Abstract
Alpha4 integrin or VLA4 (CD49d/CD29) is a multitask molecule with wide expression within and outside the hematopoietic system. Because targeted ablation of α4 integrin leads to embryonic lethality, to study its effects on adult hematopoiesis, we used animals with conditional excision of α4 integrin (α4Δ/Δ) in hematopoietic cells. In such animals, we previously documented weakened bone marrow retention of progenitor cells during homeostasis and impaired homing and short-term engraftment after transplantation. In the present study we show that long-term repopulating cells lacking α4 integrins display a competitive disadvantage in hematopoietic reconstitution compared to normal competitors. Although initial dominance of α4+ competitors is due to their better homing and proliferative expansion early after transplantation, a progressive decline in contribution of α4Δ/Δ hematopoiesis is compatible with neither normal homing nor normal function of α4Δ/Δ hematopoietic stem cells (HSCs) in post-homing hematopoiesis. In the absence of α4+ competitor cells, α4Δ/Δ HSCs can establish long-term hematopoiesis in primary recipients, however, some resurgence of host hematopoiesis is evident, and it becomes dominant in secondary transplants, so that no survivors with exclusively α4Δ/Δ cells are seen in tertiary transplants. Collectively, our data provide compelling evidence that under regenerative stress α4 integrin assumes a greater importance than for maintenance of steady-state hematopoiesis.
Introduction
The α4 integrin (CD49d), or VLA4, the heterodimer of α4 and β1 integrin (CD49d/CD29), is widely expressed within and outside the hematopoietic system and exerts important functional control over many physiologic, as well as pathologic or neoplastic, processes.1-6 Among hematopoietic cells, α4 integrin is expressed in a constitutively active stage in primitive cells and in an inactive state in several classes of mature cells.7 It exercises a decisive influence on the migration and recruitment patterns of mature cells, especially lymphocytes, to several tissues,8 whereas within bone marrow (BM), it plays a critical role in the interactions of hematopoietic cells with microenvironmental cells and their matrix.9 Because of its ability to not only serve as adhesion receptor, but also to execute bi-directional signaling (outside-in and inside-out10 ) and to interact with cytokines/chemokines and other integrins expressed by hematopoietic cells or/and microenvironmental cells, α4 integrin is uniquely poised to influence interactions of hematopoietic cells with their environment.
Early studies of α4 integrin on hematopoiesis relied on the use of antifunctional antibodies, both in vitro9 and in vivo.11 Targeted ablation of α4 integrin caused embryonic lethality unrelated to hematopoietic effects,12 but studies in chimeric mice showed modest effects on fetal liver hematopoiesis and no contribution to adult hematopoiesis beyond the first month of postnatal life.13,14
To study the effects of α4 integrin on adult hematopoiesis, we engineered conditional knockout mice in which the α4 alleles can be disrupted upon treatment of adult animals with interferon or its inducer poly(I)-poly(C).15 Adult animals with conditional excision of α4 integrin maintain a quantitatively normal hematopoiesis at homeostasis but display alterations in biodistribution of progenitor cells with sustained elevations in circulation and in the spleen. Such an effect is consequent to their compromised retention within the BM in the absence of α4 integrin, an effect reminiscent of the mobilization seen in vivo with anti-α4 antibodies.11 A counterpart of this effect is a partial impairment in BM homing and in short-term engraftment.15 To test the ability of α4-deficient cells for long-term, durable engraftment in irradiated recipients and the self-renewal properties of α4Δ/Δ hematopoietic stem cells (HSCs), in the present study we carried out a series of transplantation experiments with or without competitor cells. Our results suggest that absence of α4 integrin in hematopoietic cells greatly compromises their competitive ability for hematopoietic reconstitution and their self-renewal capacity in serial transplantations.
Materials and methods
Mice
Mx.cre+ α4f/f and Mx.cre+ α4Δ/Δ mice were generated in our laboratory as described previously.15 Three injections of poly(I)-poly(C) every other day were used for ablation of adult animals, whereas in neonates, 2 injections were used during the first week of postnatal life. Alpha4 ablation was tested 2 weeks later. Ablated animals are referred to as α4Δ/Δ, and the animals with more than 5% α4+ cells in their peripheral blood were not included in experiments. Mice were bred and maintained in the specific pathogen-free facility at the University of Washington in accordance with the Institutional Animal Care Use Committee guidelines.
Antibodies, cell staining, and FACS analysis
Cells from mouse tissues (bone marrow, peripheral blood, or spleen) were stained using the following directly conjugated antibodies from BD Biosciences (San Diego, CA): B220 (RA3-682), CD3 (17A2), CD4 (RM4-5), CD8 (53-6.7), CD45 (30F-11), TER119, Mac-1 (M1/70), Gr-1 (RB6-8C5), CD117 (c-kit, 2B8), Sca-1 (D7), CD29 (β1, Ha2/5), anti-CD48 (HM48-1), CD49e (α5, 5H10-27 [MFR5]), anti-CD150, and anti-α4β7 (DATK32), directly conjugated CD150 (SLAM, IPO-3) purchased from eBioscience (San Diego, CA) and directly conjugated CD49d (anti-α4, PS/2) antibody was purchased from Southern Biotechnology Associates, Birmingham, AL. The cocktail of antibodies used for staining of lineage-committed cells included CD3 (17A2), Mac-1, TER119, and Gr-1, B220. A FACSCalibur (BD Biosciences) and CELLQuest software were used for cell analysis.
Proliferation assessment
Assessment of proliferative status of BM cells of α4Δ/Δ and of controls (α4f/f) was done using a single injection of BrDU (250 mg/kg body weight) and analyzing the cells 3 hours later, as described.16 BrDU staining was carried out according to the manufacturer's instructions and was combined with anti-CD45-PE or anti-kit-APC.
Competitive repopulation
Pooled BM cells from α4Δ/Δ mice (= test cells) were mixed in different proportions (250 000-750 000) with α4f/f/cre- cells (250 000) (= competitor cells) and transplanted by intravenous injection (0.5-1.0 × 106) into lethally irradiated (1200 cGy at a dose rate of > 100 cGy/min) wild-type (WT) recipients using a cesium source. In addition to already ablated α4Δ/Δ, α4f/fcre+ BM cells before ablation were competed (1:1) with α4+/+ Mx.cre+ cells. Recipients of the latter pool of BM were ablated (by poly(I)-poly(C)) 4 weeks after transplantation. BM and peripheral blood (PB) cells of all recipients were evaluated at several points after transplantation by fluorescence-activated cell sorting (FACS), colony-forming unit-in culture (CFU-C) in the presence or absence of G418, and by genomic analysis (polymerase chain reaction [PCR]). At chosen times after transplantation, selected primary recipients served as donors for secondary transplants, which were similarly analyzed 3 to 6 months later.
Transplantation of α4-deficient BM or PB
Bone marrow cells (1-5 × 106 cells/recipient) from α4 ablated mice were infused into lethally irradiated recipients (1150 cGy whole body irradiation with a 137Cs source). Both splenectomized and nonsplenectomized animals were used as recipients. Recipients were analyzed 2, 10, 16, and 56 weeks later, and serial transplantations (secondary and tertiary) were carried out from selected primary and secondary recipient (α4+/+ or α4Δ/Δ) animals. Evaluation of hematopoietic reconstitution was carried out by FACS for different cell lineages, by hematopoietic-cell quantitation in BM, PB, and spleen, and through CFU-C assays with or without G418. Presence of “floxed” (f), “deleted” (Δ), or “WT” alleles were used in genomic analysis to assess donor-cell contribution. Peripheral blood also was used for transplantation (0.3 mL from α4Δ/Δ or α4+/+ animals). Recipients were analyzed 4 months later, using the approaches described earlier in “Materials and methods.” All procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Clonogenic progenitor assays
Colony-forming-unit-in culture (CFU-C) assays were performed using a methylcellulose mixture (Methocult GF, Stem Cell Technologies, Vancouver, BC, Canada), as described previously.15 BM or PB cells were inoculated for culture, and colonies were identified morphologically in culture plates as erythroid (BFUe) or granulocyte/macrophage (CFU-GM) or CFU-Mixed. Whenever appropriate, G418 was added to cultures to test for the presence of the neo-allele in α4-unexcised cells (α4f/f), providing complementary evidence to that from genomic PCR analysis.
Genomic PCR. Genomic DNA was isolated using a Gentra Kit (Gentra Systems, Minneapolis, MN). The following primer combinations were used for PCR: α4 gene-specific primers: F1 (5′-CCACCTGGTGTATGAAAGC-3′), F2 (5′-CGGGATCAGAAAGAATCCAAA-3′), and R1 (5′-CTGGCATGGGGTTAAAATTG-3′); this primer combination allows discrimination between WT, deleted (Δ), and floxed (f) alleles; neo-specific primers: sense (5′-GCACGCAGGTTCTCCGGC-3′), antisense (5′-GTCCTGATAGCGGTCCGCC-3′); cre-specific primers; sense (5′-CATTTGGGCCAGCTAAACAT-3′), antisense (5′-TAAGCAATCCCCAGAAATGC-3′).
Statistical analysis. Data shown are means ± standard error of the mean (SEM). Statistical analyses were performed by using a Student t test.
Results
Normal immunophenotypic profile of α4Δ/Δ stem cells
Previous studies in mice, in which excisions of α4 integrin in hematopoietic cells occurred early or late in their postnatal life, have shown normal quantitative hematopoiesis and normal lineage distribution at homeostasis. (Proportions of kit+ cells in BM, or of Gr-1+, Mac-1+, CD3+, and TER119+ were similar to those of normal cre(-) mice15 ). A modest deviation in the frequency of B cells (B220+) between BM and PB was seen, as well as significant and sustained augmentations in circulating progenitor (CFU-C) cells of all classes,15 as documented by clonal growth in vitro. To test whether the immunophenotypic profile of α4Δ/Δ HSCs several months after α4-integrin excision is also quantitatively preserved, we assessed the proportion in BM of Lin-/Sca-1+/kit- (LSK) cells that contain an enriched population of HSCs. As seen in Figure 1A, we found no differences in the proportions of either Lin-/kit+ or Lin-/Sca-1+/kit+ (LSK) cells between α4Δ/Δ and controls. More importantly, these populations were virtually negative for α4 expression (Figure 1B). Recently, an improved immunophenotype was described for stem cells (CD150+/CD48-), and we tested proportions of these cells between WT and α4Δ/Δ mice. No quantitative differences between controls and α4Δ/Δ BM cells were evident (CD150+/CD48+: 4.09% in controls and 6.6% in α4Δ/Δ; CD150+/CD48- were 0.014% and 0.018%, respectively, and all CD150+CD48+ cells were α4 negative (Figure 1C). In addition to the immunophenotypic profile, we tested the proliferative status of kit+ cells in BM of +/+ animals (n = 4) and of α4Δ/Δ (n = 8, before and after splenectomy). No significant differences in BrDU labeling were seen among the groups (BrDU+: 24.2% ± 3.6% vs 29.7% ± 2.0% among CD45+ cells, respectively, P = .24).
Competitive repopulation with α4Δ+/+ and α4Δ/Δ cells
To test whether the phenotypically defined α4Δ/Δ HSCs are endowed with a normal functional capacity for long-term reconstitution of lethally irradiated recipients, we performed competitive repopulation experiments.17 Initially, we mixed equal numbers of BM nucleated cells from α4Δ/Δ mice (ablated several months previously) and from BM of cre-α4f/f mice (1:1 test to competitor ratio). Equal mixing was verified by immunophenotyping of pool populations and proportions of kit+ cells (Figure 2A). This 1:1 donor pool of cells was injected (0.5 + 0.5 × 106 cells/recipient) into a cohort of 10 lethally irradiated WT recipients. In this setting, hematopoiesis by α4Δ/Δ, α4f/f, or α4 WT cells can be genetically distinguished. To assess long-term donor contributions to hematopoiesis, PB from recipient mice was sampled at 16, 30, and 42 weeks after transplantation. Blood was analyzed by FACS (proportion of α4+ cells) and for progenitor content in the presence or absence of G418 for scoring neo-resistant (α4f/f derived) or neo-sensitive (α4Δ/Δ or WT-derived) colonies. The proportion of α4+ cells (among CD45+ cells) at all times tested was between 65% and 75%. Proportions of Gr-1+ cells reflecting recent contributions by BM progenitors also were similar. One rather moribund animal was killed earlier at 12 weeks and showed 52.68% α4+ in PB (the expected contribution); however, in the BM he had 71.65% α4+ cells. All the kit+ in the BM were α4+, as was the great majority of Gr-1+, Mac-1+, TER119+, and B220+ cells (data not shown). This suggested that PB data may not accurately reflect BM hematopoiesis and that we may have an ongoing contribution by α4(+) at the expense of α4(-) cells. This concept was tested at 30 weeks by humanely killing 4 mice. All mice showed further increase in PB contribution by α4+ cells, but this was not statistically significant from earlier times. However, BM analysis from all 4 mice disclosed 97.45% ± 0.1% α4(+) cells compared to 77.49% ± 4.2% in PB (P = .02), suggesting complete replacement by α4(+) hematopoiesis. All CFU-C in vitro were G418 resistant, suggesting contribution by α4f/f competitor cells rather than host stem-cell resurgence. Finally, at 42 weeks after transplantation, 5 more mice were euthanized for analysis. Their PB cells were 73.14% ± 11.8% α4+, but the BM had 96.95% ± 1.65% α4+ cells. Again, a similar picture emerged to the one seen earlier at 30 weeks. These data suggested not only an inferior competition by α4Δ/Δ cells starting early after transplantation, but also a progressive replacement of hematopoiesis by α4(+) cells long after hematopoietic reconstitution is stably established. They also validate the α4f/f competitor cells as functionally intact. To uncover the presence of any silent α4Δ/Δ HSCs that had not contributed to hematopoiesis in the primary recipients, at 30 and 42 weeks after transplantation, 1 × 106 pooled BM cells from 4 mice (97.4% α4+) were given to each of 14 recipients, and BM pooled cells from 2 mice (90% α4[+]) were given to each of 10 recipients. The first cohort of recipients was tested at 27 weeks (11 mice) and the second at 13 weeks after (8 mice). All lineages within BM from both sets of animals showed α4+ hematopoiesis (> 98%) in BM, PB, and spleen.
FACS analysis of BM cells from α4-deficient or control mice. (A) Note the absence of α4 expression and the left shift in β1 integrin expression of BM cells from α4Δ/Δ mice compared to controls (4 left panels). Solid lines represent isotype control antibody. (ii) Proportions of Lineage-/Sca-1+/kit+ (LSK) cells containing an enriched population of stem cells. Gated Lineage-/kit+ cells (top left) were tested for Sca-1 positivity (ii). No differences are seen between the α4Δ/Δ (neonatally ablated and tested at 14 weeks) and control mice (14 weeks old), suggesting normal representation of LSK cells in α4-deficient mice. Furthermore, kit+ cells did not express α integrin (panel B). In panel C, BM cells were labeled with anti-CD150 PE and anti-CD48 FITC. Among more than 2 × 105 cells analyzed, the proportion of CD150+ CD48- cells were 0.014% and 0.02% (2 experiments) in control and 0.18% and 0.03% in neonatally ablated α4Δ/Δ animals of the same age (4-5 months). No α4+ cells were seen among CD150+CD48- cells from α4Δ/Δ BM (data not shown). The proportion of CD150+CD48+ was 4.09% in controls and 6.62% in α4Δ/Δ. It is of interest that all kit+ cells were CD48+ in both sets of animals.
FACS analysis of BM cells from α4-deficient or control mice. (A) Note the absence of α4 expression and the left shift in β1 integrin expression of BM cells from α4Δ/Δ mice compared to controls (4 left panels). Solid lines represent isotype control antibody. (ii) Proportions of Lineage-/Sca-1+/kit+ (LSK) cells containing an enriched population of stem cells. Gated Lineage-/kit+ cells (top left) were tested for Sca-1 positivity (ii). No differences are seen between the α4Δ/Δ (neonatally ablated and tested at 14 weeks) and control mice (14 weeks old), suggesting normal representation of LSK cells in α4-deficient mice. Furthermore, kit+ cells did not express α integrin (panel B). In panel C, BM cells were labeled with anti-CD150 PE and anti-CD48 FITC. Among more than 2 × 105 cells analyzed, the proportion of CD150+ CD48- cells were 0.014% and 0.02% (2 experiments) in control and 0.18% and 0.03% in neonatally ablated α4Δ/Δ animals of the same age (4-5 months). No α4+ cells were seen among CD150+CD48- cells from α4Δ/Δ BM (data not shown). The proportion of CD150+CD48+ was 4.09% in controls and 6.62% in α4Δ/Δ. It is of interest that all kit+ cells were CD48+ in both sets of animals.
Competitive repopulation with pooled α4+/+ (competitor cells) and α4Δ/Δ (test cells) BM cells. (A) Proportion of α4+ cells in the pooled BM sample used for transplantation is depicted in the left panel, and proportion of kit+/α4+ cells is shown in the right panel. (B) Blood samples from recipients who received transplants were assessed 16 to 42 weeks after transplantation for the presence of α4+ (▪) or α4- ( ) cells. At all times tested, the proportion of α4+ cells was 2:1 in PB (compared to input ratio of 1:1). (C) Comparison of PB and BM positivity (right panels) for α4 integrin in mice that received transplants and were killed at 30 (n = 4) or 42 (n = 5) weeks after transplantation (PB, ▪; BM from the same group of mice,
) cells. At all times tested, the proportion of α4+ cells was 2:1 in PB (compared to input ratio of 1:1). (C) Comparison of PB and BM positivity (right panels) for α4 integrin in mice that received transplants and were killed at 30 (n = 4) or 42 (n = 5) weeks after transplantation (PB, ▪; BM from the same group of mice,  ). Reconstitution by α4+ cells was higher in BM compared to concurrently tested PB cells (total cells or kit+ cells, left panels), suggesting ongoing replacement of hematopoiesis by α4+ competitor cells. Error bars represent standard error of the mean.
). Reconstitution by α4+ cells was higher in BM compared to concurrently tested PB cells (total cells or kit+ cells, left panels), suggesting ongoing replacement of hematopoiesis by α4+ competitor cells. Error bars represent standard error of the mean.
Competitive repopulation with pooled α4+/+ (competitor cells) and α4Δ/Δ (test cells) BM cells. (A) Proportion of α4+ cells in the pooled BM sample used for transplantation is depicted in the left panel, and proportion of kit+/α4+ cells is shown in the right panel. (B) Blood samples from recipients who received transplants were assessed 16 to 42 weeks after transplantation for the presence of α4+ (▪) or α4- ( ) cells. At all times tested, the proportion of α4+ cells was 2:1 in PB (compared to input ratio of 1:1). (C) Comparison of PB and BM positivity (right panels) for α4 integrin in mice that received transplants and were killed at 30 (n = 4) or 42 (n = 5) weeks after transplantation (PB, ▪; BM from the same group of mice,
) cells. At all times tested, the proportion of α4+ cells was 2:1 in PB (compared to input ratio of 1:1). (C) Comparison of PB and BM positivity (right panels) for α4 integrin in mice that received transplants and were killed at 30 (n = 4) or 42 (n = 5) weeks after transplantation (PB, ▪; BM from the same group of mice,  ). Reconstitution by α4+ cells was higher in BM compared to concurrently tested PB cells (total cells or kit+ cells, left panels), suggesting ongoing replacement of hematopoiesis by α4+ competitor cells. Error bars represent standard error of the mean.
). Reconstitution by α4+ cells was higher in BM compared to concurrently tested PB cells (total cells or kit+ cells, left panels), suggesting ongoing replacement of hematopoiesis by α4+ competitor cells. Error bars represent standard error of the mean.
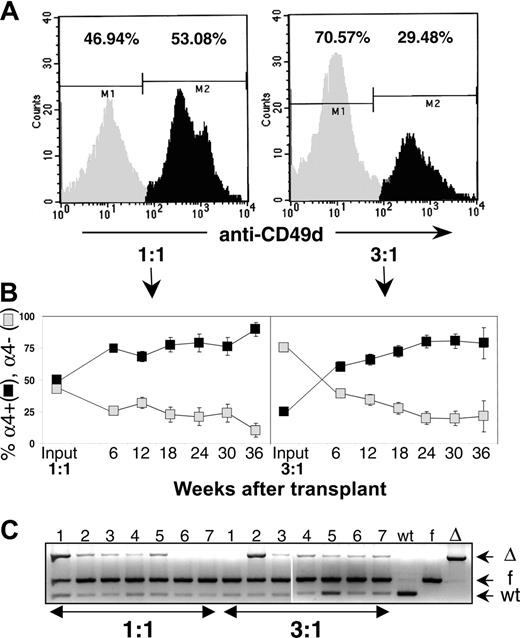
To further investigate the kinetics of the apparent competitive disadvantage of α4Δ/Δ cells and to overcome a putative partial homing defect for α4Δ/Δ HSC (long-term repopulating cells [LTRCs]), similar to the one documented for progenitor cells (CFU-C),15 we repeated the competitive repopulation experiments. Keeping the number of competitor cells constant, we used 1:1 and 3:1 (6:1 by CFU-C ratio) of test α4Δ/Δ versus α4+/+ competitor cells (Figure 3A). These cells were transplanted into splenectomized recipients to avoid contributions of α4Δ/Δ hematopoiesis by the spleen, which avidly sequesters α4Δ/Δ cells. Furthermore, we wanted to test whether contributions by the spleen could account for the differences between PB and BM seen previously, instead of evoking the postulate of progressive decline of α4Δ/Δ hematopoiesis. By 6 weeks, α4+ blood cells were above 60% in all recipients receiving either 1:1 or 3:1 donor cells (74.76% ± 2.4% for 1:1 and 60.65% ± 3.6% for 3:1). By 24 weeks (168 days) α4+ cells were 79% ± 7.0% for the 1:1 and 80.01% ± 4.9% for the 3:1 donor cohort. Thus, in accord with our earlier data, there was a progressive decline in the contribution by α4Δ/Δ cells from 6 to 36 weeks (P = .04 for the 3:1 group of recipients) (Figure 3B). When mice were killed (36 weeks), 92.55% ± 11.5% of CFU-Cs from the BM of all recipients of 1:1 ratio were G418 resistant (ie, α4f/f derived), whereas in PB were 83.6% ± 18.4%, and in the recipients of 3:1 donor pool, they were 95.5% ± 2.4% and 80.84% ± 6.0%, respectively. Moreover, DNA analysis of BM cells from all these recipients (Figure 3C) confirmed the derivation of the great majority of multilineage hematopoiesis by α4f/f competitor cells (Figure 4).
Competitive repopulation experiments with 1:1 or 3:1 α4Δ/Δ (= test) to α4+/+ (= competitive) cell ratio. (A) FACS profiles of pooled 1:1 BM or 3:1 BM samples showing proportions of α4- ( ) versus α4+ (▪) cells (CFU-C ratio of T:C cells was 6:1 in the latter). (B) PB-cell evaluation for α4 positivity shows an early and progressive reconstitution by α4+ competitor cells from 6 to 36 weeks after transplantation (P = .04,
) versus α4+ (▪) cells (CFU-C ratio of T:C cells was 6:1 in the latter). (B) PB-cell evaluation for α4 positivity shows an early and progressive reconstitution by α4+ competitor cells from 6 to 36 weeks after transplantation (P = .04,  , percent α4-; ▪, percent α4+). (C) Genomic PCR in 14 surviving mice (7 from 1:1 and 7 from 3:1) from competitive repopulation at 36 weeks. Note the predominant presence of floxed (f) allele (= competitor) compared to deleted (Δ) one and a minor presence of WT allele from hosts (likely contaminating CD45- cells). Predominant reconstitution by α4f/f (= competitors) was also corroborated by CFU-C cultures, showing mostly G418-resistant colonies (α4f/f are neo+) from competitor cells. Only 2 mice (no. 1 from 1:1 and no. 2 from 3:1) show appreciable numbers of (Δ) allele corresponding to higher proportions of α4- cells. Error bars represent standard error of the mean.
, percent α4-; ▪, percent α4+). (C) Genomic PCR in 14 surviving mice (7 from 1:1 and 7 from 3:1) from competitive repopulation at 36 weeks. Note the predominant presence of floxed (f) allele (= competitor) compared to deleted (Δ) one and a minor presence of WT allele from hosts (likely contaminating CD45- cells). Predominant reconstitution by α4f/f (= competitors) was also corroborated by CFU-C cultures, showing mostly G418-resistant colonies (α4f/f are neo+) from competitor cells. Only 2 mice (no. 1 from 1:1 and no. 2 from 3:1) show appreciable numbers of (Δ) allele corresponding to higher proportions of α4- cells. Error bars represent standard error of the mean.
Competitive repopulation experiments with 1:1 or 3:1 α4Δ/Δ (= test) to α4+/+ (= competitive) cell ratio. (A) FACS profiles of pooled 1:1 BM or 3:1 BM samples showing proportions of α4- ( ) versus α4+ (▪) cells (CFU-C ratio of T:C cells was 6:1 in the latter). (B) PB-cell evaluation for α4 positivity shows an early and progressive reconstitution by α4+ competitor cells from 6 to 36 weeks after transplantation (P = .04,
) versus α4+ (▪) cells (CFU-C ratio of T:C cells was 6:1 in the latter). (B) PB-cell evaluation for α4 positivity shows an early and progressive reconstitution by α4+ competitor cells from 6 to 36 weeks after transplantation (P = .04,  , percent α4-; ▪, percent α4+). (C) Genomic PCR in 14 surviving mice (7 from 1:1 and 7 from 3:1) from competitive repopulation at 36 weeks. Note the predominant presence of floxed (f) allele (= competitor) compared to deleted (Δ) one and a minor presence of WT allele from hosts (likely contaminating CD45- cells). Predominant reconstitution by α4f/f (= competitors) was also corroborated by CFU-C cultures, showing mostly G418-resistant colonies (α4f/f are neo+) from competitor cells. Only 2 mice (no. 1 from 1:1 and no. 2 from 3:1) show appreciable numbers of (Δ) allele corresponding to higher proportions of α4- cells. Error bars represent standard error of the mean.
, percent α4-; ▪, percent α4+). (C) Genomic PCR in 14 surviving mice (7 from 1:1 and 7 from 3:1) from competitive repopulation at 36 weeks. Note the predominant presence of floxed (f) allele (= competitor) compared to deleted (Δ) one and a minor presence of WT allele from hosts (likely contaminating CD45- cells). Predominant reconstitution by α4f/f (= competitors) was also corroborated by CFU-C cultures, showing mostly G418-resistant colonies (α4f/f are neo+) from competitor cells. Only 2 mice (no. 1 from 1:1 and no. 2 from 3:1) show appreciable numbers of (Δ) allele corresponding to higher proportions of α4- cells. Error bars represent standard error of the mean.
Multilineage reconstitution by α4+ competitor cells. Expression of α4+ cells (▪) among cells from different lineages in (A) BM, (B) lymph nodes (LN), and (C) thymus of mice transplanted with 3:1 (test:competitive) cell ratio and tested at 36 weeks after transplantation. ( show the proportion of α4- cells.) Proportions of α4+ cells (▪) in all tested tissues after transplantation show only minor deviations from data in normal, nontransplanted tissues (ie, normal BM has 95%-99% α4+ cells), suggesting multilineage engraftment.
show the proportion of α4- cells.) Proportions of α4+ cells (▪) in all tested tissues after transplantation show only minor deviations from data in normal, nontransplanted tissues (ie, normal BM has 95%-99% α4+ cells), suggesting multilineage engraftment.
Multilineage reconstitution by α4+ competitor cells. Expression of α4+ cells (▪) among cells from different lineages in (A) BM, (B) lymph nodes (LN), and (C) thymus of mice transplanted with 3:1 (test:competitive) cell ratio and tested at 36 weeks after transplantation. ( show the proportion of α4- cells.) Proportions of α4+ cells (▪) in all tested tissues after transplantation show only minor deviations from data in normal, nontransplanted tissues (ie, normal BM has 95%-99% α4+ cells), suggesting multilineage engraftment.
show the proportion of α4- cells.) Proportions of α4+ cells (▪) in all tested tissues after transplantation show only minor deviations from data in normal, nontransplanted tissues (ie, normal BM has 95%-99% α4+ cells), suggesting multilineage engraftment.
These data secured the fact that α4Δ/Δ cells are out-competed by α4+ cells early during hematopoietic reconstitution and that any remaining contribution by α4Δ/Δ cells is dwindling thereafter. Since the competitive advantage of α4+/+ cells was shown for the first few weeks after transplantation, the most likely interpretation is the superior homing of short-term engrafting α4+/+ cells. To eliminate homing differences in competitive repopulation, we decided to transplant pools composed of equal numbers of α4+/+/cre+ BM cells and α4f/f/cre+ cells that had not been ablated. This pool (1:1 ratio) consisted of 96.5% α4+ cells among kit+ at the time of transplantation. Upon hematopoietic reconstitution at approximately 4 weeks, we planned to induce α4 ablation in recipient animals (by poly(I)-poly(C) injections). Thus, at 4 weeks, we tested the blood of recipient animals and, to our surprise, instead of the expected approximately 95% α4 positivity, we had only 75.4% ± 1.9% in the entire group of 10 recipients. A proportion of 25% α4-negative cells in PB can be explained only by a partial α4 gene ablation occurring at posttransplantation period, presumably because of increased levels of endogenous interferon. Nevertheless, we ablated the animals, hoping to uncover additional contribution by α4Δ/Δ cells and tested them again approximately 3 months later (67 days after ablation). At that time, PB had 82.4% ± 1.4% α4+ cells, not significantly different from earlier determinations. These experiments suggested that no significant populations of unablated α4f/f existed before our ablation, presumably because they were out-competed earlier by normal cells. Technical issues of nonablation (related to poly(I)-poly(C)) use) were excluded by successful ablation in other concurrent control animals. To solidify contributions of α4+/+ competitor cells, secondary transplantations were carried out at the time of euthanization. secondary recipients (n = 5) analyzed 4 months later showed 86.9 % ± 0.9% α4+ cells in the BM, 78.5% ± 1.8% in the spleen, and 80.7% ± 3.8% in PB.
Repopulation by α4Δ/Δ bone marrow or peripheral blood cells
To test whether α4Δ/Δ bone marrow cells can establish long-term reconstitution in the absence of competitor cells, mice received transplants of either α4Δ/Δ or of a similar number of α4+/+ bone marrow cells per recipient (Table 1). Engraftment was assessed at 2, 10, 16, and 56 weeks later. To overcome homing problems and to test the role of the spleen in α4Δ/Δ short-term hematopoiesis, we also used splenectomized recipients (1 and 5 × 106 α4Δ/Δ cells per recipient). The data were compared to 1 × 106 α4+/+ concurrent control transplantations. As seen in Tables 1 and 2, short-term engraftment by α4Δ/Δ cells at 2 weeks was less than 6% and less than 20% of that seen in recipients of control BM splenectomized or not, respectively. Thus, engraftment in the absence of the spleen, where many α4Δ/Δ cells home and subsequently differentiate, is more severely impaired. Of interest is the peripheral blood leukocytosis in α4Δ/Δ recipients (Tables 1, 2), which does not reflect higher engraftment but only the increased release of α4Δ/Δ cells in circulation.
In the long-term donor reconstitution experiments, by 10 to 16 weeks bone marrow cellularity and progenitor content recovered to levels seen in concurrent controls (Table 1). This recovery had the hallmarks of α4Δ/Δ hematopoiesis before transplantation: increase in progenitors in circulation and in spleen. However, detailed evaluation of 3 mice at 10 weeks showed that kit+/α4+ were 46.4% in one of them, and in their spleens all 3 animals showed a significant increase in total α4+ cells (24.5%, 11.2%, and 9.6%) and among kit+ cells (31.3%, 37.6%, and 55.7%). Observations in the cohort of splenectomized recipients given the same number of α4Δ/Δ cells and evaluated at 10 weeks after transplantation also showed an increase in α4+ cells (7 recipients, 16.3% ± 3.5%) and no increase in circulating progenitors compared to nonsplenectomized recipients (118 ± 36.9/mL vs 564 ± 46.4, Table 1).
In contrast to data at 10 to 16 weeks (Table 1), hematopoiesis in the 3 mice surviving longer (56 weeks) was not maintained at levels seen at 10 to 16 weeks and was not increased further (ie, by BM-cell and progenitor numbers) with age, as seen in mice that did not receive transplants.15 (Total progenitor [CFU-C] content in BM, spleen, and PB was 815 789 ± 54 705 in 50-week-old α4+/+ animals [n = 4] and 1 363 601 ± 144 885 in α4Δ/Δ animals who did not receive transplants [n = 4] of the same age. The 3 survivors had 195 487 ± 79 380 total CFU-Cs.) Unfortunately, at this time, controls that received transplants were not available for meaningful comparisons.
To assess the self-renewal capacity of α4Δ/Δ HSCs, we carried out secondary transplantation experiments, both at 10 weeks (ie, at the recovery phase) and at 56 weeks after transplantation. As indicated in the previous paragraph, one of the 3 mice evaluated at 10 weeks had approximately 50% α4+ cells in circulation, whereas the other 2 had mostly α4Δ/Δ cells in circulation (Table 3, Figure 5). The latter 2 mice served as donors for five secondary recipients (1 × 106/recipient), and the one pseudo-chimeric mouse served as a donor for another 5 recipients. α4+/+ cells from control primary recipients also were transplanted in five secondary hosts. All recipients were evaluated about 4 months later. The data are shown in Table 3. At that time, a further increase in α4+ hematopoiesis and inferior BM CFU-C content in the recipients of α4Δ/Δ cells are seen, compared to concurrent BM controls. The data collectively suggest that α4Δ/Δ donor cells from primary recipients with phenotypically restored hematopoiesis have an inferior subsequent reconstitution capacity, as indicated by emergence of surviving host hematopoietic cells and by comparing them with concurrent controls. At 56 weeks the 3 surviving mice served as donors for ten secondary recipients (Figure 5). Evaluation of 9 of these recipients 26 weeks later showed that 5 had 33.6% ± 10.5% α4+ cells in BM, and the other 4 had 6.8% ± 1.9% α4+ cells. Each of the latter 4 mice with mostly α4Δ/Δ cells served as donors for five tertiary recipients (20 total, Figure 5A). Twenty-five weeks later, there was only a 30% survival in this group (Figure 5B). The 6 surviving mice had 66.2% ± 14.1% α4+ cells in the blood. This represented host hematopoiesis (absence of neo in 4 mice [nos. 1-4, Figure 5C], indicating WT cells), whereas presence of neo in the other 2 (nos. 5-6, Figure 5C) reflected the recipient genotype (α4f/f) and not unablated α4f/f donor cells. Taken together, our data indicate failing HSC self-renewal activity in the absence of α4 integrin.
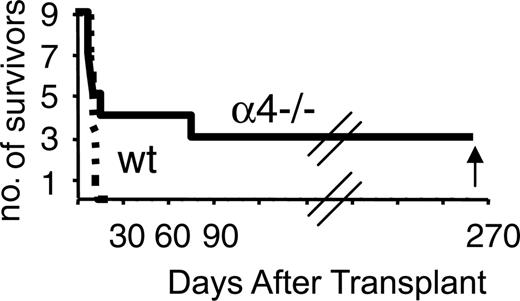
In addition to BM, PB cells from α4Δ/Δ mice were transplanted into lethally irradiated recipients to test for LTRCs in the circulation of these mice. For this purpose, blood from α4+/+ or α4Δ/Δ mice was given to 10 irradiated recipients from each group (0.3 mL/recipient). By day 30 all control recipients died; however, 3 of the recipients of α4Δ/Δ survived beyond 90 days (Figure 6). These recipients were killed 6 months later for evaluation and showed greatly diminished hematopoiesis (ie, decreased CFU-C content in BM, no increase of progenitors in circulation or in spleen; data not shown) similar to features seen long term from BM α4Δ/Δ cells. These results unequivocally suggest that the number of long-term repopulating cells is increased in circulation of α4Δ/Δ animals compared to control animals, since despite their putative homing defect, they can establish long-term hematopoiesis in primary recipients.
Discussion
Our previous studies in mice with α4 integrin gene ablation during adult life have shown no quantitative abnormalities in hematopoiesis during homeostasis for more than a year.15 However, there were aberrations in biodistribution of hematopoietic progenitors, which remained elevated in peripheral blood and were sequestered in the spleen. Furthermore, α4Δ/Δ BM cells had a partially impaired homing when given to irradiated recipients, confirming many prior studies using antifunctional α4 antibodies.18 In addition, there was a significant deficiency in short-term engraftment during the first few weeks after transplantation, suggesting impairment in expansion of hematopoiesis under stress, in agreement with independent studies of post-5FU recovery15 and engraftment studies with anti-α4-treated cells delivered intrafemorally.19 As the role of α4 integrins in the establishment and maintenance of long-term hematopoiesis was not clear from prior data, the present studies were undertaken.
Transplantation of α4- (Δ/Δ) BM or α4- (Δ/Δ) PB cells in lethally irradiated recipients. (A) Transplantation outcomes of mice given α4Δ/Δ BM donor cells killed at 10 or 56 weeks after transplantation (Table 1). Secondary transplantations (Table 3) were done at 10 or 56 weeks after primary transplantation and were tested 16 and 26 weeks after secondary transplantation (white mice, < 10% α4+ cells; gray mice, > 10% α4+ cells; black mice, dead). Note the tendency in all secondary transplant recipients for reconstitution by surviving α4+ cells from hosts (gray mice). In tertiary transplants, there was only 30% survival (B), and the 6 surviving mice (no. 1-6 in Figure 5C) were reconstituted mostly (mice no. 1-4) by host cells that were neo-negative (= WT). Two mice (no. 5, 6) showing neo+ in panel C were not reconstituted by unablated α4f/f cells, but by host cells, since these recipients were f/f/cre(-)/neo+ mice.
Transplantation of α4- (Δ/Δ) BM or α4- (Δ/Δ) PB cells in lethally irradiated recipients. (A) Transplantation outcomes of mice given α4Δ/Δ BM donor cells killed at 10 or 56 weeks after transplantation (Table 1). Secondary transplantations (Table 3) were done at 10 or 56 weeks after primary transplantation and were tested 16 and 26 weeks after secondary transplantation (white mice, < 10% α4+ cells; gray mice, > 10% α4+ cells; black mice, dead). Note the tendency in all secondary transplant recipients for reconstitution by surviving α4+ cells from hosts (gray mice). In tertiary transplants, there was only 30% survival (B), and the 6 surviving mice (no. 1-6 in Figure 5C) were reconstituted mostly (mice no. 1-4) by host cells that were neo-negative (= WT). Two mice (no. 5, 6) showing neo+ in panel C were not reconstituted by unablated α4f/f cells, but by host cells, since these recipients were f/f/cre(-)/neo+ mice.
Survival of mice that received transplants of 0.3 mL PB from α4+/+ or α4-/- (Δ/Δ) mice. All recipients of α4+/+ blood died early, whereas one third of α4-/- PB recipients survived long term.
Survival of mice that received transplants of 0.3 mL PB from α4+/+ or α4-/- (Δ/Δ) mice. All recipients of α4+/+ blood died early, whereas one third of α4-/- PB recipients survived long term.
α4-deficient donor cells display a competitive disadvantage in repopulation experiments
All experiments in which α4Δ/Δ cells were transplanted together with α4+/+ cells (at a ratio of 1:1 in 2 experiments and 3:1 test:competitor cells in one experiment) showed an early advantage of α4+/+ competitor cells over α4Δ/Δ test cells. Such an early competitive advantage likely relies, at least in part, on the better homing of α4+/+ short-term repopulating cells (CFU-Cs) compared to α4Δ/Δ ones, as documented in earlier studies.15 In addition to the inferior homing, impairment in the early expansion of α4Δ/Δ stem and progenitor cells after homing, also documented previously and further supported by post-5FU recovery data, could certainly compound the homing deficit. However, if the competitive disadvantage is consequent only to the impaired homing and early expansion of α4Δ/Δ donor cells, one would expect a stable contribution to hematopoiesis thereafter, as occurs with normally homing Rac2/CFU-C cells20 or stem-cell leukemia (SCL) conditionally ablated cells,21 or even a progressive contribution by α4Δ/Δ LTRCs, if these did not have any homing/retention defect. Instead, what was seen was a progressive contribution by α4+/+ competitor cells, as indicated by peripheral-blood evaluations comparing early and late times after transplantation (Figures 2, 3) and by differences between bone marrow and peripheral blood at the time of killing. Such a trend seen in all 3 experiments is compatible neither with a normal HSC homing nor with functionally normal HSC behavior, at least in the presence of normal competitor cells. An analogous late decline, albeit of a smaller magnitude, was seen in chimera created with DBA/2 and C57BL/6 mice and was attributed to genetic differences in stem-cell-renewal activity between the 2 murine strains.22 Furthermore, the competitive repopulation outcome is reminiscent of data with chimeric mice (from α4-/- ES cells), in which there was no contribution to hematopoiesis by α4-/- cells beyond the first month of postnatal life.13,14 These chimeric data and our data with adult cells emphasize, therefore, the competitive disadvantage of α4-deficient HSCs in the presence of normal competitors. A similar competitive disadvantage for adult HSCs was seen in chimeras with Tie2-/-23 and Sca-1-/-24 cells, whereas SCLΔ/Δ adult cells were competent in contrast to fetal ones,25 emphasizing regulatory differences between fetal and adult stem cells. It is important to point out that in the case of α4- or Tie2-deficient cells, there were no documented adverse effects on proliferation or differentiation of isolated hematopoietic cells that could complicate evaluation of engraftment rates seen in other competitive repopulations (ie, of PU.1-26 or Rac-deficient cells27 ). It was of interest that on rare occasions in the competitive repopulating experiments (Figure 3C), there was a long-term contribution by α4Δ/Δ donor cells. Such a result raised the question of whether indeed donor α4Δ/Δ can establish and support on their own long-term hematopoiesis in irradiated hosts. This issue was further explored by transplantations of bone marrow or peripheral blood from α4Δ/Δ mice.
Donor α4Δ/Δ stem cells can establish long-term hematopoiesis, but subsequent transplantability is quickly lost
Serial observations in murine recipients of α4Δ/Δ donor BM cells show that there is an initial delay in reconstitution, with eventual recovery between 10 and 16 weeks after transplantation (Table 1), signaled by an increase in the number of circulating progenitors, similar to that seen in steady-state α4Δ/Δ hematopoiesis. However, the few mice from primary transplants surviving long term (56 weeks) showed a picture of greatly diminished hematopoiesis. Of interest, there were signs of resurgence of host hematopoiesis in all late survivors (10-56 weeks, Table 1). More insight regarding the self renewal of α4Δ/Δ stem cells was gained by serial transplantation experiments. In spite of the phenotypic recovery at 10 weeks, recipients of these recovered cells (in secondary transplants) had a significant deficit in progenitor content in their bone marrow, compared to concurrent controls, and a sizeable component (∼ 1/3) of host hematopoiesis. Similarly, secondary transplant experiments from α4Δ/Δ donor cells at 56 weeks showed an increasing contribution by residual host hematopoietic cells (15 of 19 mice) several months later. Emergence of hematopoiesis by nonablated α4f/f stem cells was excluded by DNA analysis. Nevertheless, 4 of 19 secondary recipients displayed α4-deficient hematopoiesis in peripheral blood. Tertiary transplants using these phenotypically deficient cells as donor cells, however, relied on recovery of host hematopoiesis for their survival (30% survival). Dependence on host surviving cells late in transplantation is a hallmark of a reduced number of engrafting donor cells in serial transplantation experiments.28,29 We did not encounter any contribution to hematopoiesis by nonablated α4f/f cells in this setting, as seen in other examples with Mx.cre-mediated excision (PU.126 or TEL30 ).
These results collectively suggest that the number of α4Δ/Δ stem cells capable for engraftment was low in secondary transplants and virtually absent in tertiary transplants. In other words, the expected recovery, or self renewal of HSC function several months after transplantation, did not occur. Certainly some dilution of functional stem cells in the secondary and tertiary transplants is expected and may be compounded by their homing defect. However, since the latter, at least for progenitor cells, is only a partial defect, it may not easily account for the early exhaustion or the virtual absence of α4Δ/Δ HSCs in the inocula of most secondary and in all tertiary transplants. Diminished survival is not expected until after the fifth serial transplantation in this mouse strain transplanted with similar inocula.31,32
Thus, homing defects alone cannot readily explain the outcome unless a drastically reduced number of stem cells also is present or the homing and retention deficiencies of the transplanted α4Δ/Δ HSCs are more extreme than the one seen with progenitor cells. More likely, we believe, is the inability of adult α4Δ/Δ HSCs to balance self renewal versus downstream differentiation, especially under stress, either because of an intrinsic defect of α4Δ/Δ HSCs to respond to signals from the bone marrow microenvironment under stress or from their location away from short-range signals emanating from the stem-cell “niche.” Future experiments may shed light on these issues.
Is there any role of α4 integrin at the stem-cell niche?
Parameters regulating the function of stem cells and their ability to self renew are currently under intense investigation. Emerging information suggests that stem-cell renewal and/or survival is governed by a complex interplay between signals from stem cells and those emanating from the stem-cell niche.33 Several molecules actively transcribed by stem cells are shown to be important for their survival (ie, BCL2,34 MCL-1,35 TEL,30 ATM36 ), whereas other molecules interfacing with the cell-cycling machinery are important for their proliferation, directly or indirectly, and for increasing the balance of self renewal versus differentiation (HoxB4,37 Gfi-1,38 Bmi139 HoxA-9,40 Notch-1/Wnt,41 NF-Ya,42 p21,31 SHIP-1,43 Myc44 ). In addition to stem-cell-intrinsic signals, extrinsic signals from the stem-cell niche are of critical importance. If these signals act at short range, they would require intimate contact of stem cells with cells or matrix at the niche, but what secures this contact has not been worked out in detail. Recently, a couple of adhesion pathways have been described (N-cadherin/β-catenin, Tie2/Ang-1) influencing HSC retention at the niche, especially by osteoblasts, a critical structural niche component.45-47 Such a close interaction between stem cells and osteoblasts toward hypoxic areas of bone marrow (like the endosteal surface) maintains stem-cell quiescence by insulating HSCs from regional proliferative influence and protects their survival from outside injury or insults.
It is of interest that the adhesion through the Tie2 expressed by stem cells and of Ang-1 expressed by osteoblasts was inhibited by β1 and α4 integrins.23,45 Thus, the latter can presumably influence stem-cell contact at the niche downstream of the Tie2/Ang-1 molecular pathway. Furthermore, osteoblasts express an abundance of osteopontin,48 and the latter is an important ligand for α4 integrins.49,50 Additional adhesive pathways controlled by Myc expression also were postulated to affect HSC/niche interactions.44 Loss or overexpression of Myc in HSCs led to increase or decrease, respectively, of adhesion molecules involved in HSC-niche interaction.44 However, in these studies, mainly β2 integrins were involved, and no preferential maintenance of Myc-/- cells at endosteal sites was documented. Whatever the nature of adhesion molecules involved, it is presently unclear whether they function only as adhesion receptors or participate in signaling cascades required to retain cells at the niche. Moreover, it is presently unsettled whether osteoblasts lining the endosteal areas of bone marrow comprise the only unique stem-cell niches important for preservation of stem-cell function. Such a concept, for example, will not explain the survival and function of HSCs in extramedullary sites. Indeed, despite the recent resurgence of evidence favoring the endosteal niche definition, new evidence suggests that sinusoidal endothelial cells create a niche for HSCs that sustains a substantial fraction of the HSC pool.51 This concept is further bolstered by the fact that there are no frequency differences in HSCs among bones with different ratios of endosteal to central bone marrow, both when examined by modern approaches52 or by decades-old approach.53 Furthermore, it is unclear whether geographic differences in the distribution of stem cells reported in normal, nonconditioned bone marrow by old54 or by newer studies55 are abolished or disturbed in response to injury (ie, after lethal irradiation).
Whatever the anatomic location of stem-cell niche, perivascular or endosteal, a close contact likely protects the survival of stem cells and assures their quiescence. It is theoretically possible that an integrin, for instance, α4, may be one of the participating molecules securing the close contact of stem cells with cells at the niche, but further studies are needed to approach this issue.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-07-2670.
Supported by National Institutes of Health grants HL58734 and DK46557.
G.V.P. was technically involved in the execution and evaluation of all transplant experiments; L.M.S. was involved in the setup and short-term evaluation of the initial transplantation experiments; T.U. was involved in genotyping and evaluation of all transplant experiments by genomic PCR; and T.P. directed the overall study and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Betty Mastropaolo for her assistance with the figures and in editing the manuscript.
The authors declare that they have no competing financial interests.








 ). Reconstitution by α4+ cells was higher in BM compared to concurrently tested PB cells (total cells or kit+ cells, left panels), suggesting ongoing replacement of hematopoiesis by α4+ competitor cells. Error bars represent standard error of the mean.
). Reconstitution by α4+ cells was higher in BM compared to concurrently tested PB cells (total cells or kit+ cells, left panels), suggesting ongoing replacement of hematopoiesis by α4+ competitor cells. Error bars represent standard error of the mean.