Abstract
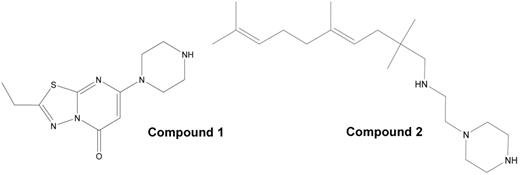
Two separate conformational changes have been proposed to accompany activation of platelet αIIbβ3: 1) leg separation leading to extension of the head region composed of the αIIb propeller and β3 βA (I-like) domains, and 2) a swing-out motion at the junction of the β3 βA (I-like) and hybrid domains. Small molecule inhibitors of αIIbβ3 competitively block the RGD ligand binding site and variably induce conformational changes in αIIbβ3 as judged by the binding of ligand-induced binding site (LIBS)-specific monoclonal antibodies. In an attempt to identify molecules that may inhibit αIIbβ3 activation without initiating the conformational changes associated with ligand binding, we screened 33,264 compounds from four different chemical libraries (Prestwick, Chembridge, Cerep and ChemDiv) for their ability to inhibit the adhesion of washed platelets in HEPES-modified Tyrode’s buffer with 1 mM Ca2+/0.5 mM Mg2+ to immobilized fibrinogen adsorbed from a 50 μg/ml solution. When tested at a final concentration of 16 μM, a total of 102 compounds (0.31%) demonstrated greater than 50% inhibition of platelet adhesion, and two of these (Figure 1) demonstrated >30% inhibition of the initial wave of ADP-induced aggregation of platelets in citrated platelet-rich plasma. IC50s for inhibition of ADP (5 μM)-induced platelet aggregation for compounds 1 and 2 were 13 ± 4.5 and 17 ± 5 μM (n=3), respectively. Compounds 1 and 2 also inhibited fibrinogen binding to platelets induced by the activating LIBS antibody AP5 with IC50s of 27 and 30 μM, and 20 and 27 μM, respectively, in two experiments. Since AP5 binds to and directly activates αIIbβ3, it is likely that the compounds’ inhibitory effects are due to direct binding to αIIbβ3 rather than inhibition of signal transduction. In two separate experiments, compound 1 at 15 - 20 μM produced variable increases in the binding of LIBS mAbs AP5, PMI-1 or LIBS1 to unactivated and ADP-activated platelets, whereas tirofiban (20 μM) consistently increased the binding of each mAb. Compound 2 did not increase the binding of any of the mAbs. Neither compound contains a negatively charged carboxyl group, which mediates the interaction of the Asp group in RGD ligands with the β3 MIDAS metal ion, but compound 1 has a carbonyl group that may potentially interact with the MIDAS metal ion. Compound 1 resembles 1,2-fused pyrimidine derivatives that have previously been demonstrated to inhibit platelet aggregation (Roma et al., Bioorg. Med. Chem. 2003, 11, 123). We conclude that high throughput screening of molecular libraries can identify novel compounds that inhibit αIIbβ3 and that one of them appears to inhibit αIIbβ3 without inducing conformational changes in the receptor.
Disclosures: Royalty interest in abciximab, a derivative of monoclonal antibody 7E3.
Author notes
Corresponding author