Abstract
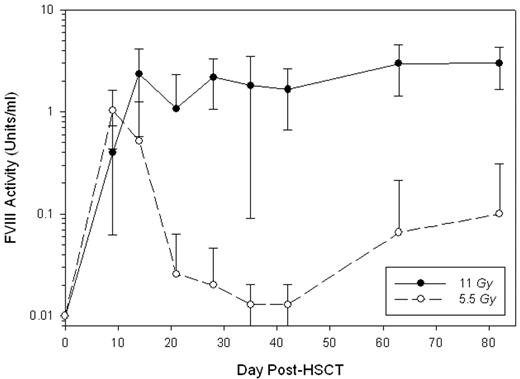
The development of inhibitory antibodies directed against human factor VIII (hfVIII) remains the most significant clinical complication associated with the treatment of hemophilia A. Recently, we demonstrated that transplantation of genetically-modified hematopoietic stem cells (HSCs) containing a high-expression porcine (HEP) fVIII transgene promoted sustained, high-level fVIII expression in naïve, genetically-immunocompetent hemophilia A mice (Gangadharan et al. 2006. Blood 107, 3859–64). In the current study, HEP-fVIII HSC transplantation (HSCT)-based gene therapy was tested in the setting of hemophilia A complicated by the presence of circulating anti-hfVIII inhibitory antibodies. Following a series of intravenous injections with recombinant hfVIII, hemophilia A mice developed anti-hfVIII antibodies with clinically-significant inhibitory titers. The median ELISA and Bethesda (inhibitory) titers were 2938 and 55 (n = 18), respectively, and a significant correlation between the anti-hfVIII ELISA and Bethesda (inhibitory) titers was observed (P < 0.001). With the exception of two mice, all plasma samples analyzed contained anti-HEP-fVIII cross-reactive antibodies by ELISA, and 5 of 16 displayed detectable anti-HEP-fVIII inhibitory activity. HSCs were transduced ex vivo with recombinant retrovirus carrying a HEP-fVIII transgene and transplanted into lethally-irradiated (11 Gy total body irradiation) hemophilia A mice (n = 11). Twelve wks post-HSCT, all mice displayed high-level donor cell engraftment in peripheral blood mononuclear cells (PBMCs) at 81 ± 22% (mean ± SD) and expressed therapeutic levels of fVIII with a mean activity of 3 ± 1.3 units/ml (Figure 1). A second cohort of mice (n = 7) underwent a similar HSCT gene therapy procedure with the exception that they received a sub-lethal dose of total body irradiation (5.5 Gy). These mice exhibited lower-level donor cell engraftment at 6.5 ± 6% (range 0.7 – 13%) in PBMCs at 12 weeks post-HSCT. At day 9 post-HSCT, the mice in this cohort contained circulating fVIII activity levels at 1 ± 0.6 units/ml (Figure 1). However in 6 of 7 mice, fVIII activity levels returned to baseline (≤0.01 units/ml) by day 28 post-HSCT, and in 4 of 7 mice, fVIII activity remained below the level of detection. The remaining 3 mice displayed 0.22 ± 0.3 (range 0.04 – 0.57) units/ml fVIII activity at 12 weeks post-HSCT and showed a trend towards greater donor PBMC engraftment, 12.8 ± 0.2% versus 1.8 ± 1.3% (P = 0.057). Analysis of the anti-hfVIII ELISA titers post-HSCT in both cohorts of mice revealed that while the titers steadily decreased in the lethally-irradiated mice (initial t1/2 ~ 12 days), the titers remained unchanged in the sub-lethally-irradiated mice possibly explaining the differences observed for donor cell engraftment and fVIII expression between the 2 groups. These data provide proof-of-concept that HSCT-based gene therapy incorporating a HEP-fVIII transgene could be utilized for the treatment of high-risk patients with refractory anti-hfVIII inhibitors.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author