stromal elements of the BM might result in a modulation of VEGF and FLT-1 and consequently result in greater dependence of the leukemia cells on FLT-1 signaling in vivo.
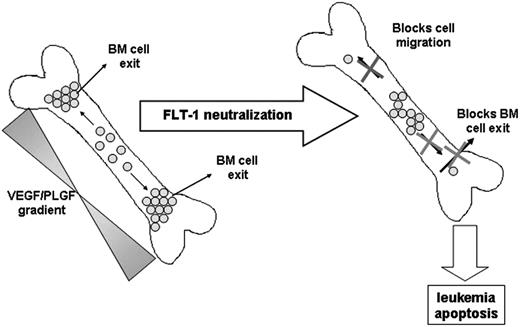
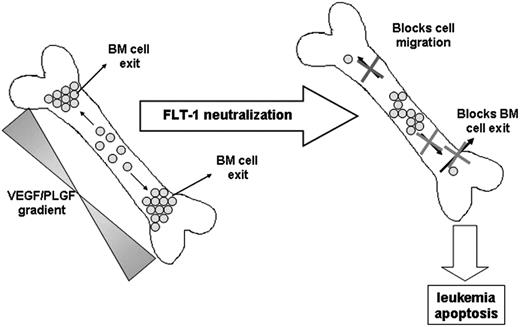
Taken together, we observed that in vivo FLT-1 neutralization in subsets of leukemias such as ALL, directly impedes cell migration into more “suitable” niches in the BM, induces leukemia apoptosis, and as a consequence delays leukemia exit into the peripheral circulation, reduces circulating human VEGF levels, and delays extramedullary growth (see model proposed in Figure 7).
To our knowledge, it is the first time that FLT-1 activation is clearly implicated in regulating leukemia-cell migration inside the BM and that neutralization of FLT-1 results in cell apoptosis, thus impeding cell exit into the peripheral circulation. Recent studies have suggested FLT-1 activation might be involved in situations of abnormal localization of immature precursors (ALIP), particularly frequent in myelodysplastic syndromes (MDSs).17 We add on to these findings and demonstrate that the paracrine activation of FLT-1 may result in migration and survival also of acute leukemia cells, such as subsets of acute lymphoblastic leukemias, within the BM microenvironment.
Proposed model for the role of FLT-1 in regulating leukemia growth and localization within the BM and in modulating the exit of ALL cells into the peripheral circulation.
Proposed model for the role of FLT-1 in regulating leukemia growth and localization within the BM and in modulating the exit of ALL cells into the peripheral circulation.
With regard to the mechanisms whereby FLT-1 may mediate ALL-cell migration, we demonstrate for the first time that this involves actin polymerization and lipid raft formation. These results are not surprising, given previously published data demonstrating the importance of actin polymerization (and focal adhesion kinase) for cell migration in other cell types32 and also a putative role for lipid rafts in cell chemotaxis and migration.33,34 Concerning the effects of nystatin, namely its capacity to block lipid raft formation and the mechanisms underlying its migration-inhibitory effect, it remains to be established whether this is required for FLT-1 redistribution along the cell membrane, modulating polarized cell movement, or whether this is connected with receptor turnover and internalization (R.F. and S.D., ongoing studies examining the molecular basis of regulating ALL-cell movement). These results not withstanding, we demonstrate active and directed cell movement in vivo and in vitro and the involvement of particular biochemical mechanisms in this process, which adds to our understanding of the biology of ALL growth within the BM microenvironment.
Our data suggest the use of FLT-1 blockers for treating subsets of acute leukemia, namely ALL, may be of therapeutic benefit. This may prove beneficial also in combination with chemotherapeutic agents used to treat patients with ALL, such as vincristine or daunorubicin (among others); because FLT-1 neutralization impedes cell movement within the BM, localizing the leukemia blasts mostly in areas of afferent (arterial) vascularization, these should be more exposed to inoculated (circulating) chemotherapy.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-06-2530.
Supported by a Fundação para a Ciencia e Tecnologia grant (POCTI/CBO/38391/2001) and fellowship (PRAXIS/SFRH/BD/14162/2003) (R.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank other members of the Angiogenesis Laboratory and the Hematology group (CIPM) for critically reading this manuscript and Dolores Bonaparte and Cristina Rodrigues (Instituto Gulbenkian Ciencia) for their help with the in vivo work.