Abstract
Overexpression of fibroblast growth factor receptor 3 (FGFR3) is a hallmark of t(4;14) multiple myeloma (MM). To dissect the mechanism of FGFR3 oncogenesis in MM, we used 3 FGFR selective kinase inhibitors—CHIR258, PD173074, and SU5402—and FGFR3-specific siRNA to modulate FGFR3 activity. Conversely, the ligand FGF was used to stimulate FGFR3 function in human MM cells. The transcriptional response to FGFR3 modification was recorded, and gene expression changes common to all 5 modifiers were documented. Ten genes were commonly regulated. Macrophage inflammatory protein-1 alpha (MIP-1α) was the single most differentially altered gene. MIP-1 α promoter function, gene expression, and protein secretion were each down-regulated following inhibition of FGFR3 signaling. Down-regulation of MIP-1 α was not, however, observed following FGFR3 inhibition in MM cells with RAS mutations implicating RAS-MAPK in MIP-1 α regulation. As confirmation, inhibition of ERK1 also down-regulated MIP-1 α in FGFR3 inhibitor-resistant cells harboring RAS mutations. MIP-1 α is implicated in the survival and proliferation of MM cells and the pathogenesis of MM bone disease. Our observation is the first to directly link an initiating IgH translocation not only to MM-cell growth and survival but also to the disease-associated bone disease.
Introduction
A primary t(4;14)(p16.3;q32.3) chromosomal translocation results in ectopic expression of fibroblast growth factor receptor 3 (FGFR3) and immunoglobulin heavy chain-MMSET transcripts in 15% of multiple myeloma (MM) patients.1,2 This translocation is present not only in MM but also in monoclonal gammopathy of unknown significance (MGUS), a precursor for MM.3
FGFR3 is a transmembrane tyrosine kinase receptor that plays an important role in normal chondrocyte and bone development.4 Indeed, activating mutations of FGFR3 cause the most common forms of human dwarfism.5-7 In MM, we have previously demonstrated that FGFR3 functions as an oncogene,8 that ectopic expression of FGFR3 promotes cytokine independence,9 and that inhibition of FGFR3 kinase function promotes cellular apoptosis.10-12 Furthermore, we have demonstrated that the presence of a t(4;14) in MM is associated with a poor clinical outcome.13-15 Of importance then, the cellular consequences of inappropriate FGFR3 kinase activity in MM are not fully understood or characterized.
Both RAS-MAPK16,17 and STAT9,18 pathways are reported as mediating the oncogenic effects of FGFR3. For example, in NIH3T3 cells, TDII mutant FGFR3 (K650E) is transforming, and inhibition of RAS or RAF signaling pathway in the same cells by a dominant-negative form of RAS can reduce TDII FGFR3 transformation efficiency, demonstrating that MAPK activation is a consequence of FGFR3 signaling.19 In support of this, we have shown potent pMAPK activation following FGF ligand stimulation of t(4;14) MM cell lines, down-regulation of pERK following FGFR3 inhibition,11 and resistance of MM cells to FGFR3 inhibition in the presence of RAS mutation.10-12
Nevertheless, since information on FGFR3 signaling in MM cells is still limited, the aim of our study was to expand understanding of the pathways regulated by FGFR3 overexpression. To this end, we used a series of known FGFR selective kinase inhibitors (CHIR258,11 PD173074,12 SU540210 ), FGFR3-specific RNAi, and the ligand acidic FGF (aFGF) to treat genetically characterized MM cell lines. Changes in gene-expression profile in response to FGFR3 perturbation were then examined. The transcriptional response to FGFR3 modification was then recorded, and genes commonly regulated by all 5 modifiers were documented. Of these, the most differentially altered gene was MIP-1α (CCL3) a known trigger of survival and proliferation in MM.20-26 Subsequent studies, reported here, confirmed that MIP-1α promoter activity, as well as MIP-1α gene and protein expression/secretion, is regulated by FGFR3 through activation of the RAS-MAPK pathway.
Materials and methods
Reagents
SU5402, a known FGFR inhibitor, was provided by Sugen (San Francisco, CA). PD173074 was obtained as a gift from Pfizer (Ann Arbor, MI). PD98059 was obtained from Cell Signaling Technology (Danvers, MA). CHIR258 was provided by Chiron (Emeryville, CA). Human recombinant acidic fibroblast growth factor (aFGF) was obtained from R&D Systems (Minneapolis, MN). Antibodies to FGFR3 (B-9, C-15) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to ERK1/2 and anti-rabbit/mouse IgG horseradish peroxidase were purchased from Cell Signaling Technology. The MIP-1 promoter-luciferase construct was a gift from Dr Sun J. Choi (Department of Medicine Hematology, University of Pittsburgh, PA).23
Cell culture and drug treatment
Human MM cell lines (KMS11, KMS18, H929, and UTMC2) were grown in Iscoves modified Dulbecco medium (IMDM) supplemented with 5% fetal calf serum (FCS), 100 μg/mL penicillin, and 100 μg/mL streptomycin (Hyclone, Logan, UT). To determine transcriptional changes associated with FGFR3 inhibition, cells lines were washed and plated in triplicate in IMDM plus 5% FCS and incubated for 48 hours with SU5402 (5 μM), CHIR258 (500 nM), or PD173074 (100 nM). Forty-eight hours was chosen because the effect of these drugs on cell viability is delayed and cellular cytotoxicity is still limited at 48 hours.10-12 For ligand stimulation assays, cells were starved overnight and induced for 8 hours with aFGF at 80 ng/mL and 30 μg/mL heparin before harvesting. Uninduced cells served as a negative control. Cells were then harvested, and RNA was extracted for subsequent expression profiling.
siRNA nucleofection
H929 and KMS11 cells were suspended in solution from nucleofector kit V following the manufacturer's (Amaxa, Cologne, Germany) guidelines for cell line transfection. Briefly, 100 μL containing 5 × 106 cells with 5 nM of either FGFR3, ERK1 and ERK2, or scrambled control siRNA oligonucleotides (Dharmacon, Lafayette, CO) was transferred to the provided cuvette and nucleofected with an Amaxa Nucleofector apparatus (Amaxa). After transfection, cells were immediately transferred into wells containing 37°C prewarmed culture medium in 6-well plates.
MTT assays
For viability assays, cells were washed and plated in triplicate in IMDM plus 5% FCS and incubated for 72 hours with SU5402 (5 μM), CHIR258 (500 nM), or PD173074 (100 nM) at concentrations of drug previously shown to inhibit FGFR3 function and to maximally inhibit the viability of FGFR3-expressing myeloma cell lines.10-12 Cells were then analyzed by MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's protocol. Each experimental condition was performed in triplicate.
Total cellular extracts and Western blots
Whole-cell lysates were made from MM cell lines by resuspending cell pellets in 2X sample buffer. The whole extracts were boiled for 5 minutes and quick chilled before loading onto a gel. Extract (30 μL) was resolved on SDS polyacrylamide gels (10%) and transferred to nitrocellulose membranes (Immobilon P; Millipore, Billerica, MA) using a semidry electroblotter. The membranes were probed with antibodies against FGFR3 and ERK1/2. Signals were detected by secondary horseradish peroxidase-conjugated antibody and enhanced chemiluminescence, as recommended by the manufacturer (Amersham Biosciences, Pittsburgh, PA). To normalize the results, blots were reprobed with an antibody against actin (Santa Cruz Biotechnology).
Intracellular immunostaining for MIP-1α
Cells in 0.5 mL media were pelletted and resuspended in PBS at a concentration of 2 to 5 × 105. Then, 0.1 mL of 10% formaldehyde was added and incubated at 37°C for 10 minutes followed by incubation on ice for 2 minutes, adding 5.4 mL ice-cold methanol gradually and incubating on ice for another 30 minutes. The cells were centrifuged and washed once with PBS supplemented with 4% fetal bovine serum (FBS). Monoclonal anti-human MIP-1α/CCL3 antibody (R&D Systems) was then added to the cells and incubated at room temperature for 15 minutes, followed by another wash with PBS and 4% FBS. Secondary antibody anti-mouse IgG-PE was added and incubated at room temperature for 15 minutes. The cells were washed once with PBS + 4% FBS and analyzed by fluorescence-activated cell sorting (FACS). Gating was performed on viable cells using forward and side scatter. Each experimental condition was repeated twice.
CCL3 promoter assay
3T3 cells that endogenously express FGFR1 (wild type) were incubated for 24 hours in the presence of PD173074 or DMSO vehicle alone and then transfected with an MIP-1α promoter-luciferase construct23 and a control vector containing green fluorescent protein (to determine transfection efficiency). Following transfection, cells were cultured for a further 48 hours with DMSO or PD173074. Luciferase activity was then measured and corrected for transfection efficiency by GFP expression and protein concentration. Normalized luciferase activity was expressed as follows: luciferase activity units/(% transfection efficiency × protein concentration). Each experimental condition was repeated 4 times.
ELISA quantitation of MIP-1 α levels
KMS11 and H929 MM cells were plated with CHIR258 or PD173074 or vehicle control for 48 hours. Cells were then washed and harvested, and viable cells were quantitated by trypan blue exclusion. Cells were then replated at 500 000 viable cells/mL, left for 8 to 10 hours, and then recounted. Supernatant was harvested and an enzyme-linked immunosorbent assay (ELISA) performed using anti-human MIP-1α/CCL3 antibody (R&D Systems). Final MIP-1α concentration was corrected for cell viability at the end of the full culture period. The experiment was performed in triplicate.
Gene-expression profiling
Gene expression on MM cell lines was analyzed on Human U133_Plus2 arrays from Affymetrix (Santa Clara, CA). Syntheses of cDNA and biotinylated cRNA, hybridizations, and scanning were conducted according to the protocols provided by the manufacturer. For patient analysis, we accessed publicly available data from the University of Arkansas on 231 patients with newly diagnosed MM, 30 with relapsed MM, 12 with MGUS, 32 human myeloma cell lines (HMCLs), and 14 control subjects.27 Gene expression intensity values, measured with the use of MAS software, version 5.01 (Affymetrix), were log transformed, normalized to the median, and analyzed using GeneSpring 7 (Silicon Genetics, Redwood City, CA).
Results
Response of MM cell lines to FGFR3 inhibitors
Previously, our lab and others10-12 have shown selective anti-FGFR3 activity for SU5402, PD173074, and CHIR258 in MM cells with t(4;14) IgH translocations. All 3 of these small-molecule receptor tyrosine kinase inhibitors compete with ATP for the specific binding site within the catalytic domain of the receptor. We were interested in using this resource to determine downstream targets of FGFR3. KMS11, H929, and KMS18 myeloma cells that harbor a t(4;14) translocation and as a result overexpress FGFR3 were treated for 48 hours with SU5402 (5 μM), CHIR258 (500 nM), or PD173074 (100 nM) at maximally effective dose concentrations. In Figure 1, we demonstrate that even at 72 hours PD173074 is not cytotoxic to myeloma cells and that cellular cytotoxicity to CHIR258 is present at 48 hours but not yet marked. RNA was extracted from drug- or vehicle-treated cells at the 48-hour time point and was analyzed on Affymetrix human HG_U133_PLUS2 arrays. Vehicle-treated KMS11, H929, and KMS18 cells as well as FGFR3-negative U266 myeloma cells served as controls.
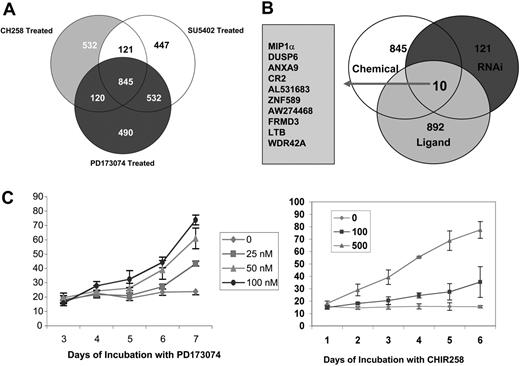
Gene-expression changes in MM cells treated with FGFR3 inhibitors. (A) Venn diagram details genes differentially altered in KMS11 myeloma cells at least 2-fold following treatment with an FGFR3-selective kinase inhibitor compared with vehicle control. Eight hundred and forty five genes were commonly dysregulated (Tables S1-S3). (B) Venn diagram maps the 2-fold differentially altered genes using FGFR3 knockdown by siRNA in KMS11 cells or chemical modulation and the 121 genes altered at least 1.5-fold following FGF ligand stimulation in UTMC2 myeloma cells (containing wild-type FGFR3 and no RAS mutations). Ten genes were found to be differentially altered under this model under all 5 experimental conditions. (C) The apoptotic effect of PD173074 and CHIR258 on KMS11 cells is not marked at 48 hours. As shown here, no increase in annexin V-positive cells is seen with PD173074 even at 72 hours compared with controls, while only early evidence of apoptosis is seen with CHIR258. Error bars show standard deviation in replicate of 3 experiments.
Gene-expression changes in MM cells treated with FGFR3 inhibitors. (A) Venn diagram details genes differentially altered in KMS11 myeloma cells at least 2-fold following treatment with an FGFR3-selective kinase inhibitor compared with vehicle control. Eight hundred and forty five genes were commonly dysregulated (Tables S1-S3). (B) Venn diagram maps the 2-fold differentially altered genes using FGFR3 knockdown by siRNA in KMS11 cells or chemical modulation and the 121 genes altered at least 1.5-fold following FGF ligand stimulation in UTMC2 myeloma cells (containing wild-type FGFR3 and no RAS mutations). Ten genes were found to be differentially altered under this model under all 5 experimental conditions. (C) The apoptotic effect of PD173074 and CHIR258 on KMS11 cells is not marked at 48 hours. As shown here, no increase in annexin V-positive cells is seen with PD173074 even at 72 hours compared with controls, while only early evidence of apoptosis is seen with CHIR258. Error bars show standard deviation in replicate of 3 experiments.
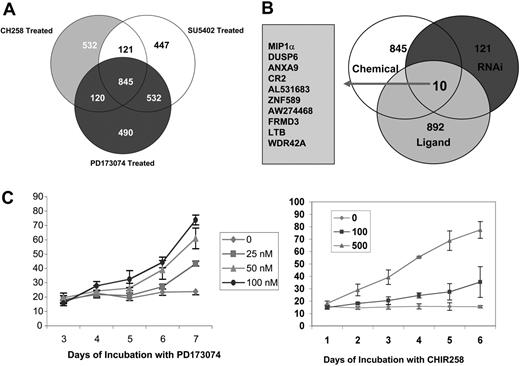
A mean of duplicate samples of CHIR258-treated versus DMSO control KMS11 cells gave a list of 1618 differentially expressed genes when quality-controlled data were set at a cutoff of 2-fold change. Using the same normalization and filtering criteria, SU5402 treatment gave a list of 1945 genes and PD173074 treatment a list of 1987 differentially regulated genes. Since each kinase inhibitor is nonspecific and has a broad range of activity against a number of classes III, IV, and V tyrosine kinases, we next sought to define what gene expression signatures are shared between the 3 compounds. For this, we used Venn diagrams to overlap the 3 differential expression gene lists and thus generated a list of regulated genes common to the 3 inhibitors (Figure 1; also see Table S1, available on the Blood website by clicking the Supplemental Tables link at the top of the online article). Only 280 of the 845 commonly dysregulated genes had a previously assigned biologic function. Among the commonly regulated genes, cell-cycle and DNA-replication genes were the most abundant and comprised more than 25% of the known genes. Other genes included a high number of ATP-binding proteins (22%), which either could indicate a nonspecificity of the ATP-binding drugs or could be a consequence of cell death.
FGFR3 target genes defined by siRNA-mediated inhibition
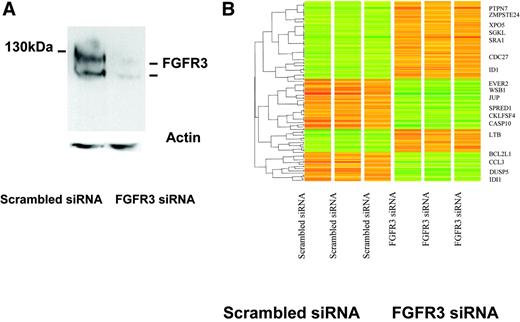
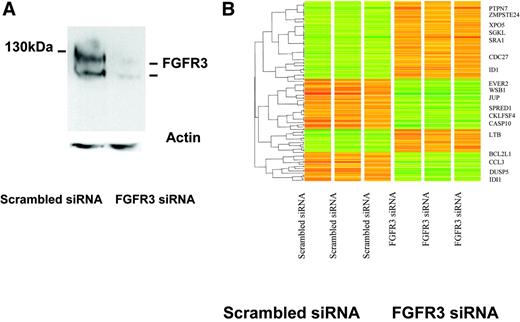
To achieve further specificity to the FGFR3 target gene list obtained from FGFR small-molecule inhibitors, we next used siRNA-mediated inhibition of FGFR3 in KMS11 cells. In these studies, FGFR3 expression was efficiently silenced (Figure 2A). Transcriptional changes in gene expression were then examined on triplicate samples 48 hours after the initial transfection with FGFR3 or control siRNA. One hundred and twenty one genes were significantly altered between the scrambled versus the FGFR3-inhibited cell lines (Figure 2B; see also Table S3).
UTMC2 myeloma cells treated with FGF ligand
To further refine expression changes associated with FGFR3 signaling, we next stimulated UTMC2 cells containing wild-type FGFR3 and no RAS mutations with the ligand aFGF, looking for genes in common that are reciprocally regulated. Cells were starved overnight and induced for 8 hours with aFGF at 80 ng/mL and 30 μg/mL heparin before harvesting. Uninduced cells served as a negative control. Transcriptional changes in gene expression were then examined on triplicate samples 48 hours after the ligand induction. Changes in gene expression associated with FGF induction were analyzed to generate a list of 892, 1.5-fold or more differentially expressed genes (Table S2).
Gene-expression changes in MM cells mediated by siRNA inhibition of FGFR3. (A) Western blot of FGFR3 protein expression in KMS11 cells at 48 hours following FGFR3-specific or scrambled control siRNA oligonucleotide transfection demonstrates successful knockdown of FGFR3. Nucleofections were done in KMS11 cells using the Amaxa apparatus and solutions. Transfection efficiencies were quantified using a green fluorescent protein (GFP) reporter construct provided by the supplier and flow cytometry. Our optimized nucleofection parameters yielded survival rates of 90% or more based on forward and side scatter parameters. Under these conditions, 70% of the surviving cells showed transgene expression 48 hours after nucleofection. (B) A heat map of triplicate experiments demonstrates differences in gene expression following FGFR3 knockdown, with representative examples of the most significant known dysregulated genes highlighted. A listing of 121 differentially regulated genes is provided in Supplemental Table S3.
Gene-expression changes in MM cells mediated by siRNA inhibition of FGFR3. (A) Western blot of FGFR3 protein expression in KMS11 cells at 48 hours following FGFR3-specific or scrambled control siRNA oligonucleotide transfection demonstrates successful knockdown of FGFR3. Nucleofections were done in KMS11 cells using the Amaxa apparatus and solutions. Transfection efficiencies were quantified using a green fluorescent protein (GFP) reporter construct provided by the supplier and flow cytometry. Our optimized nucleofection parameters yielded survival rates of 90% or more based on forward and side scatter parameters. Under these conditions, 70% of the surviving cells showed transgene expression 48 hours after nucleofection. (B) A heat map of triplicate experiments demonstrates differences in gene expression following FGFR3 knockdown, with representative examples of the most significant known dysregulated genes highlighted. A listing of 121 differentially regulated genes is provided in Supplemental Table S3.
Genes differentially changed by FGFR inhibitors, siRNA, and FGF induction
Changes in expressed gene profiles shared by all 3 FGFR small-molecule inhibitors and FGFR3 siRNA knockdown, and reciprocally affected by FGF ligand induction, were next generated by overlapping lists of genes at least 2-fold differentially regulated from each grouping (1.5-fold in the case of FGF ligand; Figure 1B). Only 10 genes fulfilled these strict criteria. Two of these genes, MIP-1α (CCL3) and DUSP6, were down-regulated by inhibition of FGFR3 and induced by FGF induction, whereas 8 genes (ANXA9, CR2, AL531683, SZF1-1, AW274468, FRMD3, LTB, and WDR42A) showed an up-regulation by inhibition of the FGFR3 receptor and down-regulation on its induction (Table 1). Since MIP-1α was the single most differentially altered gene (12-fold change), had a known association with osteolytic bone destruction, and had been shown to mediate growth, survival, and migration in MM cells, we were interested in exploring this observation further.
MIP-1 α promoter activity is regulated by FGFR signaling
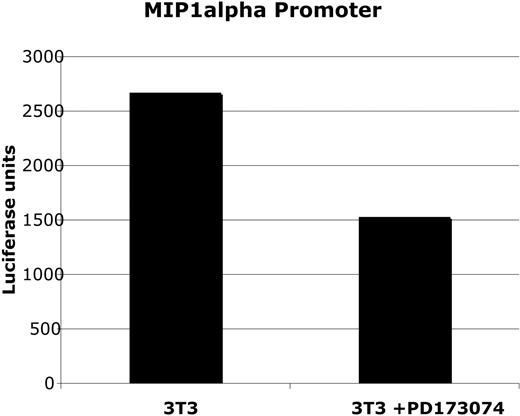
3T3 cells that endogenously express wild-type FGFR1 were incubated for 24 hours in the presence of 100 nM PD173074 (the most selective of the FGFR3 small-molecule inhibitors) or DMSO vehicle alone and then transfected with an MIP-1α promoter-luciferase construct and a control vector containing green fluorescent protein (to determine transfection efficiency). As shown in the representative experiment in Figure 3, MIP-1α promoter activity was suppressed by 33.3% to 40.5% in 4 separate experiments of FGFR inhibition compared with vehicle control.
MIP-1 α protein decreases in response to FGFR3 inhibition
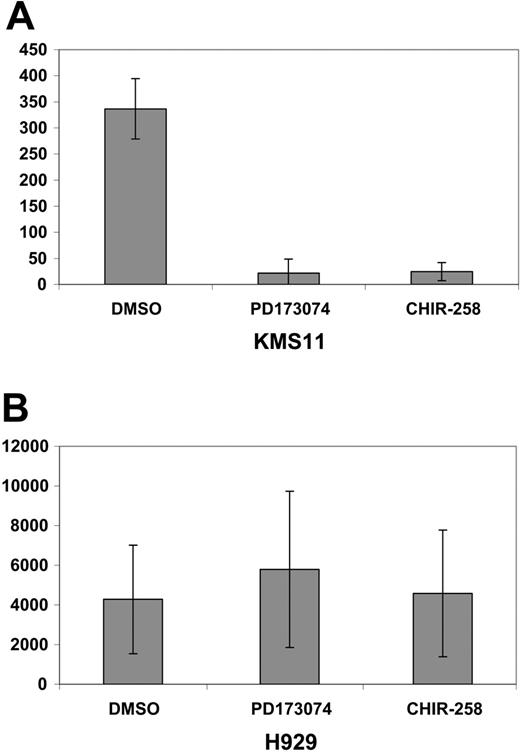
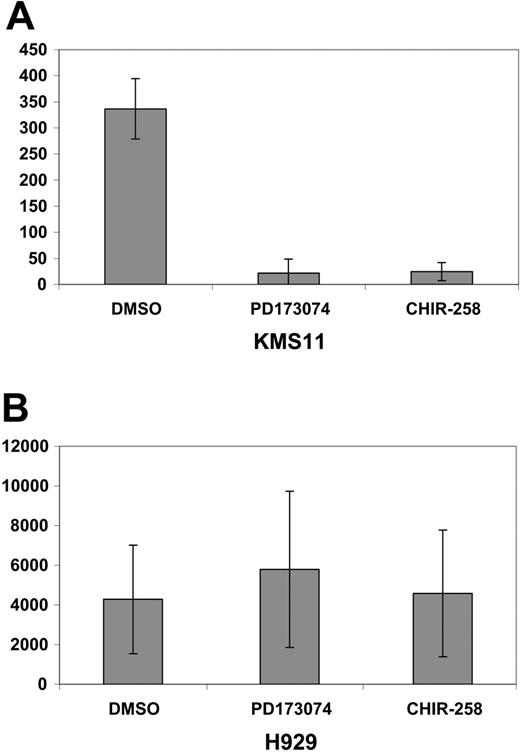
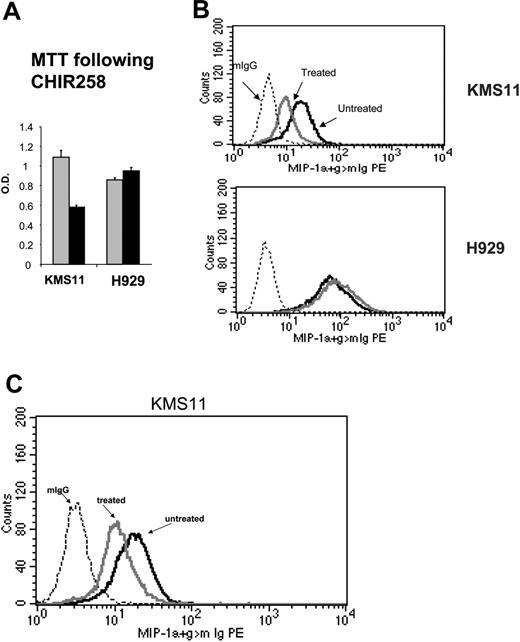
MIP-1α protein levels were determined in 2 FGFR3-expressing cell lines, KMS11 and H929. These 2 cell lines were selected on the basis of their differential sensitivity to FGFR inhibitors in MTT assays (Figure 4A and Trudel et al11,12 ). The t(4;14) KMS11 cell line was responsive, whereas H929, which harbors a downstream ras mutation in addition to expressing FGFR3, was resistant to FGFR3 inhibition in accordance with previous reports.10-12 Of interest, when both cell lines were assessed for intracellular MIP-1α protein expression by flow cytometry (Figure 4B), KMS11 cells had relatively lower baseline level of MIP-1α protein compared with the high levels of protein present in H929 cells.
In response to CHIR258 (500 nM; Figure 4B) or PD173074 (100 nM; Figure 4C), treatment of KMS11 cells lowered MIP-1α protein levels, which paralleled the observed decrease in RNA expression seen on arrays.
In contrast, H929 cells, which display minimal cytostatic response to CHIR258 or PD173074,11,12 showed no decrease in high basal levels of MIP-1α. The same result was observed for secreted MIP-1α measured by ELISA following either CHIR258 or PD173074 exposure (Figure 5).
Since FGFRs signal in part via RAS-MAPK, this finding suggested that MIP-1α is also being regulated through the RAS-MAPK pathway, and thus inhibition of FGFR3, upstream of RAS, does not affect MIP-1α levels in H929 cells. ERK1/2 has been implicated in FGFR3 signaling in our lab and by others.16,17 In order to investigate this possibility, we next decided to inhibit ERK1/2, a downstream target of RAS, in H929 cells and determine changes in MIP-1α levels.
Inhibition of ERK1/2 in myeloma cells down-regulates MIP-1 α expression
We next tested the effect of the ERK pathway inhibitor PD98059 on MIP-1α levels in H929 cells. As predicted, treatment with PD98059 (10 μM) induced a reduction of MIP-1α levels at 48 hours (Figure 6). Similarly, an inhibitory effect on MIP-1α protein levels was observed in H929 cells 48 hours after nucleofection with ERK1 and ERK2 siRNAs versus the scrambled siRNA control (not shown). These results confirm that MIP-1α regulation by FGFR3 occurs via the RAS-MAPK pathway.
MIP-1 α promoter-driven luciferase was measured in transfected NIH3T3 cells. Luciferase readings were corrected for transfection efficiency by GFP expression and protein concentration. Normalized luciferase activity was expressed as follows: luciferase activity units/(% transfection efficiency × protein concentration). Promoter activity is reduced following treatment with 100 nM PD173074. The experiment is representative of 4 independent experiments with similar results. Error bars are not applied because of large variation in luciferase quantification; however, the range of suppression over 4 independent experiments was 33.3% to 40.5%.
MIP-1 α promoter-driven luciferase was measured in transfected NIH3T3 cells. Luciferase readings were corrected for transfection efficiency by GFP expression and protein concentration. Normalized luciferase activity was expressed as follows: luciferase activity units/(% transfection efficiency × protein concentration). Promoter activity is reduced following treatment with 100 nM PD173074. The experiment is representative of 4 independent experiments with similar results. Error bars are not applied because of large variation in luciferase quantification; however, the range of suppression over 4 independent experiments was 33.3% to 40.5%.
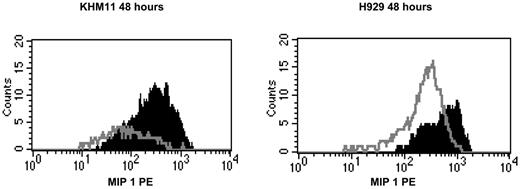
Decrease in MIP-1 α in response to FGFR3 inhibition. (A) An MTT assay performed at 72 hours following drug exposure shows inhibition of cell proliferation in CHIR258-treated cells. H929 cells harboring a t(4;14) and a RAS mutation are resistant to treatment. The error bars show standard deviation in replicate of 3 experiments.  indicates the DMSO control; ▪, cells treated with CHIR258. (B) Flow cytometry shows down-regulation of intracellular MIP-1α in CHIR258-sensitive KMS11 but not-resistant H929 MM cells. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, CHIR258-treated cells. Experiments were repeated twice with similar results. (C) Flow cytometry confirms down-regulation of intracellular MIP-1α in PD173074-sensitive KMS11. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, PD173074-treated cells. Experiments were repeated twice with similar results.
indicates the DMSO control; ▪, cells treated with CHIR258. (B) Flow cytometry shows down-regulation of intracellular MIP-1α in CHIR258-sensitive KMS11 but not-resistant H929 MM cells. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, CHIR258-treated cells. Experiments were repeated twice with similar results. (C) Flow cytometry confirms down-regulation of intracellular MIP-1α in PD173074-sensitive KMS11. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, PD173074-treated cells. Experiments were repeated twice with similar results.
Decrease in MIP-1 α in response to FGFR3 inhibition. (A) An MTT assay performed at 72 hours following drug exposure shows inhibition of cell proliferation in CHIR258-treated cells. H929 cells harboring a t(4;14) and a RAS mutation are resistant to treatment. The error bars show standard deviation in replicate of 3 experiments.  indicates the DMSO control; ▪, cells treated with CHIR258. (B) Flow cytometry shows down-regulation of intracellular MIP-1α in CHIR258-sensitive KMS11 but not-resistant H929 MM cells. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, CHIR258-treated cells. Experiments were repeated twice with similar results. (C) Flow cytometry confirms down-regulation of intracellular MIP-1α in PD173074-sensitive KMS11. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, PD173074-treated cells. Experiments were repeated twice with similar results.
indicates the DMSO control; ▪, cells treated with CHIR258. (B) Flow cytometry shows down-regulation of intracellular MIP-1α in CHIR258-sensitive KMS11 but not-resistant H929 MM cells. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, CHIR258-treated cells. Experiments were repeated twice with similar results. (C) Flow cytometry confirms down-regulation of intracellular MIP-1α in PD173074-sensitive KMS11. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, PD173074-treated cells. Experiments were repeated twice with similar results.
Decrease in secreted MIP-1 α in response to FGFR3 inhibition. An ELISA for secreted MIP-1α demonstrated a reduction in secreted MIP-1α from both CHIR258 (500 nM)- and PD173074 (100 nM)-treated KMS11 cells (A) but not in inhibitor-resistant H929 cells (B). Error bars show standard deviation in replicate of 3 experiments.
Decrease in secreted MIP-1 α in response to FGFR3 inhibition. An ELISA for secreted MIP-1α demonstrated a reduction in secreted MIP-1α from both CHIR258 (500 nM)- and PD173074 (100 nM)-treated KMS11 cells (A) but not in inhibitor-resistant H929 cells (B). Error bars show standard deviation in replicate of 3 experiments.
Treatment of RAS-mutated H929 cells demonstrates down-regulation of MIP-1 α by flow cytometric assessment following treatment with the ERK pathway inhibitor PD98059 (10 μM) at 48 hours. Gray line indicates drug treated; and the solid black line, untreated cells stained for MIP-1α expression. Experiments were performed twice with similar results; a representative experiment is shown here.
Treatment of RAS-mutated H929 cells demonstrates down-regulation of MIP-1 α by flow cytometric assessment following treatment with the ERK pathway inhibitor PD98059 (10 μM) at 48 hours. Gray line indicates drug treated; and the solid black line, untreated cells stained for MIP-1α expression. Experiments were performed twice with similar results; a representative experiment is shown here.
MIP1 α in multiple myeloma patient samples
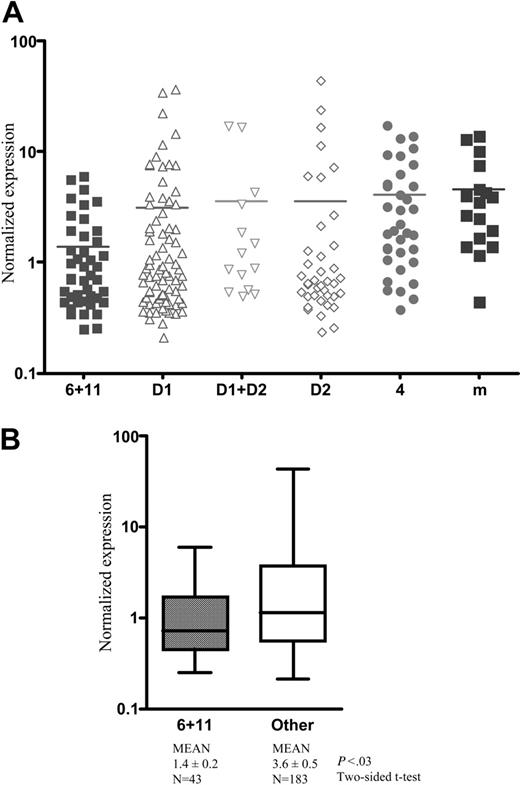
To determine whether these observations had clinical relevance, we looked at a mined expression profiling data set of 231 MM patient samples.27 MIP-1α expression was generally higher than in normal plasma cells in all MM subgroups except t(11;14) and t(6;14) patients. It was generally also higher in human myeloma cell lines, suggesting a link with proliferation of the tumor sample (not shown). Patient samples were next segregated by translocation cyclin (TC) classification27 (Figure 7A). Levels were universally high except in t(11;14) and t(6;14) patients (P = .03; Figure 7B). Mechanisms unrelated to FGFR3, but we speculate RAS-MAPK dependent, likely determine MIP-1α expression in t(4;14)-negative myeloma. Clearly, however, additional studies are required to elucidate the mechanisms of MIP-1α regulation in t(4;14)-negative myeloma patients.
MIP-1 α expression in MM patients. (A) Gene-expression profiling of 231 newly diagnosed MM samples. The newly described TC classification is used to group patients.27 6 indicates t(6;14); 11, t(11;14); 4, t(4;14); m, t(14;16) or other maf “spiked” samples; and D1 and D2, Cyclin D1- and Cyclin D2-expressing samples, respectively. Samples were normalized to a median of 1 for examination of MIP-1α. (B) Ubiquitous expression of MIP-1α is noted except in patients with a t(11;14) or t(6;14) where its expression is statistically lower. The error bars show standard deviation. Horizontal bars indicate the mean.
MIP-1 α expression in MM patients. (A) Gene-expression profiling of 231 newly diagnosed MM samples. The newly described TC classification is used to group patients.27 6 indicates t(6;14); 11, t(11;14); 4, t(4;14); m, t(14;16) or other maf “spiked” samples; and D1 and D2, Cyclin D1- and Cyclin D2-expressing samples, respectively. Samples were normalized to a median of 1 for examination of MIP-1α. (B) Ubiquitous expression of MIP-1α is noted except in patients with a t(11;14) or t(6;14) where its expression is statistically lower. The error bars show standard deviation. Horizontal bars indicate the mean.
Discussion
MIP-1α is a low-molecular-weight monokine with inflammatory and chemokinetic properties and has been characterized to be a potent osteoclast stimulatory factor in MM.28,29 It is elevated in bone marrow plasma of patients with active MM and correlates with the presence of lytic lesions.28 More recently, serum MIP-1α was reported to correlate with survival and bone resorption markers, suggesting that MIP-1α contributes to the pathogenesis of bone disease in MM and possibly in tumor growth as reflected by its impact on survival.25,30 Blocking MIP-1α by neutralizing antibodies or antisense oligonucleotides resulted in a reduction of bone disease as well as tumor burden in MM animal models.22 Also, MIP-1α is shown to stimulate proliferation, migration, and survival of plasma cells.26,31
In the current study, we found that MIP-1α/CCL3 was 1 of only 10 genes whose expression was consistently and significantly decreased in t(4;14) myeloma cells when constitutively active FGFR3 was inhibited by small-molecule tyrosine kinase inhibitors or more specifically with FGFR3 siRNA and that was reciprocally induced by FGF ligand stimulation of the cells as analyzed by gene-expression profiles. Simultaneously, we found MIP-1α/CCL3 expression to be unchanged in cells expressing wild-type FGFR3 and associated constitutive RAS mutation. This led us to speculate that MIP-1α/CCL3 is regulated by the RAS-MAPK pathway, downstream of FGFR3. We tested this hypothesis by inhibiting ERK1/2, a downstream target of the RAS-MAPK pathway, and indeed were able to decrease MIP-1α levels. These data demonstrate that ERK-MAPK inhibition is sufficient to reduce MIP-1α levels in MM cells with constitutive RAS mutations. Furthermore, these results confirm that the RAS/ERK pathway is relevant for FGFR3-mediated signaling and is involved in up-regulation of a number of critical genes that may be involved in disease severity. These findings are further supported by gene-expression profiling results of FGFR3 RNAi knockdown32 and a comparison of t(4;14)-positive versus-negative cell lines33 in which activation of the RAS/MAPK pathway is evident. Of interest, levels of MIP-1α on gene-expression profiling have also been correlated with poor outcome in diffuse large B-cell lymphoma, suggesting a broader role for this gene in cellular proliferation.34
In our study, DUSP6 was another gene that was down-regulated by FGFR3 inhibition and induced by FGF. The protein encoded by this gene is a member of the dual specificity protein phosphatase subfamily that inactivates their target kinases by dephosphorylating both phosphoserine/threonine and phosphotyrosine residues.35-37 The DUSP family negatively regulates members of the mitogen-activated protein (MAP) kinase superfamily (MAPK/ERK, SAPK/JNK, p38),38 which are associated with cellular proliferation and differentiation. Further, DUSP6 has been shown to function as a feedback attenuator of the FGF pathway in developing zebrafish embryos.39 Of interest, another DUSP family member (DUSP10) has been shown to be more highly expressed in t(4;14) MM versus t(4;14)-negative tumors,33 while DUSP22 has been shown to be associated with FGFR3 knockdown, further implicating this family in FGFR3 signaling.32
In summary, we have shown a strong association of MIP-1α/CCL3 with FGFR3 signaling using 3 selective tyrosine kinase inhibitors and siRNA-mediated knockdown of FGFR3 expression in MM cells with a t(4;14) that expresses activated FGFR3. In FGFR3-positive Ras-mutated cells, FGFR3 inhibition did not alter MIP-1α, thus suggesting that overactivation of RAS-MAPK indirectly via FGFR3 signaling or directly through constitutive activation of RAS in MM can contribute to lytic lesions associated with the pathogenesis of MM through the up-regulation of MIP-1α/CCL3. Furthermore, inhibition of FGFR3 or the RAS-MAPK pathway in patients with Ras mutations may hold therapeutic promise in controlling both MM growth and, through MIP-1α myeloma, bone disease. Of importance, these studies link for the first time an initiating oncogenic event—the t(4;14)—to the development of MM bone disease.
Prepublished online as Blood First Edition Paper, July 18, 2006; DOI 10.1182/blood-2006-04-017087.
Supported by grants from the National Cancer Institute of Canada and the Canadian Institutes for Health Research (A.K.S.) and by the American Society of Hematology (ASH) Scholar Award (S.T.), the Eli Lilly/Cancer Care Ontario/Canadian Institute of Health Research Hollenberg Award (S.T.), and the Ontario Cancer Research Network through funding provided by the Province of Ontario (S.T.).
A.K.S. has a consulting contract with Chiron of less than $10 000 annual value. S.T. has received research support in excess of $10 000 from Chiron.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





 indicates the DMSO control; ▪, cells treated with CHIR258. (B) Flow cytometry shows down-regulation of intracellular MIP-1α in CHIR258-sensitive KMS11 but not-resistant H929 MM cells. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, CHIR258-treated cells. Experiments were repeated twice with similar results. (C) Flow cytometry confirms down-regulation of intracellular MIP-1α in PD173074-sensitive KMS11. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, PD173074-treated cells. Experiments were repeated twice with similar results.
indicates the DMSO control; ▪, cells treated with CHIR258. (B) Flow cytometry shows down-regulation of intracellular MIP-1α in CHIR258-sensitive KMS11 but not-resistant H929 MM cells. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, CHIR258-treated cells. Experiments were repeated twice with similar results. (C) Flow cytometry confirms down-regulation of intracellular MIP-1α in PD173074-sensitive KMS11. Cells were examined at 48 hours after drug exposure. The dotted line indicates isotype control; black line, vehicle-treated cells; and gray line, PD173074-treated cells. Experiments were repeated twice with similar results.