Abstract
Background: THAL, whose activity in MM was discovered in the setting of advanced and refractory disease in the late 1990’s (Singhal, NEJM, 2000), has become the standard front-line therapy in combination with dexamethasone (DEX). In a randomized phase III tandem transplant trial, TT2, a higher complete response (CR) rate and longer event-free survival (EFS) had been observed on the THAL arm (Barlogie, NEJM, 2006). The similar overall survival (OS) on THAL and control arms had been attributed to the routine use of THAL as salvage therapy for the patients randomized to the No-THAL arm and the shorter post-relapse OS among patients randomized to the THAL arm.
Patients and Methods: With a median follow-up on TT2 of 53mo, 107 patients have relapsed and 219 died. Subset analyses were performed to determine whether THAL confers an OS advantage in any subgroup of patients.
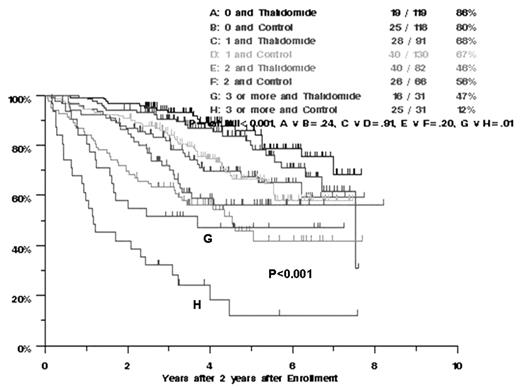
Results: 6-yr EFS and OS rates are 48%/63% on THAL and 38%/58% on control arm (p=0.01/0.67). Post-relapse OS is now similar with median durations of 5.3mo/4.3mo among control/THAL arms (p=0.11). According to multivariate analyses of 11 standard prognostic factors, EFS was shorter among patients treated without THAL, in the presence of cytogenetic abnormalities (CA), B2M and LDH elevations and low albumin, whereas CR was favorable; OS was inferior with CA, high LDH, low albumin and in patients not receiving 2nd transplant or not achieving CR. Randomization to THAL was beneficial only in the >2 risk factor group: 6-yr OS was 47% in 31 patients on THAL and 12% in 31 control patients (Figure 1, p=0.01). When examined in the context of GEP (70 gene model-based high versus low risk groups) and inter-phase FISH data (amp1q21), available in 260 patients, the 57 with GEP low risk and absence of amp1q21 receiving THAL had 5-yr OS of 90% compared to 74% among 73 controls (p=0.13).
Conclusion: With longer follow-up of 53mo on TT2, EFS remains superior among patients randomized to THAL; post-relapse survival is no longer inferior among those randomized to THAL; THAL benefited a high-risk subgroup with >2 standard risk factors, whereas no significant `difference has yet emerged among genetically defined subgroups.
Disclosures: Millennium, Zymogenetics, Novartis.; Millennium, Novartis, Serono.; Millennium, Novartis.
Author notes
Corresponding author