Abstract
Reactivation of latent human cytomegalovirus (HCMV) following allogeneic transplantation is a major cause of morbidity and mortality and predisposes to severe complications, including superinfection by Aspergillus species (spp). Antimicrobial polypeptides, including defensins and mannan-binding lectin, are known to block viral fusion by cross-linking sugars on cell surface. Pentraxin 3 (PTX3), a member of the long pentraxin family, successfully restored antifungal immunity in experimental hematopoietic transplantation. We assessed here whether PTX3 binds HCMV and murine virus (MCMV) and the impact on viral infectivity and superinfection in vivo. We found that PTX3 bound both viruses, reduced viral entry and infectivity in vitro, and protected from MCMV primary infection and reactivation as well as Aspergillus superinfection. This occurred through the activation of interferon (IFN) regulatory factor 3 (IRF3) in dendritic cells via the TLR9/MyD88-independent viral recognition sensing and the promotion of the interleukin-12 (IL-12)/IFN-γ–dependent effector pathway.
Introduction
Human cytomegalovirus (HCMV), a member of the Herpesviridae family, is a ubiquitous opportunistic pathogen that has an intimate lifelong relationship with its human host and establishes latency after clearance of primary infection.1-3 Reactivation of latent virus following allogeneic transplantation immune responses results in progressive tissue damage manifesting as overt HCMV disease or complications of this infection, including acute and chronic graft rejection, graft-versus-host disease, and superinfection by other viruses, bacteria, and fungi, particularly Aspergillus species (spp).3 Efforts have focused on the development of adoptive immunotherapeutic strategies to hasten host immune reconstruction, and cellular immunotherapy appears to be an attractive approach.4,5
The immune control of murine CMV (MCMV) infection requires elements from both innate and adaptive immune systems.6-8 Through the participation of members of the Toll-like receptors (TLRs) 9-11 and interferon (IFN) regulatory factor 3 (IRF) families, IRF3 in particular,12,13 MCMV induces early dendritic cell (DC)–dependent type I IFN and interleukin-12 (IL-12) responses essential for mouse resistance to MCMV.10,11,14-16 The TLR9/MyD88 signaling pathway mediates antiviral cytokine responses by plasmacytoid DCs (pDCs) that, through their unique capacity to secrete IFN-α, and to a lesser extent IL-12 and other innate cytokines, are a cornerstone in the initiation of both innate and adaptive immune responses to MCMV.15-19 However, conventional CD11b+ DCs also produce IFN-α independently of TLR9 and MyD88.10,20 In addition to directly interfering with viral replication through ubiquitous cellular mechanisms, IFN-α controls natural killer (NK) cell cytotoxic activity15 and regulates T-cell functions by activating classical DCs to more efficiently present antigens (Ags).15 IL-12 and IL-18 secretion are instead required to prime a strong NK cell–dependent IFN-γ response,17,21,22 a process that is essential to counteract MCMV infection in the liver, in contrast to a perforin-dependent mechanism in the spleen.23
Pentraxin 3 (PTX3) is a member of a superfamily of conserved proteins characterized by a cyclic multimeric structure and a conserved C-terminal domain.24,25 Levels of PTX3, produced by passed different cells, are very low in serum and tissues of healthy subjects but are rapidly increased in response to a variety of inflammatory stimuli.24 PTX3 played a nonredundant role in antifungal immune responses and showed therapeutic activity against aspergillosis in experimental hematopoietic stem cell transplantation (HSCT).26 Except for the potential involvement of PTX3 in dengue and its aptitude to predict more severe disease or poor clinical outcome,27 the role of PTX3 in viral infections has not been studied. Recent studies have demonstrated that antimicrobial polypeptides, including defensins and mannan-binding lectin, may block viral fusion by cross-linking sugars on the surface of cells.28,29 For enveloped viruses, such as herpesviruses, fusion of viral envelope with membrane target cells is a prerequisite for viral replication and infectivity.30
Based on these premises, we sought to determine whether PTX3 could play a role in MCMV infection. For this purpose, PTX3 was assessed for binding to human and murine CMV and inhibition of viral infectivity in vitro as well as in experimental infection or reactivation and ability to activate antiviral programs in DCs.
Materials and methods
Mice
Female 8- to 12-week-old wild-type (WT) inbred C57BL6, 129/Sv, and BALB/c mice were purchased from Charles River Breeding Laboratories (Calco, Italy). Homozygous TLR2 (TLR2–/–)–, TLR3 (TLR3–/–)–, TLR4 (TLR4–/–)–, TLR9 (TLR9–/–)–, MyD88 (MyD88–/–)–, and IL-12p40 (IL-12p40–/–)–deficient mice (on the C57BL6 background), and IFN-γ–/– (on BALB/c background), were bred under specific pathogen–free conditions at the breeding facilities of the University of Perugia, Italy. PTX3–/– (on 129/Sv-C57BL6 mixed background) were bred at the Department of Immunology and Cell Biology, Mario Negri Institute for Pharmacological Research, Milan, Italy). Mice deficient for the IFN-αβ receptor (IFN-αβR–/–; on A129 background) were obtained through Dr Manfred Kopf. Experiments were performed following protocols approved by the institutional animal committee of the University of Perugia, Italy, and in accordance with European Economic Community Council Directive as well as institutional animal care and use guidelines.
Pathogens, infections, and treatments
Stocks of Smith strain MCMV salivary gland extracts were prepared from BALB/c mice and titered in a standard plaque assay on murine embryonic fibroblast (MEF) cells.31 Highly purified HCMV stocks propagated in low-passage human embryonic lung (HEL) fibroblasts were used.32 Low-passage HEL cells and the laboratory type strain AD169 from American Type Culture Collection (VR-538; ATCC, Manassas, VA) were propagated at low virus-to-cell ratios (about 0.001 plaque-forming units [PFUs]/cells) to minimize generation of defective particles. Infections were initiated by intraperitoneal injection of 105 (BALB/c), 5 × 105 (C57BL6, PTX3+/+, and PTX3–/–) PFUs of MCMV. Virus titers were quantified on permissive cells by standard plaque assay on tissues removed at different times. PTX3 (Sigma-Tau, Pomezia, Rome, Italy) was obtained under endotoxin-free conditions by immunoaffinity of culture supernatants of Chinese hamster ovary (CHO) cells transfected with PTX3.26 Gancyclovir (GCV) was from Recordati (Milan, Italy). Both treatments started the day of the infection. For histology, sections of paraffin-embedded tissues were stained with the periodic acid–Schiff procedure. The strain of A fumigatus and the culture conditions were as described.33 MCMV-infected mice received 5 × 105Aspergillus conidia intravenously 2 weeks after the viral infection and subsequent treatment with PTX3 (1 mg/kg) daily for a week. Quantification of fungal growth was done by the chitin assay and results are expressed as micrograms of glucosamine per organ.33
Experimental HSCT model
Recipient mice were exposed to a lethal dose of 8 Gy and infused with 107/mL of T-cell–depleted donor cells (< 1% of contaminating T cells) from donor allogeneic mice, as described.33
MCMV reactivation following HSCT
Mice were infected with MCMV as described in “Pathogens, infections, and treatments”; 3 months later, latency was confirmed by the absence of acute MCMV infection in spleen34 and lung,35 both organs considered primary sites of molecular MCMV latency. Infected mice were used either as recipients of allogeneic donor uninfected bone marrow cells (MCMV+ recipients) or as donors of bone marrow cells (MCMV+ donors) to be injected into uninfected recipients. PTX3 (1 mg/kg) was given daily for 2 weeks, starting the day after HSCT. Dying or surviving mice (killed 30 days after HSCT) were assessed for MCMV viral loads in the lungs by the plaque assay.
DC subset generation
Murine DCs were obtained from bone marrow cells cultured in Iscove modified medium33 in the presence of 150 U/mL mouse recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; Sigma, St Louis, MO) and 75 U/mL rIL-4 (R&D Systems, Minneapolis, MN) for 7 days to obtain CD11b+ DCs or 200 ng/mL FLT3-L (Immunex, Seattle, WA) for 9 days to obtain pDCs.33 Final maturation was done as described.33 CD11b+ DCs were discriminated on CD11chigh expression and were distinctly composed of CD8α+ DCs and CD11b+ DCs. pDCs were defined as CD11blow, Ly6G+ CD8α+/– cells. Spleen DCs were purified by magnetic-activated sorting using CD11c MicroBeads and MidiMacs (Miltenyi Biotec, Bergisch Gladbach, Germany). Photographs were taken using a high-resolution microscopy color camera AxioCam, using AxioVision software Rel. 3.1 (Carl Zeiss, Milan, Italy).
Flow cytometry analyses
After blocking of Fc receptors (FcRs) with the anti-CD16/32 (2.4G2) antibody, cells were analyzed for antigen expression with a FACScan flow cytofluorometer (Becton Dickinson, Mountain View, CA) equipped with CELLQuest software 02-61496-00 Rev A (Becton Dickinson). Control staining of cells with irrelevant antibody (Ab) was used to obtain background fluorescence values. Abs were from Pharmingen (San Diego, CA).
Plaque assay
Plaque assay was determined on cells grown to subconfluence and incubated with serially diluted virus samples for 2 hours at 37°C.36 All organs from uninfected animals were negative viruses. Virus titers are expressed as log10 (mean ± SE).
PTX3 binding assays to immobilized viruses
Plates were coated overnight at 4°C with a 0.05 M carbonate solution containing 104 PFU MCMV. Nonspecific binding sites were blocked by 5% bovine serum albumin in phosphate-buffered saline (PBS). PTX3 binding to HCMV was measured using HCMV Ag-coated plates (AID GmbH, Strasburg, Germany). Binding was performed by mixing viruses and biotin-labeled PTX3 (PTX3bio+) for 2 hours at 37°C. Inhibition was performed by preincubation with unbiotinilated PTX3 (PTX3bio–) for 2 hours at 37°C prior to the addition of PTX3bio+. The optical density at 450 nm was read using the Horseradish Peroxidase Substrate Kit (Bio-Rad Laboratories, Life Science Group, Segrate, Italy). Nonspecific binding of PTX3 to virus-uncoated plates was minimal.
Inhibition of viral replication
MEF and HEL cells (2 × 104/well) grown to subconfluence were either preincubated for 2 hours at 37°C with PTX3 and then added to 104 PFU MCMV or HCMV, respectively, or left untreated and infected with 104 PFU CMV pretreated with PTX3 for 2 hours at 37°C. After a 2-hour adsorption period, the inoculum was removed and cells were washed with PBS and incubated in complete medium. In selected experiments, a PTX3-neutralizing monoclonal antibody (70 ng/100 μL)37 was used to minimize carry-over effects. Infectivity was measured 72 hours later. One well per plate was mock infected and served as a cell control. In the case of DCs, 106/cell were either preincubated with 5 μg/mL PTX3 and then added to 105 PFU MCMV or were untreated and infected with 105 PFU MCMV pretreated with 5 μg/mL PTX3. Infectivity was measured 48 hours later.
Transmission electron microscopy
Electron microscopy on MEF cells exposed for 1 and 2 hours to 104 PFU PTX3-treated MCMV was done as described.38 Sections were examined with a Philips transmission electron microscope (TEM; Philips Medical Systems, Bothell, WA) 208 equipped with a digital camera.
Cytotoxicity assays
NK cells, purified from spleens by DX5 microbeads (Miltenyi Biotec), were defined as NK1.1+CD3– cells. NK cytolytic activity was assessed against 51Cr-labeled YAC-1 lymphoma cells.5 CD8+ and CD4+ T cells were isolated from spleens by positive selection using magnetic-activated cell sorting (MACS) enrichment kits (Miltenyi Biotec). The purity of enriched samples was more than 90%. T-cell cytolytic activity was assessed on 51Cr-labeled, IFN-γ–treated MEF cells as described.39 Cells were infected with 50 PFU virus for 16 hours in the presence of 300 mg/mL phosphonoacetic acid to prevent L gene expression.
Immunoblot analysis of IRF3 phosphorylation
DCs were exposed for 3 hours to 5 μg/mL PTX3 or 105 PFU MCMV, either alone or in combination. Immunoblot analysis was conducted by a standard procedure in conditions in which the phosphorylation status of proteins is maintained.13,40 The antibodies used were rabbit anti-IRF3 and bovine anti–rabbit horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). This procedure allows the simultaneous detection of the 2 nonactivated IRF3 forms (I and II) and the activated, C-terminally phosphorylated IRF3 (P). Visualization was performed with the electrochemiluminescence (ECL) Western blotting analysis system from Amersham (Arlington Heights, IL) and Kodak Biomax films (Kodak, Rochester, NY).
Quantification of CMV mRNA and DNA
A highly sensitive reverse transcription–polymerase chain reaction (RT-PCR) assay was used for amplification of the 356-bp segment of MCMV glycoprotein B (gB) DNA from total cellular RNA.41 Synthesis and PCR of cDNA were done as described.33 Synthetic DNA oligonucleotide primers were selected from the published sequence of the MCMV gB gene.9 The sense and antisense primers were as follows: 5′-AAG-CAG-CAC-ATC-CGC-ACC-CTG-AGC-GCC-3′ (sense), and 5′-CCA-GGC-GCT-CCC-GGC-GGC-CCG-CTC-TCG-3′ (antisense). Cycling conditions were initial denaturation for 3 minutes at 95°C, followed by cycles of 1 minute at 95°C, 1 minute at 50°C, and 20 seconds at 72°C, and a final extension for 10 minutes at 72°C. To determine HCMV DNA copy number, total DNA was isolated as described32 and viral DNA levels were measured by real-time RT-PCR using the Q-CMV Real Time Complete Kit (Nanogen Advanced Diagnostics, Turin, Italy), which amplifies the major immediate early antigen, HCMVUL123. The RT-PCR products were detected by measuring fluorescence with passive reference dye in Sequence Detection System ABI Prism 7300 (Applied Biosystems-Applera Italia-Monza, Milan, Italy). Control threshold values were calculated by determining the point at which the fluorescence exceeded a background limit of 0.04. Distilled water also served as a negative control. Amounts of genomic equivalents (GE)/mL in each sample were determined by means of a quantification software (ABI Prism 7300).
Quantification of cytokines by real-time RT-PCR, ELISA, and ELISPOT assay
Real-time RT-PCR was performed using the iCycler iQ detection system (Bio-Rad Laboratories, Hercules, CA) and SYBR Green chemistry (Finnzymes Oy, Espoo, Finland).42 Cell were lysed and total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Milan, Italy) and was reverse transcribed with Sensiscript Reverse Transcriptase (QIAGEN) according to the manufacturer's directions. PCR primers were obtained from Invitrogen (Carlsbad, CA). The PCR primers used were: forward primer, 5′-CACCCTTGCCCTCCTAAACC, and reverse primer, 5′-CAAGGCACAGGGTCATCATC, for IL-12p35; forward primer, 5′-GCACTGGGTGGAATGAGACT and reverse primer 5′-AGTGGAGAGCAGTTGAGGACA, for IFN-β; and forward primer, 5′-CGCAAAGACCTGTATGCCAAT and reverse primer, 5′-GGGCTGTGATCTCCTTCTGC for γ-actin. PCR amplification of the housekeeping γ-actin gene was performed for each sample to control for sample loading and to allow normalization between samples as per the manufacturer's instructions (Applied Biosystems). Water controls were included to ensure specificity. The thermal profile for SYBR Green real-time PCR was 95°C for 3 minutes, followed by 40 cycles of denaturation for 15 seconds at 95°C and an annealing/extension step of 1 minute at 60°C. Each datapoint was examined for integrity by analysis of the amplification plot. The mRNA-normalized data were expressed as relative cytokine mRNA (ΔΔCt) in treated cells compared with that of mock-infected cells. The levels of cytokines in the culture supernatants of mitogen-stimulated spleen cells (48 hours of stimulation with 10 μg/mL concanavalin A [ConA]) or MCMV-pulsed DCs (24 hours) were determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems and PBL Biomedical Lab, Milan, Italy). The detection limits of the assays were less than 16 pg/mL for IL-12 p70, less than 10 pg/mL for IFN-γ, less than 3 pg/mL for IL-10, and less than 10 pg/mL for IFN-α. IFN-γ–producing cells were enumerated by enzyme-linked immunospot (ELISPOT) assay on purified cells from spleens.33 Results are expressed as the mean number of cytokine-producing cells (± SE) per 105 cells, calculated using replicates of serial 2-fold dilutions of cells.
Quantification of PTX3 by ELISA
Quantification of PTX3 in sera, lung homogenates (a week after the infection), and culture supernatants of DCs was done by ELISA.25
Statistical analyses
Student paired t test was used to determine the significance of values in experimental groups (significance was defined as P < .05). Survival data were analyzed using the Mann-Whitney U test. In vivo groups consisted of 6 animals. Unless otherwise indicated, data are mean plus or minus SE.
Results
PTX3 binds CMV, inhibits viral infection in vitro, and activates IRF3
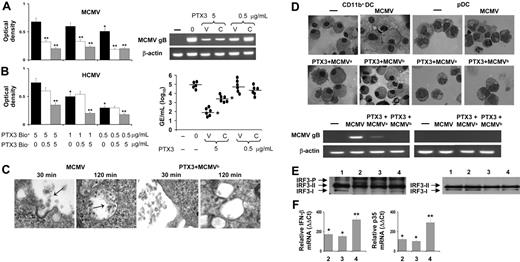
To test whether PTX3 affects CMV infection in vitro, we evaluated the ability of PTX3 to bind to HCMV or MCMV, the effects of PTX3 exposure on productive infection of permissive MEF and HEL cells, respectively, and the effects of cell treatment with PTX3 on subsequent viral infection. PTX3 bound both HMCV and MCMV in a dose-dependent manner and the binding was significantly reduced in the presence of unlabeled PTX3 (Figure 1A-B). The binding of PTX3 to HCMV was not inhibited in the presence of human antibodies directed against the 150-kDa (late), 65- and 52-kDa (early), or 28-kDa (specific) antigens (data not shown), a finding suggesting the diversity of viral molecules recognized by PTX3 and human-specific antibodies. Exposure to PTX3 appeared to inhibit viral cell fusion and internalization as revealed by electron microscopy (Figure 1C). This resulted in a strong dose-dependent inhibition of viral infection, as assessed by the reduced levels of MCMV gB transcripts in MEF cells (Figure 1A) or HCMV genomes (Figure 1B) on HEL cells paralleled by reduced infectious viral particles in culture supernatants (data not shown). The inhibitory effect was rapid, and inactivation was obtained already after 30 to 45 minutes of exposure (data not shown). Interestingly, pretreatment of cells with the highest concentration of PTX3 also inhibited the infection. Because experiments in which residual PTX3 was neutralized by specific antibodies ruled out the possible carry over effects of free PTX3 on either cells or the virus (data not shown), these findings suggest that PTX3 affects viral infectivity as well as the permissivity of cells to the infection.
PTX3 binds to CMV, inhibits viral replication, and activates IRF3. Binding of biotin-labeled PTX3 (PTX3bio+) to MCMV (A) or HCMV (B) virus. Different concentrations of unbiotinylated PTX3 (PTX3bio–) were added for 2 hours at 37°C to MCMV Ag-coated or HCMV-coated plates followed by the addition of different concentrations of PTX3bio+ for an additional 2 hours at 37°C. The optical density at 450 nm was read using the Horseradish Peroxidase Substrate Kit. *P < .05, 1 or 0.5 μg/mL vs 5 μg/mL PTX3bio+;**P < .05, PTX3bio+ with and without PTX3bio–. Error bars indicate SE. For inhibition of viral replication, MEF or HEL cells were (0) infected with MCMV or HCMV, respectively; (V) untreated and infected with PTX3-treated CMV; or (C) pretreated with PTX3 and added to untreated CMV. MCMV gB transcript expression or amounts of genomic equivalents (GE)/mL of HCMV DNA were assessed by RT-PCR 72 hours after the infection. The results shown are from 1 representative experiment out of 3. (C) Electron microscopy of MEF cells after 30 or 120 minutes' exposure to untreated or PTX3-treated MCMVs (2 hours at 37°C; PTX3 + MCMVb). Arrows indicate virions. Magnification (right to left), × 36 000; × 23 000; × 29 000; and × 19 000. (D) CD11b+ DCs or pDCs, generated from bone marrow progenitors of BALB/c mice, were mock infected (–), infected with untreated (MCMV) or PTX3-treated virions (PTX3 + MCMVb), or pretreated with PTX3 (PTX3 + MCMVa) and then infected before being assessed, 48 hours later, for morphology by light microscopy and viral replication by RT-PCR. Images were visualized with a 100×/1.25 NA oil-immersion objective lens, using cedar oil. (E) IRF3 phosphorylation in CD11b+ DCs or pDCs mock-infected (lane 1) or after 3 hours' exposure to MCMV (lane 2), PTX3 (lane 3), or both (lane 4). The 2 nonactivated IRF3 forms (I and II) and the activated, C-terminally phosphorylated IRF3 (P) are indicated. (F) Induction of Ifnγ and Il12p35 mRNA in CD11b+ DCs after 3 hours' exposure to MCMV (lane 2), PTX3 (lane 3), or both (lane 4). Data are expressed as relative cytokine mRNA (ααCt) in treated cells compared with that of mock-infected cells.
PTX3 binds to CMV, inhibits viral replication, and activates IRF3. Binding of biotin-labeled PTX3 (PTX3bio+) to MCMV (A) or HCMV (B) virus. Different concentrations of unbiotinylated PTX3 (PTX3bio–) were added for 2 hours at 37°C to MCMV Ag-coated or HCMV-coated plates followed by the addition of different concentrations of PTX3bio+ for an additional 2 hours at 37°C. The optical density at 450 nm was read using the Horseradish Peroxidase Substrate Kit. *P < .05, 1 or 0.5 μg/mL vs 5 μg/mL PTX3bio+;**P < .05, PTX3bio+ with and without PTX3bio–. Error bars indicate SE. For inhibition of viral replication, MEF or HEL cells were (0) infected with MCMV or HCMV, respectively; (V) untreated and infected with PTX3-treated CMV; or (C) pretreated with PTX3 and added to untreated CMV. MCMV gB transcript expression or amounts of genomic equivalents (GE)/mL of HCMV DNA were assessed by RT-PCR 72 hours after the infection. The results shown are from 1 representative experiment out of 3. (C) Electron microscopy of MEF cells after 30 or 120 minutes' exposure to untreated or PTX3-treated MCMVs (2 hours at 37°C; PTX3 + MCMVb). Arrows indicate virions. Magnification (right to left), × 36 000; × 23 000; × 29 000; and × 19 000. (D) CD11b+ DCs or pDCs, generated from bone marrow progenitors of BALB/c mice, were mock infected (–), infected with untreated (MCMV) or PTX3-treated virions (PTX3 + MCMVb), or pretreated with PTX3 (PTX3 + MCMVa) and then infected before being assessed, 48 hours later, for morphology by light microscopy and viral replication by RT-PCR. Images were visualized with a 100×/1.25 NA oil-immersion objective lens, using cedar oil. (E) IRF3 phosphorylation in CD11b+ DCs or pDCs mock-infected (lane 1) or after 3 hours' exposure to MCMV (lane 2), PTX3 (lane 3), or both (lane 4). The 2 nonactivated IRF3 forms (I and II) and the activated, C-terminally phosphorylated IRF3 (P) are indicated. (F) Induction of Ifnγ and Il12p35 mRNA in CD11b+ DCs after 3 hours' exposure to MCMV (lane 2), PTX3 (lane 3), or both (lane 4). Data are expressed as relative cytokine mRNA (ααCt) in treated cells compared with that of mock-infected cells.
Acute infection with MCMV induces a transient, but profound, immunosuppression in susceptible BALB/c mice, which can be linked to infection of CD11b+ DCs.14 CD11b+ DCs support productive infection of MCMV both in vitro and in vivo,14 whereas MCMV does not replicate in pDCs.16 To assess whether PTX3 would affect MCMV infection and the antiviral program of DC, PTX3-treated MCMV was added to CD11b+ DCs and pDCs from BALB/c mice, and infectivity was assessed in terms of viral replication. The results showed that MCMV replicates in CD11b+ DCs. However, PTX3 treatment of either the virus or DCs greatly reduced viral replication. No viral replication whatsoever could be revealed in pDCs (Figure 1D). PTX3 also reduced viral replication in CD11b+ DCs derived from C57BL6 mice and, interestingly, in conventional DCs derived from human peripheral CD14+ cells cultured in GMCSF + IL-4 (data not shown).
CMV is known to either activate13 or subvert43 IRF3 that, upon activation, localizes to the nucleus, where it promotes transcription of antiviral genes necessary for host response to the virus.13 We verified whether PTX3 would affect IRF3 activation by assessing the phosphorylation status in response to the virus. Western blot analysis showed that along with the 2 species (I and II) of IRF3, an activated, phosphorylated form (P) of IRF3 was also present in CD11b+ DCs, but not pDCs, exposed to the virus or PTX3, either alone or in combination (Figure 1E). IRF3 initiates transcription of a subset of interferon-stimulated genes, including Ifnβ12,13 and Il12p35.44 Both IFN-β and IL-12p35 mRNAs were up-regulated in CD11b+ DCs upon exposure to either the virus or PTX3 separately or to PTX3-treated virus (Figure 1F), a finding suggesting that IFR3 activation is associated with the initiation of antiviral programs in CD11b+ DCs. These results suggest that PTX3 may prevent MCMV infection by inhibiting viral entry, curtailing the subsequent stages of the infection and activating the antiviral program of CD11b+ DCs.
PTX3 protects from CMV infection and reactivation in vivo
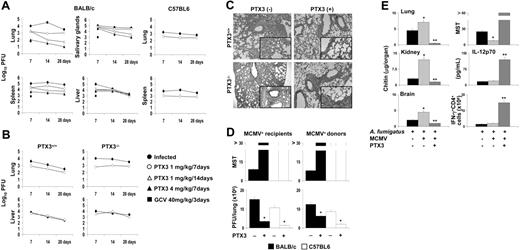
To assess whether PTX3 would have antiviral effects in vivo, PTX3 was administered to susceptible (BALB/c) or resistant (C57BL6) mice with primary MCMV infection or reactivation. Mice were infected with a sublethal dose of MCMV, treated with different doses of PTX3 or GCV, and the titer loads in spleen, lung, liver, and salivary glands were determined at different weeks after the infection (Figure 2A). In line with previous reports,41 MCMV replicated to high titers in the visceral organs of susceptible BALB/c than C57BL6 mice, particularly in the early phase of the infection. PTX3, however, significantly and persistently decreased the viral load, particularly in the lung and spleen, where the effect was similar to that of GCV a week after the infection. The antiviral effect was more pronounced in the lung and spleen of susceptible (more than 2 logs of difference) than resistance mice. The viral titer in the livers of C57BL6-resistant mice was lower than that of susceptible BALB/c mice and was almost unaffected by PTX3 treatment (data not shown). Prolonged treatment (2 weeks) with PTX3 was more effective, particularly in the lung and spleen (Figure 2A). Treatment with PTX3 also ameliorated inflammatory pathology and cellular recruitment in lung, spleen, and liver of susceptible mice (data not shown). These results suggest that PTX3 could be an important component of the host antiviral immune response. To directly address this issue, we measured levels of PTX3 produced during infection and assessed the susceptibility of PTX3–/– mice to MCMV as well as the responsiveness to exogenous PTX3. Circulating levels of PTX3 were not increased after the infection (from 16.0 to 16.7 ng/mL in BALB/c and from 14.0 to 16.0 ng/mL in C57BL6 mice). However, local levels in the lung were significantly increased, particularly in BALB/c mice (from 0.5 to 2.13 ng/mL). Consistent with these findings, PTX3–/– mice were more susceptible to infection than PTX3+/+ mice, particularly in the lung, where viral titer was greatly reduced upon treatment with PTX3. PTX3 did not modify the low viral titer in the liver of PTX3–/– mice (Figure 2B), as opposed to the great reduction in the salivary glands (data not shown). Histologic examination of lung of infected mice revealed a more severe inflammatory pathology in PTX3–/– than PTX3+/+ mice, consisting of heavy cellular recruitment associated with signs of parenchimal destruction, peribrochial fibrosis, and Globet cell hyperplasia. In both types of mice, however, treatment with PTX3 greatly ameliorated the inflammatory response (Figure 2C). Together, these data suggest that PTX3 contributes to host immune response to MCMV and that exogenous supply of PTX3 may have decisive antiviral effects.
PTX3 protects from CMV infection and reactivation in vivo. (A-B) BALB/c or C57BL6, PTX3+/+, and PTX3–/– mice were infected intraperitoneally with MCMV. Virus titers, expressed as log10 (mean ± SE), were quantified on MEF cells by standard plaque assay on tissues removed at different times. PTX3 and GCV were administered intraperitoneally beginning on the day of the infection. Controls received the diluent alone. Results are representative of 4 independent experiments. (C) Histologic analysis of periodic acid–Schiff–stained lung sections from PTX3+/+ and PTX3–/– mice infected with MCMV and treated (+) or not (–) with PTX3 for a week. Cellular recruitment associated with signs of parenchimal destruction, peribrochial fibrosis, and Globet cell hyperplasia (magnified × 20 in the insets; a 20×/0.45 objective lens was used) were seen in PTX3–/– more than PTX3+/+ mice and were ameliorated by PTX3 treatment. Histology was done 1 day after treatment. Magnification, × 10 in all panels; obtained with a10×/0.25 objective lens. (D) BALB/c or C57BL6 mice were infected with MCMV as described for panel A. Three months later, MCMV latency was confirmed by the absence of acute MCMV infection in spleen and lung. Infected mice were used either as recipients of allogeneic donor uninfected bone marrow cells (MCMV+ recipients) or as donors of bone marrow cells (MCMV+ donors) to be injected into uninfected recipients. PTX3 (1 mg/kg intraperitoneally) was given daily for 2 weeks, starting the day after HSCT. Dying or surviving mice (killed 30 days after HSCT) were assessed for MCMV viral loads in the lungs by the plaque assay. MST indicates median survival time (days). Bars indicate the standard errors. *P < .05, treated versus untreated mice. (E) MCMV-infected BALB/c mice received Aspergillus conidia intravenously 2 weeks after the viral infection and subsequent treatment with PTX3 (1 mg/kg intraperitoneally) daily for 1 week. Quantification of fungal growth was done 3 days after infection by the chitin assay and results are expressed as chitin content (micrograms of glucosamine/organ). MST indicates median survival time. IL-12p70 levels were assessed in lung homogenates and the frequency of IFN-γ–producing cells was assessed in purified CD4+ T cells from spleens 3 days after Aspergillus-infection by specific ELISA or ELISPOT assays. Bars indicate the standard errors. *P < .05, MCMV-infected versus uninfected mice. **P < .05, PTX3-treated versus untreated MCMV-infected mice.
PTX3 protects from CMV infection and reactivation in vivo. (A-B) BALB/c or C57BL6, PTX3+/+, and PTX3–/– mice were infected intraperitoneally with MCMV. Virus titers, expressed as log10 (mean ± SE), were quantified on MEF cells by standard plaque assay on tissues removed at different times. PTX3 and GCV were administered intraperitoneally beginning on the day of the infection. Controls received the diluent alone. Results are representative of 4 independent experiments. (C) Histologic analysis of periodic acid–Schiff–stained lung sections from PTX3+/+ and PTX3–/– mice infected with MCMV and treated (+) or not (–) with PTX3 for a week. Cellular recruitment associated with signs of parenchimal destruction, peribrochial fibrosis, and Globet cell hyperplasia (magnified × 20 in the insets; a 20×/0.45 objective lens was used) were seen in PTX3–/– more than PTX3+/+ mice and were ameliorated by PTX3 treatment. Histology was done 1 day after treatment. Magnification, × 10 in all panels; obtained with a10×/0.25 objective lens. (D) BALB/c or C57BL6 mice were infected with MCMV as described for panel A. Three months later, MCMV latency was confirmed by the absence of acute MCMV infection in spleen and lung. Infected mice were used either as recipients of allogeneic donor uninfected bone marrow cells (MCMV+ recipients) or as donors of bone marrow cells (MCMV+ donors) to be injected into uninfected recipients. PTX3 (1 mg/kg intraperitoneally) was given daily for 2 weeks, starting the day after HSCT. Dying or surviving mice (killed 30 days after HSCT) were assessed for MCMV viral loads in the lungs by the plaque assay. MST indicates median survival time (days). Bars indicate the standard errors. *P < .05, treated versus untreated mice. (E) MCMV-infected BALB/c mice received Aspergillus conidia intravenously 2 weeks after the viral infection and subsequent treatment with PTX3 (1 mg/kg intraperitoneally) daily for 1 week. Quantification of fungal growth was done 3 days after infection by the chitin assay and results are expressed as chitin content (micrograms of glucosamine/organ). MST indicates median survival time. IL-12p70 levels were assessed in lung homogenates and the frequency of IFN-γ–producing cells was assessed in purified CD4+ T cells from spleens 3 days after Aspergillus-infection by specific ELISA or ELISPOT assays. Bars indicate the standard errors. *P < .05, MCMV-infected versus uninfected mice. **P < .05, PTX3-treated versus untreated MCMV-infected mice.
As reactivation of latent HCMV following allogeneic transplantation is a major clinical problem,3,45 the effect of PTX3 was also assessed in MCMV reactivation in experimental HSCT. As HCMV seropositivity of either donor or recipient could be associated with an increased risk of immune-mediated complications,1 the activity of PTX3 was assessed in either MCMV+ recipients or MCMV+ donors, using either susceptible or resistant mice. In each combination, MCMV reactivation occurred within 10 to 20 days after engraftment, as revealed by the decreased survival and the elevated viral replication in the lung. Treatment with PTX3, however, completely prevented viral reactivation, as revealed by long-term survival and almost absent viral replication (Figure 2D).
PTX3 protects MCMV-infected mice from invasive pulmonary aspergillosis
HCMV reactivation predisposes to severe complications, including superinfection by Aspergillus spp.46 We have already shown that PTX3 plays a nonredundant role in host antifungal immunity and that PTX3 treatment prevented aspergillosis in experimental HSCT.26 To assess whether treatment of MCMV-infected mice with PTX3 also decreases the risk of invasive aspergillosis, MCMV-infected mice were treated with PTX3 for a week and infected with Aspergillus conidia intravenously a week later. Mice were monitored for survival, fungal growth, and activation of protective antifungal T-helper 1 (Th1) responses, such as IL-12p70 production and frequency of IFN-γ–producing CD4+ T cells. The results showed that preinfection with MCMV increased fungal infectivity, as revealed by increased fungal burden in target organs and decreased survival. PTX3 treatment, however, rescued the mice from the infection, as indicated by the long-term survival, the almost completely reduced fungal growth, and the restoration of protective antifungal Th1 resistance, as revealed by the increased IL-12 production and frequency of IFN-γ–producing splenic CD4+ T cells (Figure 2E).
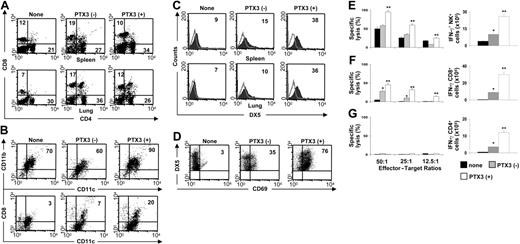
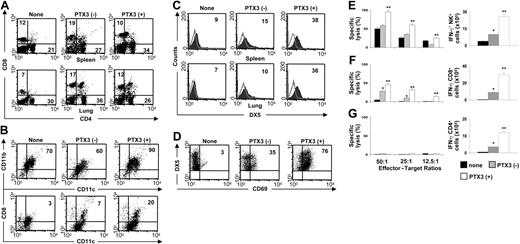
PTX3 supports DC, NK cell, and T-cell activation in vivo. Phenotypic analysis of total spleen and lung cells (A,C), spleen DCs (B), and spleen NK cells (D) from untreated MCMV-infected BALB/c mice (–) or mice a day after 1 week of treatment (+) with PTX3 (1 mg/kg intraperitoneally). None indicates uninfected mice. Numbers refer to the percentage of positive cells on FACS analysis. Histograms are representative of 1 of 4 independent experiments. Open areas represent control cells stained with irrelevant antibody. (E) Cytotoxic activity (by standard 51Cr-release assay against YAC-1 targets) and frequency of IFN-γ–producing splenic NK cells by ELISPOT assay from BALB/c mice infected and treated as described in “Pathogens, infections, and treatment.” (F-G) Cytotoxic activity (against 51Cr-labeled MEF cells) and frequency of IFN-γ–producing splenic CD8+ or CD4+ T cells by ELISPOT assay from BALB/c mice infected and treated as described in “Pathogens, infections, and treatment.” Bars indicate the standard errors. *P < .05, infected versus uninfected mice. **P < .05, PTX3-treated versus untreated infected mice. The results shown represent 3 representative experiments of 5.
PTX3 supports DC, NK cell, and T-cell activation in vivo. Phenotypic analysis of total spleen and lung cells (A,C), spleen DCs (B), and spleen NK cells (D) from untreated MCMV-infected BALB/c mice (–) or mice a day after 1 week of treatment (+) with PTX3 (1 mg/kg intraperitoneally). None indicates uninfected mice. Numbers refer to the percentage of positive cells on FACS analysis. Histograms are representative of 1 of 4 independent experiments. Open areas represent control cells stained with irrelevant antibody. (E) Cytotoxic activity (by standard 51Cr-release assay against YAC-1 targets) and frequency of IFN-γ–producing splenic NK cells by ELISPOT assay from BALB/c mice infected and treated as described in “Pathogens, infections, and treatment.” (F-G) Cytotoxic activity (against 51Cr-labeled MEF cells) and frequency of IFN-γ–producing splenic CD8+ or CD4+ T cells by ELISPOT assay from BALB/c mice infected and treated as described in “Pathogens, infections, and treatment.” Bars indicate the standard errors. *P < .05, infected versus uninfected mice. **P < .05, PTX3-treated versus untreated infected mice. The results shown represent 3 representative experiments of 5.
PTX3 recovers DC/NK-cell reactivity and activates T cells in MCMV infection
One of the most striking features of MCMV infection of susceptible BALB/c mice is the early disappearance of CD8α+ DCs from the spleen, likely due to the lack of NK cells supporting this DC subset.14,47 The expansion of both CD8α+ DCs and Ly49H+ NK-cell populations are indeed reciprocally regulated in infection.48 We looked therefore for the effect of PTX3 on the expansion and functional activity of DC subsets and NK cells in the spleen and lung of MCMV-infected mice. Figure 3 shows that PTX3 treatment, while not affecting the expansion of CD4+ or CD8+ T cells in both organs (Figure 3A), expanded CD11b+ DCs and CD8α+ DC subsets in the spleen (Figure 3B), and NK1.1+ NK cells in both spleen and lung (Figure 3C). NK cells were fully activated as revealed by the increased expression of the activation marker CD69 (Figure 3D). The frequency of IFN-γ–producing cells and cytotoxic activity of ex vivo–purified splenic NK cells were both significantly up-regulated upon PTX3 treatment (Figure 3E).
In primary infected BALB/c mice, CD8+ T cells play a major and protective role by clearing productive infections in visceral organs, with the exception of the epithelial cells of the salivary glands, where persistent productive infection is controlled by CD4+ T cells and IFN-γ.49 To evaluate whether treatment with PTX3 also affects CD8+ and CD4+ T-cell–dependent antiviral activity, we assessed the cytotoxic and IFN-γ–producing activity of purified splenic CD8+ and CD4+ T cells from infected mice after PTX3 treatment. Figure 3F and 3G show that both activities were up-regulated in CD8+ T cells from PTX3-treated mice, whereas the IFN-γ–producing activity was increased only in CD4+ T cells. Therefore, despite the fact that the absolute number was not increased, the effector antiviral activity of both T-cell subsets was increased by treatment with PTX3. Together, these findings suggest an activity of PTX3 on both the innate and adaptive antiviral immune responses.
PTX3 promotes cytokine production in MCMV infection
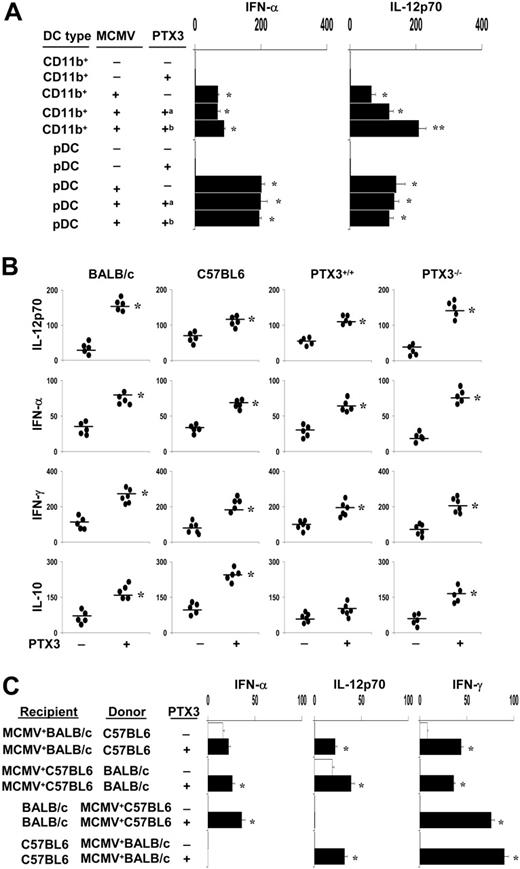
As early activation of NK cells in MCMV infection is mediated by IFN-α/β, which promotes cytotoxicity and proliferation of NK cells, and IL-12, which induces IFN-γ production,15-17,19,21 we evaluated the pattern of cytokine production by DC subsets exposed to MCMV in the presence of PTX3. We resorted to bone marrow–derived CD11b+ DC and pDC subsets from uninfected BALB/c mice to distinguish between the effect of PTX3 on DCs and on the virus itself. DCs were either pretreated with PTX3 before viral infection or untreated and infected with PTX3-treated MCMV. In line with previous findings,20 both DC subsets produced IFN-α and IL-12p70 in response to the virus, although pDCs produced more than CD11b+ DCs. PTX3 increased both cytokine productions, but particularly IL-12p70, either after cell or viral treatment but only on CD11b+ DCs (Figure 4A). Similar effects were induced, although to a lesser extent, in DCs from C57BL6 mice (data not shown). These data, together with those of Figure 1, indicate that PTX3 neither affects infectivity nor the activation program of pDCs in response to MCMV, as opposed to CD11b+ DCs, in which infectivity and cytokine production were greatly affected by PTX3.
To correlate the pattern of cytokine production in vitro with that occurring in vivo, we measured IL-12p70, IFN-α, IFN-γ, and IL-10 production in culture supernatants of spleen cells from mice with primary MCMV infection and treated with PTX3. We also compared levels of cytokine production between susceptible and resistant mice as well as PTX3–/– and PTX3+/+ mice. We found that treatment with PTX3 resulted in an increased production of all cytokines, but particularly IL-12, in both susceptible (BALB/c and PTX3–/–) and resistant (C57BL6 and PTX3+/+) mice, although to a lesser degree in the latter (Figure 4B). Together, these data suggest that PTX3 promotes the IL-12–dependent more than the IFN-α–dependent pathway in response to MCMV. This was also confirmed in the reactivation model in which protection by PTX3 correlated with the activation of the IL-12p70/IFN-γ–dependent pathway, particularly in the condition of seropositivity of the recipients (Figure 4C).
PTX3 promotes cytokine production. (A) CD11b+ DCs or pDCs, generated from bone marrow progenitors of BALB/c mice, were pre-exposed to 5 μg/mL PTX3 before infection (a) or were untreated and infected with 5 μg/mL PTX3-treated virus (b). Cytokines were determined in culture supernatants by ELISA assay and are expressed as picograms per milliliter. Bars indicate the standard errors. *P < .05, cytokine production in MCMV-infected DCs versus uninfected DCs. **P < .05, DCs infected with PTX3-treated virions versus PTX3-treated DCs. (B) Cytokine production in MCMV infection. Cytokine levels (pg/mL) in culture supernatants of spleen cells from mice with primary MCMV infection and treated with PTX3. *P < .05, PTX3 treated versus untreated mice. (C) Cytokine production in the MCMV reactivation model. BALB/c or C57BL6 mice were infected with MCMV. Infected mice were used either as recipients of allogeneic donor uninfected bone marrow cells (MCMV+ recipients) or as donors of bone marrow cells (MCMV+ donors) to be injected into uninfected recipients. PTX3 (1 mg/kg intraperitoneally) was given daily for 2 weeks, starting the day after HSCT. Cytokine (pg/mL) levels in culture supernatants of spleen cells were determined by ELISA assay. Bars indicate the standard errors. *P < .05, PTX3 treated versus untreated mice.
PTX3 promotes cytokine production. (A) CD11b+ DCs or pDCs, generated from bone marrow progenitors of BALB/c mice, were pre-exposed to 5 μg/mL PTX3 before infection (a) or were untreated and infected with 5 μg/mL PTX3-treated virus (b). Cytokines were determined in culture supernatants by ELISA assay and are expressed as picograms per milliliter. Bars indicate the standard errors. *P < .05, cytokine production in MCMV-infected DCs versus uninfected DCs. **P < .05, DCs infected with PTX3-treated virions versus PTX3-treated DCs. (B) Cytokine production in MCMV infection. Cytokine levels (pg/mL) in culture supernatants of spleen cells from mice with primary MCMV infection and treated with PTX3. *P < .05, PTX3 treated versus untreated mice. (C) Cytokine production in the MCMV reactivation model. BALB/c or C57BL6 mice were infected with MCMV. Infected mice were used either as recipients of allogeneic donor uninfected bone marrow cells (MCMV+ recipients) or as donors of bone marrow cells (MCMV+ donors) to be injected into uninfected recipients. PTX3 (1 mg/kg intraperitoneally) was given daily for 2 weeks, starting the day after HSCT. Cytokine (pg/mL) levels in culture supernatants of spleen cells were determined by ELISA assay. Bars indicate the standard errors. *P < .05, PTX3 treated versus untreated mice.
The efficacy of PTX3 depends on the IL-12p70/IFN-γ–dependent pathway
To directly evaluate the relative role of IFN-α, IL-12p70, and IFN-γ production in the protective efficacy of PTX3 in the acute MCMV infection, we assessed the relative efficacy of PTX3 in mice deficient for IFN-γ, IL-12p40, and IFN-αβR. As already reported,15-17,19 deficiency in IFN-γ or IFN-αβR greatly increased the susceptibility to the infection, as revealed by more than 1 log increase of the viral load in the lung compared with the corresponding WT mice. In contrast, deficiency of IL-12p40 did not increase significantly the viral load (from 3.4 × 103 to 4.2 × 103, WT versus IL-12p40–/– mice; Figure 5). PTX3 inhibited by more than 1 log the viral load in IFN-αβR–/–, an effect that was superior to that seen in WT mice. In contrast, the inhibitory activity was significantly reduced in IFN-γ–/– or IL-12p40–/– compared with the corresponding WT control mice (from 6.3 to 6 log10 in IFN-γ–/– vs 4.8 to 3.4 log10 in BALB/c WT, and from 3.6 to 3.4 log10 in IL-12p40–/– vs 3.4 to 2.9 log10 in C57BL6 WT after PTX3 treatment; Figure 5). Both IL-12 and IFN-γ were produced at high levels in IFN-αβR–/– mice treated with PTX3 (Figure 5), a finding confirming the preminent role of the IL-12p70/IFN-γ axis in the protective effect of PTX3.
The efficacy of PTX3 is TLR9/MyD88 independent but TLR2/TLR3 /TLR4 dependent
Effective anti-MCMV immune surveillance required functional TLR2, TLR3, and TLR9 signaling through MyD88-dependent and -independent pathways.7,9-11 To assess the role of TLR and MyD88 in the efficacy of PTX3 in the acute infection, TLR- or MyD88-deficient mice were challenged with MCMV and followed for viral replication in the lung. In accordance with published data,9-11 TLR9–/–, MyD88–/–, and TLR3–/– mice were more susceptible to MCMV than C57BL6 mice, while deficiency for TLR2 and TLR4 did not significantly affect mouse resistance (Figure 5). Not only was PTX3 still effective in TLR9–/– and MyD88–/– mice, but its efficacy was apparently increased, particularly in MyD88–/– mice, compared with C57BL6 mice (from 3.4 to 2.9 log10 in WT vs 4.8 to 3.2 log10 in MyD88–/– mice and 3.9 to 3 log10 in TLR9–/– mice after PTX3 treatment). In contrast, PTX3 was completely ineffective in TLR3–/– mice, a finding implicating TLR3 as a major factor contributing to both susceptibility to the infection and PTX3 activity. Interestingly, PTX3 was completely ineffective in TLR2–/– or TLR4–/– mice, a finding suggesting the possible involvement of these TLRs also in the activation of the antiviral immune response by PTX3. Here again, the efficacy of PTX3 directly correlated with levels of IL-12 and IFN-γ that were significantly increased in supernatants of splenocytes from MyD88–/– and TLR9–/– mice, whose production was low otherwise, as already shown by others,9 and ablated in TLR2–/–, TLR3–/–, and TLR4–/– mice (Figure 5). Therefore, because the MyD88 adaptor is required for the signal transduction of TLR, except TLR3,50 MyD88-independent pathways are crucially involved in the MCMV sensing and subsequent response induced by PTX3.
PTX3 activity is IL-12/IFN-γ dependent and TLR9/MyD88 independent. Viral load and cytokine production in different types of mice upon MCMV infection and PTX3 treatment. Animals were infected intraperitoneally with 105 (BALB/c, IFN-γ–/–) or 5 × 105 (C57BL6, IL-12p40–/–, IFN-αβ–/–, TLR2–/–, TLR3–/–, TLR4–/–, TLR9–/–, and MyD88–/–) PFU of MCMV. Virus titers, expressed as log10, were quantified on MEF cells by standard plaque assay on lung tissues removed at 7 days after infection. PTX3 (1 mg/kg intraperitoneally) was administered daily beginning on the day of the infection and continuing for 1 week. Controls received the diluent alone. Cytokine (pg/mL) levels in culture supernatants of spleen cells (day 7) were determined by ELISA assay. Bars indicate the standard errors. Results are representative of 3 independent experiments. *P < .05, PTX3-treated versus untreated mice.
PTX3 activity is IL-12/IFN-γ dependent and TLR9/MyD88 independent. Viral load and cytokine production in different types of mice upon MCMV infection and PTX3 treatment. Animals were infected intraperitoneally with 105 (BALB/c, IFN-γ–/–) or 5 × 105 (C57BL6, IL-12p40–/–, IFN-αβ–/–, TLR2–/–, TLR3–/–, TLR4–/–, TLR9–/–, and MyD88–/–) PFU of MCMV. Virus titers, expressed as log10, were quantified on MEF cells by standard plaque assay on lung tissues removed at 7 days after infection. PTX3 (1 mg/kg intraperitoneally) was administered daily beginning on the day of the infection and continuing for 1 week. Controls received the diluent alone. Cytokine (pg/mL) levels in culture supernatants of spleen cells (day 7) were determined by ELISA assay. Bars indicate the standard errors. Results are representative of 3 independent experiments. *P < .05, PTX3-treated versus untreated mice.
Discussion
Pathogen recognition is a common feature among the members of the pentraxin family. PTX3 binds a number of different soluble ligands with high affinity, including microbial products.51 This study broadens the spectrum of antimicrobial activity of PTX3, which includes an ability to bind CMV viruses, inhibit their infectivity, and protect from infection via the production of IL-12/IFN-γ through a TLR9/MyD88-independent pathway.
Herpesviruses use a complex route of entry into cells that involves multiple interactions between several distinct viral envelope glycoproteins and cellular receptors.30 This may account for the ability of HCMV to bind, penetrate, and replicate in a wide range of cells. We found here that PTX3 binds HCMV and MCMV and inhibits viral-cell fusion and internalization. Thus, similar to other lectin components of the innate immune system,28,29 cross-linking of glycoproteins found on viral or cellular sources could be a mechanism by which PTX3 may block viral fusion and entry. The finding that binding of PTX3 to HCMV was not inhibited by human antibodies recognizing early and late viral antigens confirms that PTX3 acts at an earlier stage than viral replication. As the gB of HCMV is one of the most important glycoproteins involved in attachment and fusion with host cells,52 studies are under way to elucidate the role of gB in the binding of PTX3 to the virus and the influence of PTX3 deglycosylation on this interaction. Intriguingly enough, PTX3 also bound and inhibited replication of orthomyxoviruses, such as influenza viruses, that, unlike herpesviruses, use a single glycoprotein, the hemoagglutinin, for membrane fusion.53 PTX3 efficiently bound and inhibited replication of the human H3N2 virus, whereas it did not bind or inhibit the A/PR8/34 (H1N1) virus (S.B., unpublished observation, December 2005), a finding suggesting the possible involvement of hemoagglutinin glycosylation in the action of PTX3.
Regardless of the molecular mechanism(s) by which PTX3 interacts with viruses, our findings are consistent with a role of PTX3 as an opsonin and imply the existence of a cellular receptor. In this regard, the finding that PTX3 inhibited viral infection in DCs is of interest. Not only is PTX3 produced by DCs of the myelomonocytic lineage and not by pDCs, but PTX3 also regulates the maturation, secretion, and antiviral program of DCs, thus behaving as a flexible regulator of the functions of this cell population.54 We found that PTX3, while limiting viral replication in CD11b+ DCs, activated in these cells the key transcription factor IRF3, which is responsible for antiviral gene induction,12,13 including the Ifnβ and Il12p35 genes.44 Accordingly, IL-12p70 and, to a lesser extent, IFN-α production were induced by PTX3 in these cells. It appears, therefore, that an autocrine loop may be at work in the PTX3/DC interaction with the virus, whereby the production of PTX3 contributes to antiviral defense by acting on both sites, viral inhibition and activation of an antiviral state on DCs. As a matter of fact, PTX3 was produced, although at a low level, by CD11b+ DCs exposed to the virus (data not shown).
The finding that DCs are sensitive to PTX3 activity may account for the increased recovery of splenic DC and NK cell number and functions (IFN-γ production and cytotoxicity) upon PTX3 treatment. Although the production of type I IFN by pDCs after infection is critical for the early control of the infection through the activation of NK-cell cytotoxicity,18 CD11b+ DCs have recently been found to contribute to this control by producing IL-12, IFN-α, and activating NK cells.20 NK-cell recovery was also observed in the lung, which is considered to be 1 of the major organs of CMV infection, persistence, and reactivation,55,56 and also a site of PTX3 production.57 However, optimal activation of NK cells in MCMV infection also requires the engagement of cell-surface receptors,48 such as the activating receptor NKG2D, the ligation of which can override the inhibitory signals provided by major histocompatibility complex (MHC) class I engagement.58 In MCMV-susceptible BALB/c mice, early NK-cell responses are ineffective because of viral interference with the expression of ligands for NKG2D ligands (NKG2DLs).59 Therefore, it is possible that viral interference with NKG2D functions can be effectively bypassed by PTX3-stimulated MCMV-activated CD11b+ DCs. This may account for the ability of PTX3 to control the viral load in the liver of susceptible BALB/c more than resistant C57BL6 mice.
Large DNA viruses such as MCMV use an array of immune evasion strategies to ensure that viral replication proceeds successfully despite antiviral host immune responses. It is therefore not unexpected that the host uses multiple TLRs to recognize MCMV and to ensure that an appropriate immune response is mounted. TLR9, TLR3, and TLR2, in combination with CD14, have been linked to viral recognition and activation of immune responses in infection.9-11,60 In line with the notion that CD11b+ DC production of type I interferons occurs independently of TLR9,20 PTX3 activity was independent of TLR9/MyD88 but dependent on TLR2, TLR3, and TLR4. The elevated viral load together with the failure of PTX3 treatment to modify it suggests a major role for the TLR3-dependent pathway in the control of the infection and activation of PTX3-dependent antiviral activity. Consistently, preliminary results indicate the lack of IFR3 activation following virus and/or PTX3 exposure in the condition of TLR3 deficiency. That TLR4 uses a MyD88-independent pathway for type I IFN production is known.61
The involvement of TLR2 is of potential interest for several reasons. First, TLR2 recognition of HCMV occurs in a replication-independent manner, as a viral envelope glycoprotein is the specific molecular trigger for TLR2.62 This would suggest an ability of the host to detect viruses during entry but before the onset of any replication events. Second, TLR2 has been shown to mediate DC activation by PTX3 bound to the outer membrane protein A from Klebsiella pneumonia.51 The activation program of DCs also included induction of PTX3. Therefore, TLR2 may participate in the amplification loop between PTX3 and CD11b+ DCs during the antiviral response to MCMV in vivo and implies that PTX3 may act early during MCMV infection.
One interesting observation of the present study is the finding that IL-10 is also produced after treatment with PTX3 in both susceptible and resistant mice. Adaptive immunity is required for the termination of the productive infection and the establishment of latency.63 Whether IL-10 can be considered an integral component of the antiviral immune responses, as seen in other microbial infections, is presently unclear. Undoubtedly, the effects of PTX3 goes beyond an activity on the innate immune system to include the activation of appropriate adaptive T-cell responses that, as a matter of fact, are believed to occur independently of MyD88-dependent events.9 Both CD4+ and CD8+ T-cell–effector functions were up-regulated following PTX3 treatment, a finding that may explain the relevant antiviral activity of PTX3 in the salivary glands, where persistent productive infection is controlled by CD4+ T cells and IFN-γ.64,65 Moreover, an effect on the adaptive immunity is also consistent with the ability of PTX3 to prevent reactivation, given the crucial role played by IFN-γ for the establishment of latency.66 As CD11b+ DCs are known to efficiently prime for CD4+ T-cell responses, PTX3 has the unique ability to decrease viral load yet to promote the initiation of the acquired T-cell responses. Alternatively, as IL-12 production by CD11b+ DCs is inhibited by type I IFN-producing pDCs,16 PTX3 may come into play in conditions in which the action of pDCs may be overwhelming.
Finally, the finding that PTX3 is effective in preventing MCMV infection and reactivation as well as the subsequent Aspergillus infection, all being severe complications in patients who received transplants, points to a major role of PTX3 not only as marker of pathology and prognostic factor but also as a therapeutic agent in viral infections and superinfections in the transplantation settings.
Prepublished online as Blood First Edition Paper, July 13, 2006; DOI 10.1182/blood-2006-03-009266.
Supported by the National Research Project on AIDS, contract 50F.30, “Opportunistic Infections and Tuberculosis,” Italy (L.R.); by Ministero Istruzione Università e Ricerca (L.R. and A.M.) and by the European Commission (A.M.).
The authors declare no competing financial interests.
L.R. and A.M. devised the study, critically evaluated the data at regular intervals, and drafted the paper. L.R. takes responsibility for integrity of the work as a whole. S.B., R.G., and L.P. developed the experimental models of MCMV infection. K.P. developed the reactivation model in HSCT. T.Z. performed all the DC studies in vitro. P.B. performed the IRF3 study. S.B. and M.C. did the electron microscopy. M.N. and A.M.I. made a major contribution with virus handling and titration. A.D. contributed with PTX3 ELISA. C.G. provided the PTX3-deficient mice. G.S. and R.D.S. contributed practical support of purified PTX3. F.B., as Head of Microbiology, made a major intellectual and critical contribution to the study design.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Lara Bellocchio for dedicated editorial assistance, Dr Paolo Mosci for histology, Dr Roberto Castronari for RT-PCR, Dr Manfred Kopf (Swiss Federal Institute of Technology Zurich, Switzerland) for providing us with mice, and Drs Santo Landolfo and Patrizia Caposio (University of Turin, Italy) for the supply of the Smith strain MCMV salivary gland extracts.