Abstract
Background: Non-myeloablative conditioning can avoid early post-transplant toxicity associated with myeloablative conditioning prior to allogeneic hematopoietic progenitor cell transplantation (HPCT). In order to achieve bidirectional host vs. donor and donor vs. host tolerance, we developed a transplant model utilizing a pre-transplant dose of “tolerizing” donor cells in combination with anti-CD40L monoclonal antibody (mAb) treatment (
Methods: we tested the effect of graded doses of ionizing gamma irradiation (7.5, 15, and 30 Gy) on donor splenocytes administered as a tolerizing dose of alloantigen on 6 days pre-BMT. Mixed lymphocyte reactions (MLR) were performed using host-type lymphocytes as responders and irradiated cells as stimulators in the presence or absence of anti-CD40L mAb. Secretion of inflammatory cytokine (IFN-γ and TNF-α) and anti-inflammatory cytokine (IL-10) and cell proliferation (CFSE dilution) were measured.
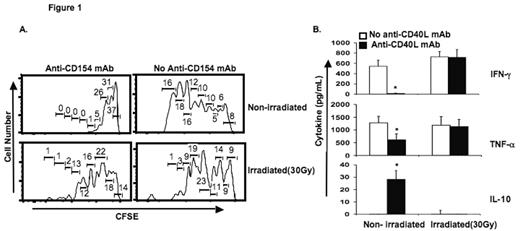
Results: Graded doses of irradiation (0, 7.5, 15, and 30 Gy) produced increasing frequencies of apoptosis in murine splenocytes (4, 26, 41, 49, and 27 % apoptotic cells, respectively). Irradiation of MLR stimulators abrogated the immunosuppressive effect of anti-CD40L mAb. Using MLR with untreated (non-irradiated stimulators), proliferation of CFSE-stained responder T-cells (Figure 1A) and synthesis of IFN-γ and TNF-α were decreased, while production of IL-10 increased in the presence of anti-CD40L mAb (Figure 1B). Irradiated, apoptotic stimulators led to graded increases in IFN-γ and TNF-α synthesis (Figure 1B), increased proliferation of CFSE-stained responder T-cells (Figure 1A), and decreased production of IL-10 in MLR containing anti-CD40L mAb (Figure 1B). Contrary to our predictions, mixed-chimerism without GVHD was enhanced by pre-transplant administration of viable allogeneic splenocytes, and diminished in mice with prior exposure to apoptotic/necrotic donor splenocytes at test points up to 250 days post-transplant. Psoralen plus ultraviolet A treated splenocytes had a similar frequency of apoptotic cells and led to a similar level of donor chimerism as 7.5 Gy irradiation-treated cells. The highest level of donor chimerism was observed when viable donor CD11b+ splenocytes were administered pre-transplant as the “tolerizing” cell infusion.
Conclusion: The pre-transplant administration of donor splenocytes rendered apoptotic/necrotic by irradiation doses of 15 Gy or higher led to lower levels of donor BM-derived chimerism compared to transplant regimens containing viable donor splenocyte administered on prior to BMT or day −6 regimens with no “ tolerizing ” pre-transplant donor cells in a non-myeloablative model of murine BMT utilizing low-dose busulfan and anti-CD40L mAb. Bidirectional tolerance induced by anti-CD40L mAb is an active process involving viable donor cells. The mechanisms whereby anti-CD40L mAb induces tolerance via participation of donor and host regulatory elements will be discussed.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author