Polycythemia vera (PV) is a clonal myeloproliferative disorder characterized by excessive erythrocyte production. Most patients with PV harbor an activating JAK2 mutation, but the molecular links between this mutation and erythrocyte overproduction are unknown. The interaction between death receptors and their ligands contributes to the physiological regulation of erythropoiesis through the inhibition of erythroblast proliferation and differentiation. With the use of an in vitro culture system to generate differentiating erythroid cells, we found that erythroblasts derived from patients with PV harboring the JAK2 V617F mutation were able to proliferate and generate higher numbers of mature erythroid cells in the presence of inhibitory signals delivered by CD95 (Fas/Apo-1) and TRAIL receptor stimulation. JAK2-mutated PV erythroblasts showed lower levels of CD95-induced caspase activation and incomplete caspase-mediated cleavage of the erythroid transcription factor GATA-1, which was entirely degraded in normal erythroblasts on CD95 stimulation. JAK2 mutation was associated in PV erythroblasts with cytokine-independent activation of the JAK2 effectors Akt/PKB and ERK/MAP and with a deregulated expression of c-FLIPshort, a potent cellular inhibitor of death receptor–induced apoptosis. These results show the presence in PV erythroblasts of proliferative and antiapoptotic signals that may link the JAK2 V617F mutation with the inhibition of death receptor signaling, possibly contributing to a deregulation of erythropoiesis.
Introduction
Polycythemia vera (PV) is a clonal myeloproliferative disorder that originates from the transformation of a multipotent hematopoietic progenitor and results in the overproduction of erythroid cells often accompanied by thrombocytosis, granulocytosis, or both. Patients with PV display an increased red blood cell mass despite normal or depressed erythropoietin (EPO) levels, trilineage marrow hyperplasia, splenomegaly, and a predisposition to myelofibrosis and acute leukemia.1 The observation that hematopoietic progenitors from patients with PV can form hemoglobinized colonies in vitro in the absence of exogenous EPO2-4 fostered the search for abnormalities in the EPO receptor (EpoR) that would produce a constitutive signal or would decrease the threshold of EPO stimulation. However, no alteration was found in EpoR sequence or expression or EPO-binding affinity.5-8 Several molecular alterations have been reported to associate with PV, including impaired platelet expression of the thrombopoietin receptor c-mpl, increased activity of PTP-MEG2 phosphatase, deregulated Bcl-XL expression, increased levels of the cyclin-dependent kinase inhibitor INK4a/ARF, and elevated granulocyte expression of the PRV-1 (polycythemia rubra vera-1) receptor.9-13 However, the most compelling evidence for a molecular alteration associated with the myeloproliferative phenotype has been provided recently by showing the occurrence of a unique V617F activating mutation in the Janus kinase 2 (JAK2) gene that has been found in most patients with PV.14-19
Homeostasis of the erythroid compartment is finely regulated by positive signals mainly delivered by EPO, interleukin-3 (IL-3), and stem cell factor (SCF) and negative signals generated by tumor necrosis factor (TNF) family members. Members of the TNF receptor family, such as CD95 (Fas/Apo-1) and TNF-related apoptosis inducing ligand (TRAIL) receptors, are expressed on immature erythroblasts, whereas their cognate ligands are expressed by erythroblasts at advanced stages of maturation.20-23 The interaction between death receptors and their ligands triggers the formation of an intracellular signaling complex called death-inducing signaling complex (DISC) that includes adaptor proteins, caspases, and regulatory proteins.24 The balance between proapoptotic and antiapoptotic factors at the DISC has been demonstrated to be crucial for the final outcome of death receptor–generated signals. For example, increased levels of cellular FLICE-inhibitory protein (c-FLIP), a catalytically inactive homolog of caspase-8, have been demonstrated to render cells resistant to the extrinsic apoptotic pathway. More recently, it has been shown that the long form of c-FLIP can act as an inhibitor or as an inducer of cell death,25-27 whereas the short form of c-FLIP is primarily effective at inhibiting death receptor–mediated apoptosis.28-30
In immature erythroblasts, stimulation of the CD95 receptor results in caspase activation and cleavage of the major erythroid transcription factor GATA-1.21 These events ultimately lead to a reversible inhibition of cell proliferation and differentiation or in apoptosis, depending on the levels of EPO present in the microenvironment. Therefore, the interaction between death receptors and their ligands has been proposed as a feedback mechanism that regulates red blood cell production in response to changes in serum EPO levels.20-22 This hypothesis is consistent with the diffuse erythrocyte accumulation observed in mice embryos defective for caspase-8, the only common upstream effector of CD95 and TRAIL receptors in mice.31 Similarly, depletion of the hematopoietic death receptor in zebrafish results in an erythroid dysregulation phenotype reminiscent of human polycythemia.32
The mutated JAK2 form found in most patients with PV is able to generate constitutive JAK2-mediated signals when exogenously expressed in cell lines and to induce erythrocytosis in mice.14 We hypothesized that in PV erythroid progenitor cells, the presence of JAK2 V617F may result in the increased generation of signals responsible for cell survival and proliferation and may interfere with death receptor–mediated inhibition of erythropoiesis. Thus, using a serum-free culture system to obtain pure differentiating erythroid populations, we characterized the functional and molecular events related to death receptor activation in erythroblasts derived from patients with PV in relation to the presence of the JAK2 V617F mutation.
Materials and methods
Cytokines and antibodies
Human recombinant SCF, IL-3, and granulocyte macrophage–colony-stimulating factor (GM-CSF) were purchased from Peprotech (Rocky Hill, NJ). Recombinant human erythropoietin was supplied by Amgen (Thousand Oaks, CA). Anti-CD95 activating antibody (clone CH11), polyclonal antibody against caspase-3, and monoclonal anti–caspase-8 antibody (clone 5F7) were purchased from Upstate Biotechnology (UBI, Lake Placid, NY). Anti–GATA-1 (N1), anti-Akt (C20), and anti-ERK antibodies (E4 and K23) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–phospho-Akt was from Promega (Madison, WI). Anti-FADD was from BD PharMingen (San Diego, CA), and antiactin monoclonal antibody was from Oncogene (San Diego, CA). Anti-FLIP antibody (clone NF6) was purchased from Alexis (Lausen, Switzerland) and was used for Western blot experiments in conjunction with an isotype-specific secondary antibody. Recombinant human TRAIL (KillerTRAIL) was purchased from Alexis.
Adult peripheral blood human progenitor cell purification and culture
Peripheral blood was obtained from healthy donors and phlebotomy-treated patients with PV after their informed consent and approval by the institutional Committee for Human Studies were given. Patients with PV (12 men, 1 woman; age range, 30-78 years; median age, 60 years) met the criteria established by the Polycythemia Vera Study Group and did not have history of any other disease. Blood samples were collected in sodium heparin and processed within 3 hours. CD34+ hematopoietic progenitors were purified by positive selection using the midi-MACS immunomagnetic separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. CD34+ cells were cultured in serum-free medium prepared as follows: Iscove modified Dulbecco medium (Euroclone, West York, United Kingdom) was supplemented with delipidated bovine serum albumin (10 mg/mL), pure human transferrin (0.7 mg/mL), human low-density lipoprotein (40 μg/mL), insulin (10 μg/mL), sodium pyruvate (10–4 M), L-glutamine (2 × 10–3 M), rare inorganic elements supplemented with iron sulfate (4 × 10–8 M), and nucleosides (10 μg/mL each). Serum-free medium was supplemented with 0.01 U/mL IL-3, 0.001 ng/mL GM-CSF, and 3 U/mL EPO (standard erythroid medium) to induce unilineage erythroid differentiation, routinely yielding 98% ± 2% glycophorin A–positive cells.33 The differentiation stage of erythroid precursor cells was routinely evaluated by May-Grünwald-Giemsa staining and cytologic analysis.
Western blotting
Protein extracts were prepared by resuspending cell pellets in 1% NP40 lysis buffer (20 mM Tris HCl, pH 7.2, 200 mM NaCl, 1% NP40) supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktails I and II (all from Sigma-Aldrich, St Louis, MO). Lysate concentrations were determined by the Bradford assay (Bio-Rad Laboratories, Richmond, CA), and equal amounts of proteins were used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Samples were analyzed by standard immunoblot procedure and visualized by chemiluminescence (Super Signal West Pico Pierce, Rockford, IL). In some patients, the yield of CD34+ cells isolated from phlebotomy-derived blood was too low to produce a number of day 7 erythroblasts sufficient for Western blotting experiments.
JAK2 V617F genotyping
Genomic DNA was extracted by standard procedures from peripheral blood granulocytes. The presence of the JAK2 V617F mutation was determined with the JAK2 Activating Mutation Assay (InVivoScribe Technologies, San Diego, CA) based on BsaXI digestion of a PCR product encompassing the site of mutation.
Evaluation of caspase activity and apoptosis
Caspase 3/7 activity in CD95-stimulated erythroblasts was determined with the Apo-ONE Homogeneous Caspase 3/7 Assay (Promega), based on cleavage of the z-DEVD-rhodamine 110 substrate, in accordance with the manufacturer's instructions. Fluorescence values were read on a Victor 2 plate fluorometer (Wallac, Perkin Elmer, Norwalk, CT) at a wavelength of 435/585 nm. Apoptosis in CD95-treated erythroblasts was assessed by ethidium bromide/acridine orange staining and fluorescence microscopy.
Flow cytometry analysis
For flow cytometry analysis of CD95 expression, erythroblasts were incubated for 1 hour on ice in PBS 1% BSA with anti-CD95 monoclonal antibody (clone DX2; BD PharMingen, San Diego, CA) or control IgG1, then washed and incubated 45 minutes on ice with a phycoerythrin (PE)–conjugated antimouse secondary antibody (Invitrogen-Molecular Probes, Eugene, OR). For flow cytometry detection of TRAIL receptors, erythroblasts were incubated with anti–TRAIL receptor-1 and -2 from R&D Systems (Minneapolis, MN) in combination with a PE-labeled antigoat secondary antibody from Chemicon (Temecula, CA). To detect glycophorin A expression, cells were incubated for 30 minutes with PE-conjugated anti–glycophorin A (CD235a; BD PharMingen). Samples were analyzed on a FACScan flow cytometer (Becton Dickinson).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 for Windows (GraphPad Software, San Diego, CA). Unpaired t test (with unequal variances correction where needed) was used to analyze the statistical significance of cell proliferation, of apoptotic cells in CD95-treated cultures, and differentiation status. The statistical significance of caspase 3/7 activation and death receptor–induced growth inhibition results was analyzed by Mann-Whitney nonparametric test. The percentage of anti-CD95–induced growth inhibition was determined as 100 – (100 × treated cells/untreated cells).
Results
Growth and differentiation of PV erythroblasts in serum-free liquid culture
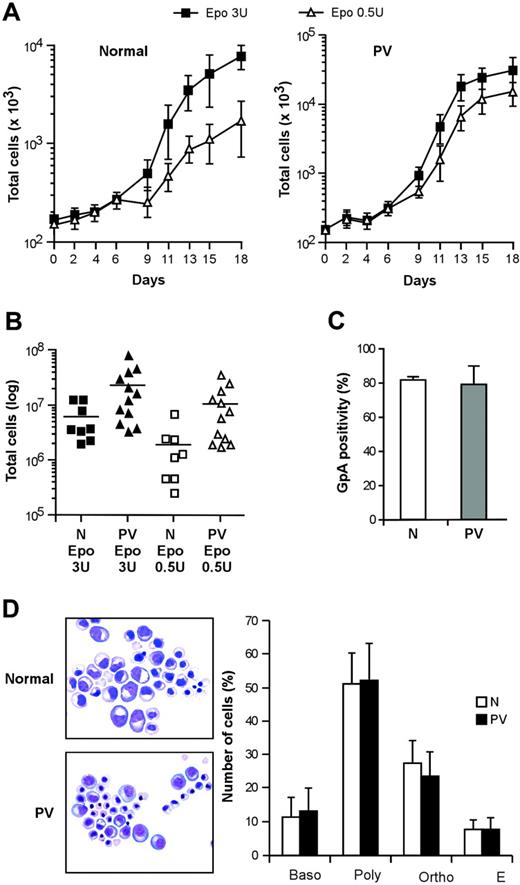
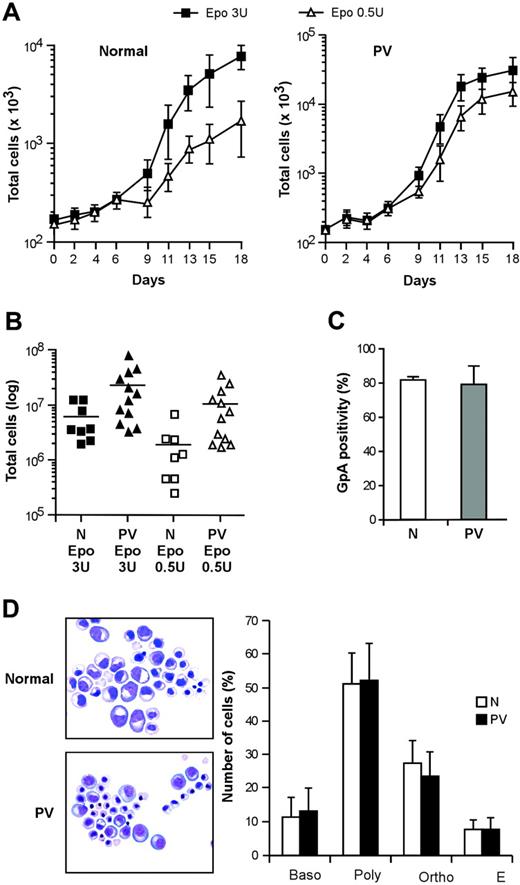
Virtually pure populations of erythroid progenitor and precursor cells can be obtained by culturing CD34+ cells purified from the peripheral blood of healthy persons in a serum-free medium containing very low doses of IL-3 and GM-CSF and high concentrations of EPO.33 These culture conditions avoid the use of SCF (which delays erythroid maturation while promoting cell expansion) and therefore are particularly suitable to study the proliferation and differentiation of primary erythroblasts. We obtained pure populations of differentiating erythroblasts from CD34+ hematopoietic progenitors of patients with PV and compared their growth and differentiation with those of normal erythroblasts in this serum-free/SCF-free culture system. Erythroblasts derived from PV CD34+ cells showed an increased proliferative capacity in the presence of saturating EPO concentrations (Figure 1A), in agreement with earlier reports.34,35 In line with the previously reported EPO hypersensitivity of PV erythroid progenitors, culture in the presence of suboptimal levels of EPO (0.5 U/mL) significantly reduced the expansion of normal erythroblasts, whereas PV cells were scarcely affected (Figure 1A-B). The in vitro differentiation of PV erythroblasts was similar to the differentiation of control erythroid cells as routinely checked by the evaluation of glycophorin A (GpA), a specific marker for the late stages of erythroid maturation, and by Giemsa staining (Figure 1C-D). Interestingly, GpA expression at day 7 of erythroid culture showed slightly higher variability in PV erythroblasts than in healthy controls, possibly reflecting individual disease-related differences in the rate of erythroid maturation. However, erythroblasts from all the patients with PV examined reached 98% ± 2% GpA positivity at later stages of differentiation.
Increased resistance of PV erythroblasts to death receptor–mediated inhibition of erythropoiesis
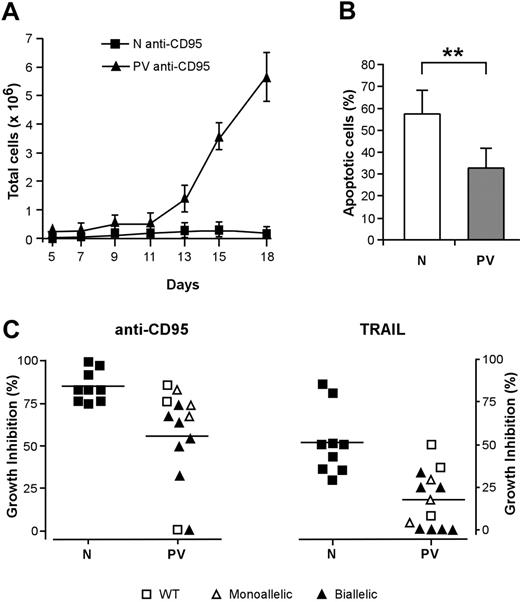
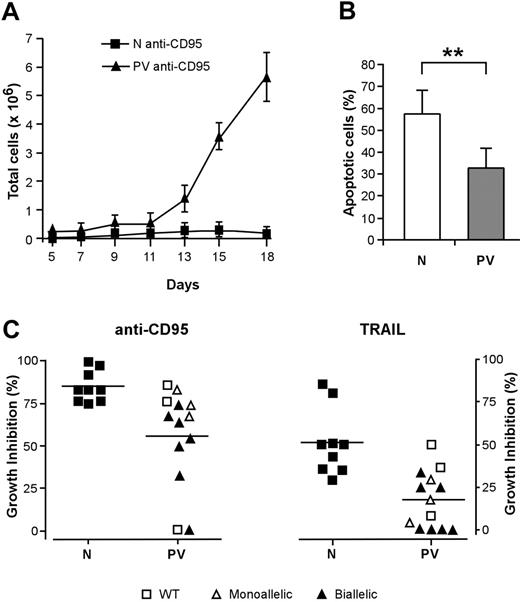
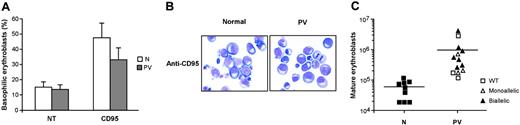
The activation of death domain–containing receptors on the surfaces of immature erythroblasts results in a reversible arrest of proliferation and differentiation or in cell death and has been proposed to restrain the production of erythroid cells in physiologic and pathologic circumstances.20,21,36 To investigate whether the effects of death receptor activation were altered in patients with PV, we cultivated immature erythroblasts derived from normal and PV CD34+ cells with low doses of CD95-activating antibody, which inhibits the growth and maturation of normal erythroid precursor cells.21 The proliferation of normal erythroblasts was strongly reduced by CD95 stimulation, whereas erythroid precursors derived from patients with PV exhibited a significantly higher resistance to CD95-mediated growth inhibition (Figure 2A). Erythroblasts whose maturation was hindered by chronic low-dose CD95 stimulation eventually undergo apoptosis at later times of culture. We evaluated the number of apoptotic cells in anti-CD95–treated normal and PV erythroblasts at day 14 of culture and found a reduced presence of apoptotic cells among PV erythroblasts (Figure 2B). To determine whether death receptor sensitivity was related to the presence of the JAK2 V617F mutation, we analyzed patients with PV for JAK2 mutational status using genomic DNA extracted from peripheral blood granulocytes. Most (10 of 13; 76.9%) of the patients used for this study harbored the JAK2 V617F mutation, with an unexpected number in either homozygosis or heterozygosis of homozygous individuals. The presence of biallelic mutated JAK2 was mostly found in erythroblasts with a higher-than-normal resistance to the growth inhibitory effects of anti-CD95 and TRAIL (Figure 2C). Taken together, these results suggest that the resistance of PV erythroblasts to death receptor–mediated inhibitory signals may be related to the presence of the JAK2 V617F mutation and may concur with an increased proliferative ability to the abnormal expansion of the erythroid compartment that occurs in PV.
Proliferation and differentiation of normal and PV erythroblasts in serum-free liquid culture. (A) Growth of erythroblasts derived from peripheral blood CD34+ cells of healthy donors (normal, left panel) and patients with PV (PV, right panel) cultivated in standard erythroid medium containing 3 U/mL EPO (▪) or 0.5 U/mL EPO (▵). Data are represented as mean ± SEM of experiments performed with 5 healthy donors and 10 patients with PV. (B) Number of erythroblasts obtained at day 18 of serum-free liquid culture from normal (N) and PV (PV) CD34+ cells derived from patients and healthy controls and cultivated as described. P < .05 for N versus PV in 3 U/mL EPO, for N versus PV in 0.5 U/mL EPO, and for N 3 U/mL EPO versus N 0.5 U/mL EPO, whereas the difference between PV 3 U/mL EPO and PV 0.5 U/mL EPO was not statistically significant. (C) Glycophorin A (GpA) expression of erythroblasts at day 7 of unilineage culture derived from CD34+ cells of healthy donors (N) and patients with PV (PV). (D) May-Grünwald-Giemsa staining of normal (N) and PV (PV) erythroblasts at day 14 of erythroid differentiation in serum-free liquid culture and relative percentage of cells belonging to different erythroid differentiation stages. Baso indicates basophilic erythroblasts; poly, polychromatophilic erythroblasts; ortho, orthochromatic erythroblasts; E, erythrocytes. Data represent the mean ± SD of experiments performed with cells derived from 13 patients. Images were taken with a Nikon Eclipse E1000 upright microscope equipped with a Nikon Plan Fluorite 40 ×/1.3 NA oil immersion objective and a Nikon DXM 1200 digital camera with dedicated acquisition software (Nikon ACT-1 v. 2.1; all from Nikon Instruments, Sesto Fiorentino, Firenze, Italy). Subsequent image editing was performed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Proliferation and differentiation of normal and PV erythroblasts in serum-free liquid culture. (A) Growth of erythroblasts derived from peripheral blood CD34+ cells of healthy donors (normal, left panel) and patients with PV (PV, right panel) cultivated in standard erythroid medium containing 3 U/mL EPO (▪) or 0.5 U/mL EPO (▵). Data are represented as mean ± SEM of experiments performed with 5 healthy donors and 10 patients with PV. (B) Number of erythroblasts obtained at day 18 of serum-free liquid culture from normal (N) and PV (PV) CD34+ cells derived from patients and healthy controls and cultivated as described. P < .05 for N versus PV in 3 U/mL EPO, for N versus PV in 0.5 U/mL EPO, and for N 3 U/mL EPO versus N 0.5 U/mL EPO, whereas the difference between PV 3 U/mL EPO and PV 0.5 U/mL EPO was not statistically significant. (C) Glycophorin A (GpA) expression of erythroblasts at day 7 of unilineage culture derived from CD34+ cells of healthy donors (N) and patients with PV (PV). (D) May-Grünwald-Giemsa staining of normal (N) and PV (PV) erythroblasts at day 14 of erythroid differentiation in serum-free liquid culture and relative percentage of cells belonging to different erythroid differentiation stages. Baso indicates basophilic erythroblasts; poly, polychromatophilic erythroblasts; ortho, orthochromatic erythroblasts; E, erythrocytes. Data represent the mean ± SD of experiments performed with cells derived from 13 patients. Images were taken with a Nikon Eclipse E1000 upright microscope equipped with a Nikon Plan Fluorite 40 ×/1.3 NA oil immersion objective and a Nikon DXM 1200 digital camera with dedicated acquisition software (Nikon ACT-1 v. 2.1; all from Nikon Instruments, Sesto Fiorentino, Firenze, Italy). Subsequent image editing was performed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Effect of death receptor activation on the proliferation and survival of PV erythroid precursors. CD34+ cells purified from the peripheral blood of healthy donors and patients with PV were cultivated in standard erythroid medium. From day 5 of erythroid culture, cells were treated with the indicated stimuli in medium containing 0.5 U/mL EPO. Anti-CD95 or TRAIL was maintained at the indicated concentrations throughout the time of culture. (A) Growth of normal (N, ▪) and PV (PV, ▴) erythroblasts in the presence of 50 ng/mL anti-CD95 antibody. Data are expressed as mean ± SD of experiments performed with erythroblasts derived from 5 healthy donors and 10 patients with PV. (B) Percentage of apoptotic cells in cultures of normal (N) and PV erythroblasts (PV) at day 14 in the presence of 50 ng/mL anti-CD95 antibody from day 5 of culture. Data represent the mean ± SD of experiments performed with cells derived from 13 patients (**P < .01). (C) Growth inhibition exerted by 50 ng/mL anti-CD95 antibody (left) or by 200 ng/mL TRAIL (right) on normal (N) and PV (PV) erythroblasts in relation to the presence of JAK2 V617F mutation. WT indicates wild-type JAK2; monoallelic, monoallelic JAK2 V617F mutation; biallelic, biallelic JAK2 V617F mutation. The percentage of growth inhibition was calculated on cells at day 18 of culture, as described in “Materials and methods.” P ≤ .001 for anti-CD95 antibody; P ≤ .005 for TRAIL. Horizontal bars indicate the mean value of the N and PV percentage growth inhibition.
Effect of death receptor activation on the proliferation and survival of PV erythroid precursors. CD34+ cells purified from the peripheral blood of healthy donors and patients with PV were cultivated in standard erythroid medium. From day 5 of erythroid culture, cells were treated with the indicated stimuli in medium containing 0.5 U/mL EPO. Anti-CD95 or TRAIL was maintained at the indicated concentrations throughout the time of culture. (A) Growth of normal (N, ▪) and PV (PV, ▴) erythroblasts in the presence of 50 ng/mL anti-CD95 antibody. Data are expressed as mean ± SD of experiments performed with erythroblasts derived from 5 healthy donors and 10 patients with PV. (B) Percentage of apoptotic cells in cultures of normal (N) and PV erythroblasts (PV) at day 14 in the presence of 50 ng/mL anti-CD95 antibody from day 5 of culture. Data represent the mean ± SD of experiments performed with cells derived from 13 patients (**P < .01). (C) Growth inhibition exerted by 50 ng/mL anti-CD95 antibody (left) or by 200 ng/mL TRAIL (right) on normal (N) and PV (PV) erythroblasts in relation to the presence of JAK2 V617F mutation. WT indicates wild-type JAK2; monoallelic, monoallelic JAK2 V617F mutation; biallelic, biallelic JAK2 V617F mutation. The percentage of growth inhibition was calculated on cells at day 18 of culture, as described in “Materials and methods.” P ≤ .001 for anti-CD95 antibody; P ≤ .005 for TRAIL. Horizontal bars indicate the mean value of the N and PV percentage growth inhibition.
Inefficient CD95-induced caspase activation and GATA-1 cleavage in PV erythroblasts
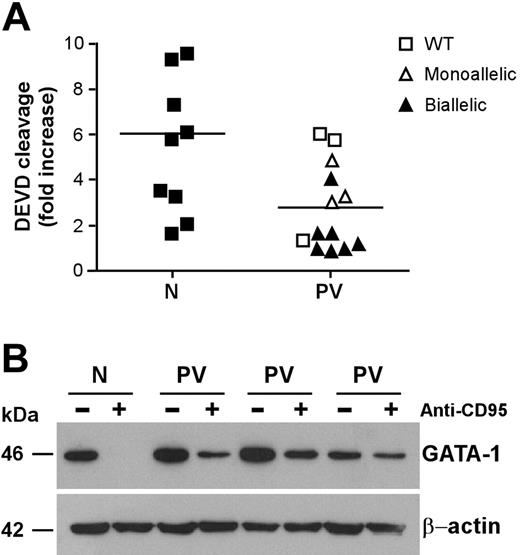
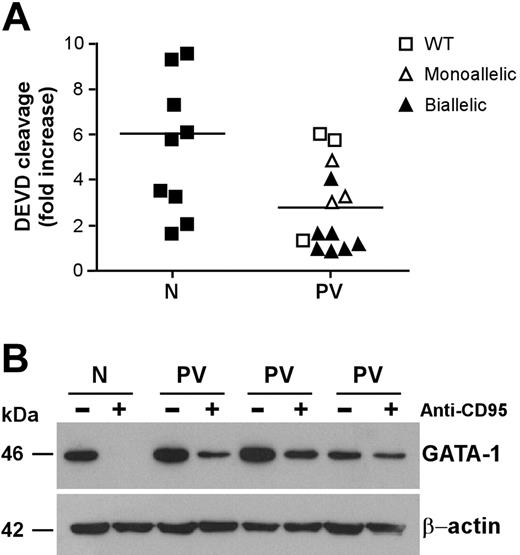
CD95 stimulation of immature erythroblasts results in caspase activation and cleavage of the transcription factor GATA-1, which is essential for regular erythroid development.21,37 Analysis of executioner caspase activity revealed a lower CD95-induced caspase activation in CD95-stimulated immature PV erythroid precursors than in their normal counterparts (Figure 3A). The relation between CD95-induced caspase activity and JAK2 mutation was similar to that found in experiments of death receptor–induced growth inhibition shown in Figure 2C. Erythroblasts with lower CD95-induced caspase activation also harbored the JAK2 V617F mutation, with the exception of those derived from one patient with wild-type JAK2 that were also highly resistant to anti-CD95 and TRAIL. Because executioner caspases are responsible for GATA-1 degradation in erythroblasts exposed to CD95 stimulation, we sought to investigate whether lower caspase activation in PV erythroblasts would result in inefficient GATA-1 cleavage. Therefore, we assessed GATA-1 levels in normal and PV erythroblasts untreated and treated with anti-CD95 antibodies. Basal GATA-1 expression was comparable between untreated normal and PV erythroblasts. However, on CD95 stimulation, GATA-1 was completely degraded in normal erythroblasts but not in PV erythroblasts, which retained a consistent fraction of the GATA-1 protein (Figure 3B). We also observed a residual presence of the GATA-1 protein in PV erythroblasts after 4 days in the presence of anti-CD95 antibodies (data not shown), suggesting that GATA-1 persistence in CD95-treated PV erythroblasts may play a role in the ability of these cells to resist the inhibitory effects of prolonged death receptor stimulation.
CD95 stimulation delays the differentiation of PV erythroblasts but does not completely inhibit mature cell production
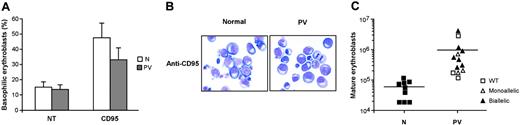
CD95 activation has been shown to restrain erythroid maturation, extending the permanence of immature erythroblasts in the basophilic stage of differentiation.20,21 As a result, normal erythroblasts can face 2 different fates. If CD95 stimulation is removed, cells can reenter the differentiation process and achieve terminal maturation. Conversely, if CD95 activation persists, normal erythroblasts remain locked at the immature stage of differentiation and eventually undergo apoptosis. We cultivated normal and PV erythroblasts in the presence of CD95-activating antibodies and assessed the effects on erythroid differentiation and final maturation. CD95 stimulation inhibited the differentiation of normal and PV erythroblasts (Figure 4A-B), though PV erythroid precursors were slightly less sensitive to CD95-induced inhibition of differentiation (Figure 4A). On prolonged CD95 stimulation, normal erythroblasts remained blocked at early differentiation stages and could not achieve terminal maturation, whereas PV erythroblasts were able to produce variable numbers of mature erythroid precursors (Figure 4C). Erythroblasts derived from patients with normal JAK2 produced lower numbers of mature erythroblasts in the presence of CD95 stimulation, with the exception of the patient whose erythroblasts showed low caspase activation and high resistance to death receptor–induced growth inhibition. Altogether, these findings suggest that erythroblasts derived from patients with PV harboring the JAK2 V617F mutation retain the capacity to produce mature red blood cells even in the presence of inhibitory stimuli delivered by death receptors.
Caspase activation and GATA-1 cleavage in normal and PV erythroblasts. Erythroid precursors derived from CD34+ cells of healthy donors (N) and patients with PV (PV) characterized for the JAK2 V617F mutation (as in Figure 2) were cultivated in standard erythroid medium. At day 7 of culture, cells were stimulated with 250 ng/mL anti-CD95 antibodies for 24 hours in standard erythroid medium containing 0.5 U/mL EPO and were analyzed for caspase activation (P < .01) (A) and GATA-1 protein levels (B). Results shown in panel B were obtained with a pool of erythroblasts obtained from 3 healthy donors (N) and are representative of experiments performed with erythroblasts derived from 6 patients with PV harboring the JAK2 V617F mutation. Horizontal bars in panel A indicate the mean value of the N and PV caspase activation.
Caspase activation and GATA-1 cleavage in normal and PV erythroblasts. Erythroid precursors derived from CD34+ cells of healthy donors (N) and patients with PV (PV) characterized for the JAK2 V617F mutation (as in Figure 2) were cultivated in standard erythroid medium. At day 7 of culture, cells were stimulated with 250 ng/mL anti-CD95 antibodies for 24 hours in standard erythroid medium containing 0.5 U/mL EPO and were analyzed for caspase activation (P < .01) (A) and GATA-1 protein levels (B). Results shown in panel B were obtained with a pool of erythroblasts obtained from 3 healthy donors (N) and are representative of experiments performed with erythroblasts derived from 6 patients with PV harboring the JAK2 V617F mutation. Horizontal bars in panel A indicate the mean value of the N and PV caspase activation.
Differentiation of normal and PV erythroblasts in the presence of death receptor stimulation. (A) Percentages of basophilic erythroblasts in normal (N) and PV (PV) erythroid cell populations at day 14 of culture, untreated (NT), or treated with 50 ng/mL anti-CD95 antibody (CD95) from day 5 in standard erythroid medium containing 0.5 U/mL EPO. Data represent the mean ± SD of cell percentages obtained from May-Grünwald-Giemsa stainings prepared with erythroblasts from 13 patients and 9 healthy controls. The difference between normal and PV erythroblasts is not statistically significant. (B) May-Grünwald-Giemsa staining of erythroblasts derived from 1 healthy donor (N) and 1 PV patient (PV) at day 14 of differentiation in the presence of 50 ng/mL anti-CD95 antibody. Images were captured as described in the legend of Figure 1D. (C) Absolute number of mature (orthochromatic) erythroblasts obtained from healthy controls (N) and patients with PV (PV) characterized for the presence of the JAK2 V617F mutation at day 14 of culture in the presence of 50 ng/mL anti-CD95. Horizontal bars indicate the mean value of the N and PV erythroblasts.
Differentiation of normal and PV erythroblasts in the presence of death receptor stimulation. (A) Percentages of basophilic erythroblasts in normal (N) and PV (PV) erythroid cell populations at day 14 of culture, untreated (NT), or treated with 50 ng/mL anti-CD95 antibody (CD95) from day 5 in standard erythroid medium containing 0.5 U/mL EPO. Data represent the mean ± SD of cell percentages obtained from May-Grünwald-Giemsa stainings prepared with erythroblasts from 13 patients and 9 healthy controls. The difference between normal and PV erythroblasts is not statistically significant. (B) May-Grünwald-Giemsa staining of erythroblasts derived from 1 healthy donor (N) and 1 PV patient (PV) at day 14 of differentiation in the presence of 50 ng/mL anti-CD95 antibody. Images were captured as described in the legend of Figure 1D. (C) Absolute number of mature (orthochromatic) erythroblasts obtained from healthy controls (N) and patients with PV (PV) characterized for the presence of the JAK2 V617F mutation at day 14 of culture in the presence of 50 ng/mL anti-CD95. Horizontal bars indicate the mean value of the N and PV erythroblasts.
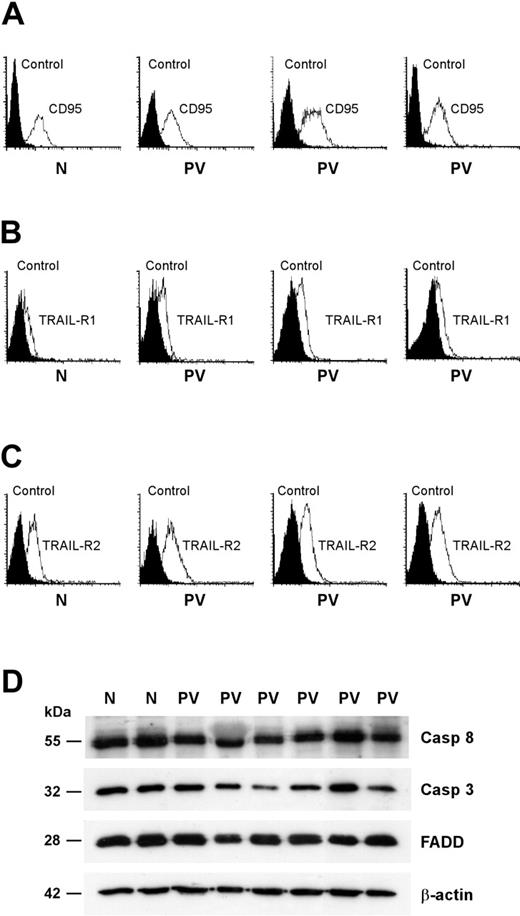
PV erythroblasts have normal expression of death receptors and effectors
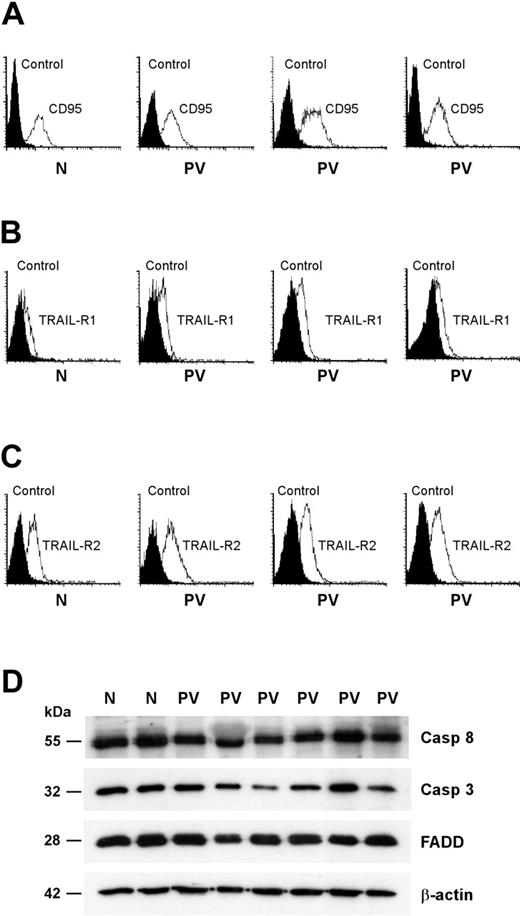
Death receptor trimerization is followed by the formation of a cytoplasmic DISC that is responsible for the activation of executioner caspases and the subsequent cleavage of selected substrates.24 Given that a reduced expression of death receptors or DISC components may be responsible for a defective response to death receptor–mediated signals, we assessed the presence of key components of the CD95 and TRAIL pathways in PV erythroid precursor cells. The expression of CD95 and death domain–containing TRAIL receptors (TRAIL-R1 and TRAIL-R2) was comparable between normal and PV erythroblasts (Figure 5A-C). To rule out the presence of alterations in downstream components of the CD95 signaling pathway, we compared the expression of the DISC-associated proteins FADD and caspase-8 and of the executioner caspase-3 in normal and PV erythroid precursors. Western blot analysis of FADD, caspase-8, and caspase-3 did not reveal gross abnormalities in PV erythroblasts (Figure 5D). We observed small individual variations in the levels of the 3 proteins that, however, were also present among healthy controls (Figure 5D; data not shown).
Cytokine-independent kinase activation and altered regulation of c-FLIPshort expression in PV erythroblasts
The V617F form of JAK2 has been shown to constitutively activate several cytokine-regulated pathways when exogenously expressed in cell lines, resulting in growth factor–independent proliferation and survival.14 Among the effectors that can be activated by JAK2 are ERK/MAP kinases and Akt/PKB, which are implicated in resistance to apoptosis induced by several stimuli, including those generated by death receptor activation. We analyzed the activation of Akt/PKB and ERK/MAP kinases in the presence of different EPO concentrations in erythroblasts obtained from CD34+ cells of healthy donors and patients with PV harboring the JAK2 V617F mutation. In the presence of saturating EPO levels, Akt/PKB and ERK/MAP kinase phosphorylation was comparable between normal and PV erythroblasts. EPO reduction or short-term EPO deprivation produced a progressive decrease in the levels of phosphorylated Akt/PKB and ERK 1/2 in normal erythroblasts (Figure 6A-B). Conversely, PV erythroblasts maintained high levels of phosphorylated Akt/PKB and ERK 1/2 in the presence of low EPO concentrations, even after 24 hours without EPO (Figure 6A-B), a condition producing a 15% ± 5% mortality rate in normal erythroblasts and a 5% ± 5% mortality rate in PV erythroblasts (not shown). Akt/PKB and ERK/MAP kinases have been shown to protect cells from death receptor–induced apoptosis through multiple mechanisms, including the up-modulation of c-FLIP, a key inhibitor of CD95-induced apoptosis.38-40 We have previously demonstrated that exogenous c-FLIP expression protects immature erythroblasts from death receptor–mediated antidifferentiative effect and GATA-1 cleavage.21 Thus, we investigated whether erythroblasts derived from patients with PV had alterations in c-FLIP basal expression or regulation in the presence of different EPO levels. In the presence of saturating EPO concentrations, normal and PV erythroblasts had comparable average levels of the long and the short forms of c-FLIP (Figure 6C; data not shown). When EPO was reduced or removed from the culture medium, the levels of c-FLIP long (c-FLIPlong) were scarcely affected in normal or PV erythroblasts. However, the short form of c-FLIP (c-FLIPshort) rapidly decreased along with EPO concentration in normal but not in PV erythroblasts. The lower responsiveness of c-FLIPshort to EPO variations, which may result from constitutive kinase signaling downstream mutated JAK2, might contribute to death receptor resistance of PV erythroblasts and ultimately lead to a blunted response to death receptor–mediated regulation of erythropoiesis.
Expression of CD95 and TRAIL receptors and effectors in normal and PV erythroblasts. Erythroid precursors derived from CD34+ cells of healthy donors (N) and patients with PV (PV) were cultivated in standard erythroid medium and, at day 8 of differentiation, analyzed for membrane expression of CD95 (A), TRAIL receptor-1 (B), and TRAIL receptor-2 (C). Data are representative of the results obtained with the 13 patients with PV shown in the previous figures. (D) Western blot analysis of the CD95 signal transducers FADD, caspase-3, and caspase-8. Results shown are representative of experiments performed with erythroblasts derived from CD34+ cells of 5 healthy donors and 9 patients with PV.
Expression of CD95 and TRAIL receptors and effectors in normal and PV erythroblasts. Erythroid precursors derived from CD34+ cells of healthy donors (N) and patients with PV (PV) were cultivated in standard erythroid medium and, at day 8 of differentiation, analyzed for membrane expression of CD95 (A), TRAIL receptor-1 (B), and TRAIL receptor-2 (C). Data are representative of the results obtained with the 13 patients with PV shown in the previous figures. (D) Western blot analysis of the CD95 signal transducers FADD, caspase-3, and caspase-8. Results shown are representative of experiments performed with erythroblasts derived from CD34+ cells of 5 healthy donors and 9 patients with PV.
Akt/PKB and ERK kinase phosphorylation and c-FLIP levels in normal and PV erythroblasts. (A) Western blot analysis of phospho-Akt/PKB levels in normal (N) erythroblasts and in erythroblasts derived from patients with PV harboring the JAK2 V617F mutation (PV). Erythroblasts at day 7 of unilineage erythroid culture were incubated for 48 hours in standard erythroid medium with 3 U/mL EPO or 0.5 U/mL EPO or for 24 hours without EPO. (B) Western blot analysis of phospho-ERK 1/2 levels in normal erythroblasts (N) and in erythroblasts derived from patients with PV harboring the JAK2 V617F mutation (PV), treated as in panel A. (C) Expression of the c-FLIPlong (FLIPL) and c-FLIPshort (FLIPS) proteins in normal (N) and PV (PV) erythroblasts. Cells were cultivated for 7 days in standard erythroid medium and treated as in panel A. Results shown in panels A to C are representative of experiments performed with erythroblasts derived from CD34+ cells of 5 healthy donors and 5 patients with PV harboring the JAK2 V617F mutation.
Akt/PKB and ERK kinase phosphorylation and c-FLIP levels in normal and PV erythroblasts. (A) Western blot analysis of phospho-Akt/PKB levels in normal (N) erythroblasts and in erythroblasts derived from patients with PV harboring the JAK2 V617F mutation (PV). Erythroblasts at day 7 of unilineage erythroid culture were incubated for 48 hours in standard erythroid medium with 3 U/mL EPO or 0.5 U/mL EPO or for 24 hours without EPO. (B) Western blot analysis of phospho-ERK 1/2 levels in normal erythroblasts (N) and in erythroblasts derived from patients with PV harboring the JAK2 V617F mutation (PV), treated as in panel A. (C) Expression of the c-FLIPlong (FLIPL) and c-FLIPshort (FLIPS) proteins in normal (N) and PV (PV) erythroblasts. Cells were cultivated for 7 days in standard erythroid medium and treated as in panel A. Results shown in panels A to C are representative of experiments performed with erythroblasts derived from CD34+ cells of 5 healthy donors and 5 patients with PV harboring the JAK2 V617F mutation.
Discussion
Myeloproliferative disorders are characterized by the accumulation of myeloid cells, usually with the prevalence of one lineage over the others. An excessive production of erythrocytes is the hallmark of PV, indicating the presence of alterations in the mechanisms that control erythropoiesis.1,41 In the normal erythroid compartment, multiple signals cooperate to tune erythrocyte production according to physiologic requirements. EPO is the main switch that regulates erythroblast proliferation, promoting the growth and survival of erythroid progenitors and immature precursors.42 A recently proposed model for the negative control of erythropoiesis suggests that TNF family ligands expressed by mature erythroid precursors can interact with their cognate receptors expressed on the surfaces of immature erythroblasts.20,22,23 This interaction results in proliferative and differentiative arrest or apoptosis of immature erythroblasts, depending on the levels of EPO available within the erythroblastic island.20,21 Thus, death receptors may act as an effector arm of EPO fluctuations, influencing the rates of erythroblast expansion and maturation in the bone marrow.
The deregulated erythroid expansion that occurs in PV may be attributed to a greater proliferative ability of PV hematopoietic progenitors or to an increased resistance to signals that restrain erythroblast expansion, such as those mediated by death receptors. Recently, a clonal mutation in the JAK2 gene has been found in most patients with PV, providing the first compelling evidence for a molecular mechanism underlying this disease.14-19 JAK2 is normally activated downstream of the EPO receptor and is responsible for generating signals necessary for erythroblast proliferation and survival. Specifically, JAK2 activates ERK/MAP kinases, PI3 kinase, and STAT5, resulting in cell proliferation and protection from apoptotic stimuli. JAK2 activation resulting from the V617F mutation has been shown to result in sustained phosphorylation of STAT5, Akt/PKB, and ERK kinases in the absence of EPO, which in turn result in growth factor independence of hematopoietic cell lines.14 However, it is unknown whether the activation of multiple pathways by JAK2 V617F in primary erythroid progenitors may interfere with signals responsible for the negative control of erythropoiesis, such as those delivered by death receptors. Previous studies have reported an increased expression of the antiapoptotic protein Bcl-XL in PV erythroid cells,9 which is consistent with the constitutive STAT5 activation produced by mutated JAK2. Because Bcl-XL represents a downstream effector of EPO-mediated survival signals,43-45 it is likely that increased levels of this protein play an important role in sustaining the survival of PV erythroblasts in the presence of suboptimal EPO levels or in the absence of EPO. It has been reported that, in some cell types, increased Bcl-XL expression also confers protection toward death receptor–induced apoptosis.46,47 However, exogenous Bcl-XL expression is unable to protect erythroid precursors from CD95-induced apoptosis (A.Z., unpublished results, October 2002); therefore, this protein represents an unlikely candidate for the protection of PV erythroblasts from death receptor–mediated inhibitory signals.
We have found that erythroblasts derived from patients with PV harboring the JAK2 V617F mutation have an increased resistance to death receptor–induced proliferation arrest and apoptosis and are able to produce higher numbers of mature erythroid cells in the presence of death receptor stimulation. After the activation of CD95 and TRAIL receptors, PV erythroid progenitors derived from patients with the JAK2 V617F mutation showed lower levels of executioner caspase activation and incomplete degradation of the essential erythroid transcription factor GATA-1. The effects of the residual GATA-1 presence in CD95-stimulated PV erythroblasts are difficult to evaluate. However, recent work on the consequences of graded GATA-1 expression in embryonic stem cell–derived erythroid populations showed that, though the complete absence of GATA-1 induces an apoptotic response, reduced GATA-1 levels are compatible with cell survival and proliferation.48 Therefore, it may be speculated that the inefficient caspase-mediated GATA-1 cleavage occurring in CD95-stimulated PV erythroblasts may contribute to the survival and growth of these cells in the presence of death receptor activation.
High levels of EPO, and thus a consistent activation of EpoR downstream signaling pathways, protect immature erythroblasts from death receptor–induced apoptosis.20 In PV erythroblasts derived from patients with the JAK2 V617F mutation, we found a constitutive activation of the downstream kinases Akt/PKB and ERK in the presence of reduced EPO levels and in the absence of EPO. This may result in a proliferative advantage of JAK2-mutated PV erythroblasts and in protection from death receptor–mediated inhibition of erythropoiesis in vivo, in which EPO levels are on the order of 0.02 U/mL.
Three patients who participated in this study had normal JAK2 genes and displayed variable response to death receptor stimulation. Erythroblasts derived from 2 patients with wild-type JAK2 displayed a sensitivity to anti-CD95 and TRAIL similar to that of controls, whereas erythroblasts derived from the third patient were highly resistant to the effects of CD95 and TRAIL receptor stimulation. The molecular causes of erythroid expansion in patients with PV with normal JAK2 are unknown, and several alterations may concur to promote proliferation and apoptosis resistance. However, we found that erythroblasts with wild-type JAK2 that were more sensitive to death receptor activation displayed low endogenous c-FLIP levels, whereas erythroblasts with wild-type JAK2 that were refractory to death receptor stimulation had extremely high levels of c-FLIPlong and c-FLIPshort in basal culture conditions (data not shown). Therefore, it can be hypothesized that c-FLIP overexpression may contribute to death receptor resistance and possibly to deregulated erythropoiesis in some patients with PV with wild-type JAK2.
The fact that the short form of c-FLIP is predominantly affected by EPO concentrations in normal erythroblasts may result from its fast turnover rates, which render c-FLIPshort protein levels extremely responsive to external stimuli.49 Recently, c-FLIPshort has been indicated as the main regulator of CD95-activated signals at the DISC level because its expression correlates with the resistance of T cells to CD95-induced apoptosis.29,30 Interestingly, an increased expression of c-FLIPshort has been recently implicated in the aberrant expansion of autoreactive T lymphocytes in the chronic inflammatory disorder Behçet disease,50 suggesting that c-FLIPshort deregulation may be common to conditions of altered hematopoietic cell proliferation. Because apoptotic defects that affect the death receptor pathway can result in myeloid leukemogenesis,51,52 it would be interesting to investigate death receptor functionality and c-FLIPshort regulation in other myeloproliferative diseases with the JAK2 V617F mutation.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-07-3037.
Supported by the Italian Association for Cancer Research (AIRC). C.M. is the recipient of an Italian Foundation for Cancer Research (FIRC) fellowship.
A.Z. designed the experiments, performed research, and wrote the paper. F.P. performed research and assembled the figures. M.S. performed research and analyzed data. G.R. and C.M. performed research. A.T. provided important analytical tools. G.G. provided blood samples. C.P. supervised the project and critically reviewed the manuscript. R.D.M. designed the project, supervised the work, and critically reviewed the manuscript.
F.P. and M.S. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Paola Di Matteo and Stefano Guida for excellent technical assistance.