Abstract
Previous studies have demonstrated that sickle cell disease (SCD) can be corrected in mouse models by transduction of hematopoietic stem cells with lentiviral vectors containing antisickling globin genes followed by transplantation of these cells into syngeneic recipients. Although self-inactivating (SIN) lentiviral vectors with or without insulator elements should provide a safe and effective treatment in humans, some concerns about insertional mutagenesis persist. An ideal correction would involve replacement of the sickle globin gene (βS) with a normal copy of the gene (βA). We recently derived embryonic stem (ES) cells from a novel knock-in mouse model of SCD and tested a protocol for correcting the sickle mutation by homologous recombination. In this paper, we demonstrate the replacement of the human βS-globin gene with a human βA-globin gene and the derivation of mice from these cells. The animals produce high levels of normal human hemoglobin (HbA) and the pathology associated with SCD is corrected. Hematologic values are restored to normal levels and organ pathology is ameliorated. These experiments provide a foundation for similar studies in human ES cells derived from sickle cell patients. Although efficient methods for production of human ES cells by somatic nuclear transfer must be developed, the data in this paper demonstrate that sickle cell disease can be corrected without the risk of insertional mutagenesis.
Introduction
Sickle cell disease (SCD) is an autosomal recessive disorder that affects a significant proportion (approximately 1 in 500 individuals) of the African-American population. Hispanic, Arabic, Mediterranean, and some Asian populations are also affected. More than 300 000 individuals worldwide and more than 70 000 in the United States suffer from the disease. The molecular basis for sickle cell disease is an A to T transversion in the sixth codon of the human β-globin gene.1,2 This simple transversion changes a polar glutamic acid residue to a nonpolar valine in the βs-globin chain on the surface of HbS (α2βS2) tetramers. The valine creates a hydrophobic projection that fits into a natural hydrophobic pocket formed on Hb tetramers after deoxygenation.3,4 The interaction of tetramers results in the formation of HbS polymers/fibers that cause red blood cells (RBCs) to become rigid and nondeformable and to occlude small capillaries.5-10 These vasoocclusive events cause severe tissue damage that can result in strokes, splenic infarction, kidney failure, liver and lung disorders, painful crises, and other complications. Cycles of erythrocyte sickling also cause the cells to become fragile, and lysis produces chronic anemia.
Sickle cell disease is normally a relatively benign disorder in the first few months of life because human fetal hemoglobin (HbF) has potent antisickling properties. HbF, which comprises 70% to 90% of total hemoglobin at birth, is gradually replaced by HbS during the first few months of life. Rising HbS levels result in the onset of disease between 3 and 6 months of age. We recently produced a knock-out/transgenic mouse model that mimics this switch from HbF to HbS. The LCR γ-βS transgene in these animals was designed to switch hemoglobins after birth11-13 rather than before birth14-19 as observed in animals produced with cosmid, BAC, or YAC transgenes. The LCR γ-βS transgenic animals are relatively healthy at birth and then develop severe anemia when the switch to HbS is completed at approximately 3 weeks of age. More recently, we used the same γ-βS configuration to produce a knock-in mouse model of sickle cell disease (T.M.R. and T.M.T., unpublished data, November 2003). These animals complete the switch of human hemoglobins (HbF to HbS) after birth and develop the same severe anemia as the knock-out/transgenic mice during the first week of life. We derived embryonic stem (ES) cells from these sickle knock-in mice for the present studies.
Two groups have corrected SCD in mouse models by transduction of hematopoietic stem cells with lentiviral vectors containing antisickling globin genes followed by transplantation of these cells into syngeneic recipients.20,21 Although self-inactivating (SIN) lentiviral vectors with or without insulator elements should provide a safe and effective treatment for hemoglobinopathies,22 some concerns about insertional mutagenesis persist.23 If viral integration inhibits a tumor-suppressor gene or activates an oncogene, leukemia can result. Recent studies of children who received a transplant of retrovirally transduced hematopoietic cells in France reveal that this outcome can occur.24 An approach that bypasses this problem is replacement of the sickle globin gene (βS) with a normal copy of the gene (βA). In this paper, we demonstrate the replacement of the human βS-globin gene with a human βA-globin gene by homologous recombination, and the derivation of mice from these cells. The results demonstrate successful correction of the hemolytic anemia and organ pathology that characterize sickle cell disease in humans.
Materials and methods
Production of the –383 γ-βA-globin gene construct
A plasmid containing the human Aγ globin gene with 383 bp of 5′ flanking sequence (4.6 kb total) and a human βA globin gene with 815 bp of 5′ flanking sequence (4.1 kb total) was digested with SalI to produce an 8.7-kb –383 γ-βA DNA fragment. The SalI sites were changed to ClaI by adding adaptors and the 8.7-kb ClaI fragment was subcloned into pBlueScript. A herpes simplex virus (HSV) thymidine kinase (TK) gene, which is driven by a phosphoglycerate kinase (PGK) promoter, was digested with XbaI and a 2.0-kb XbaI fragment was isolated. This fragment was subcloned into the plasmid pTR18 that contains XhoI and SalI sites on either side of XbaI. This plasmid was digested with XhoI and SalI to generate a 2.0-kb XhoI/SalI fragment. Another plasmid, containing 1.7 kb of mouse 5′ homology, a floxed PGK/Hygromycin (Hygro) gene, and 7 kb of mouse 3′ homology, was linearized with SalI, and the 13.3-kb DNA fragment was isolated from an agarose gel. The 2.0-kb XhoI/SalI fragment containing PGK/TK was inserted into the SalI site of pTR401. The resulting plasmid was then digested with ClaI, which cuts between the mouse 5′ homology and the floxed PGK/Hygro gene, and the 8.7-kb γ-βAClaI fragment was inserted. This final plasmid containing TK/mouse 5′ homology/–383 γ-βA/floxed PGK/Hygro/mouse 3′ homology was digested with multiple enzymes to verify that the 24-kb plasmid was correct.
ES cell culture and homologous recombination to repair DNA lesion
The production of a novel, knock-in mouse model of sickle cell disease and the derivation of ES cells from these mice will be described elsewhere (T.M.R. and T.M.T., unpublished data, November 2003). The –383 γ-βA DNA construct was linearized by NotI digestion and electroporated into the knock-in sickle ES cells (hα/hα, –1400 γ-βS/–1400 γ-βS). The cells were plated onto mitomycin C–treated mouse embryonic fibroblasts (MEFs) in Dulbecco modification of Eagle medium (DMEM) containing 16.7% fetal bovine serum (FBS; HyClone, Logan, UT), 1× nucleosides, 2 mM l-glutamine, 1× nonessential amino acids, 50 IU/mL penicillin, 50 μg/mL streptomycin, 0.1 mM β-mercaptoethanol, and 1000 U/mL leukemia inhibitory factor (LIF). Positive/negative selection in hygromycin (125 μg/mL) and gancyclovir (2 μM) was used to enrich for homologous recombinants.25 DNA isolated from individual ES cell colonies was analyzed by polymerase chain reaction (PCR). Homologous recombinants were identified with primer 1 and primer 2 to identify correct 5′ sequences (primer 1 is outside of the vector homology region) and with primers 5 and 6 to identify correct 3′ sequences (primer 6 is outside of the vector homology region). PCR with primers 3 and 4 followed by Bsu36I digestion was used to distinguish βS and βA alleles. Primer sequences are as follows: primer 1, 5′-CTCCTGACTCGGTATCCTGC-3′; primer 2, 5′-GAAGTTCTCAGGATCCACATGC-3′; primer 3, 5′-GATATATCTTAGAGGAGGGC-3′; primer 4, 5′-CCAACTTCATCCACGTTCAC-3′; primer 5, 5′-CAGAGCTTGGTTGACGGCAATTTCG-3′; and primer 6, 5′-TGAGCTCCGGAGGTACCCAGG-3′.
Positive colonies were electroporated with a cytomegalovirus (CMV)/Cre plasmid, and individual colonies containing a correctly deleted marker gene were identified by PCR.
Blastocyst injection and globin protein analysis
Correctly targeted ES cell lines were injected into C57BL/6 blastocysts. Chimeric males obtained from these injected blastocysts were mated with hα/hα, –1400 γ-βS/mβ females, and offspring were screened for the corrected genotypes (–383 γ-βA/–1400 γ-βS and –1400 γ-βA/–1400 γ-βS; “Results”) by PCR of tail DNA and Bsu36I digestion.
Fifty microliters of whole blood was washed in 500 μL PBS. Cell pellets were resuspended in 100 μL lysis solution (5 mM sodium phosphate, 0.5 mM EDTA, pH 7.4) and incubated on ice for 15 minutes. One tenth volume of 10% NaCl was added and the sample was centrifuged at maximum speed in an Eppendorf microfuge (Hamburg, Germany). Approximately 1 μL of the supernatant was analyzed on an isoelectric focusing (IEF) gel. IEF was performed using the Isothermal Controlled Electrophoresis system (Fisher Scientific, Pittsburgh, PA) with precast agarose IEF gels (RESOLVE from PerkinElmer, Helsinki, Finland). Hemoglobin bands were quantitated by densitometry with a BioRad (Hercules, CA) GS-800 scanner using Quantity One software (BioRad).
Hematologic indices and histopathology
Blood was collected from anesthetized animals into Microtainer EDTA collection tubes (Becton Dickinson, Franklin Lakes, NJ). RBC count was measured on a HemaVet 1700 (CDC Technology, Oxford, CT) hematology analyzer. Hemoglobin concentration was determined spectrophotometrically after conversion to cyanmethemoglobin with Drabkin reagent (Sigma, St Louis, MO). Before determining the hemoglobin concentration, red cell membranes were pelleted at 16 000g for 5 minutes in an Eppendorf microfuge. Packed cell volume (PCV) was measured with a JorVet J503 (Jorgenson Laboratories Systems, Loveland, CO) microhematocrit centrifuge. Reticulocyte counts were determined by flow cytometry after staining with thiazole orange. Urine osmolality was measured with the Wescor Vapro Vapor Pressure Osmometer 5520 (Logan, UT) after food and water were withheld from the mice for 16 hours. Tissue preparations from the spleen, liver, and kidney were fixed in 70% alcoholic formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin stain by standard methods.
Results
Production of a human βA-globin gene targeting construct
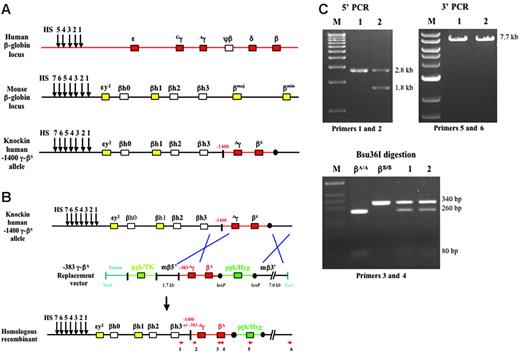
We recently produced a knock-in mouse model of sickle cell disease by replacing the mouse α-globin genes with a human α-globin gene (hα/hα) and by replacing the mouse β-globin genes with human Aγ- and βS-globin genes (–1400 γ-βS/–1400 γ-βS; T.M.R. and T.M.T., unpublished data, November 2003). We also derived ES cells from these knock-in sickle mice. The ES cells are homozygous for the human α-globin gene (not shown) and homozygous for the Aγ- and βS-globin genes as indicated in Figure 1A. The Aγ-globin gene in the knock-in sickle ES cells contains 1400 bp of 5′ flanking sequence; therefore, the genotype is designated as –1400 γ-βS. The targeting construct that we produced for gene replacement contained a human –383 γ-βA-globin gene fragment that is similar to –1400 γ-βS except that the γ gene has only 383 bp of 5′ flanking sequence. This-24 kb replacement vector contained 1.7 kb of mouse 5′ flanking sequence, the –383 γ-βA fragment (8.7 kb), a floxed PGK/Hygro gene, 7 kb of mouse 3′ flanking sequence, and a PGK/TK gene (Figure 1B).
Replacement of the human βS-globin gene with the human βA-globin gene in ES cells derived from knock-in sickle mice
The targeting construct was electroporated into knock-in sickle ES cells, and the cells were grown in hygromycin and gancyclovir for 2 weeks. One hundred thirteen colonies were picked and homologous recombinants were identified by PCR of genomic DNA. In 16 (14.2%) of the 113 colonies, the βS-globin gene was successfully replaced with the βA-globin gene. In 1 of the 16 colonies, recombination at the 5′ end occurred upstream of the –383 γ sequence; therefore, the replacement allele contained the –383 Aγ-globin gene promoter (Figure 1B-C, 5′ PCR, lane 2; 1.8-kb PCR product). In the remaining 15 colonies, recombination at the 5′ end occurred downstream of the –383 γ sequence; therefore, the replacement allele maintained the –1400 γ promoter (Figure 1B-C, 5′ PCR, lane 1; 2.8-kb PCR product). Genomic DNA from all 16 colonies was analyzed by PCR with primers 3 and 4, which amplify both βS and βA alleles producing 340-bp amplicons. These amplicons were digested with Bsu36I, which does not cut the βS allele but digests the βA allele into 260- and 80-bp fragments (Figure 1B-C). Homologous recombination at the 3′ end of all 16 colonies was correct (Figure 1B-C, 3′ PCR; 7.7-kb PCR product). Cell lines containing the –1400 γ-βA replacement allele or the –383 γ-βA replacement allele were transiently transfected with a CMV/Cre plasmid, and colonies containing a deletion of the PGK/Hygro marker gene were identified by PCR.
Replacement of the βS-globin gene with a βA-globin gene in knock-in sickle ES cells. (A) Schematic representation of the human β-globin locus, mouse β-globin locus, and the human –1400 Aγ-βS knock-in locus in ES cells derived for this study. Arrows indicate DNase I hypersensitive sites (HSs) that mark the locus control region (LCR). Red and yellow boxes represent functional human and mouse genes, respectively. White boxes represent pseudogenes. Black circles indicate loxP sites. (B) Schematic representation of gene replacement in knock-in sickle ES cells. The 24-kb replacement vector contains a 2.1-kb PGK/TK marker gene, 1.7 kb of mouse 5′ flanking sequence, a –383 γ-βA fragment (8.7 kb), a 1.8-kb floxed PGK/Hygro gene, and 7 kb of mouse 3′ flanking sequence. Homologous recombinants were identified by PCR with primers 1 and 2 to identify correct 5′ sequences (primer 1 is outside of the vector homology region) and with primers 5 and 6 to identify correct 3′ sequences (primer 6 is outside of the vector homology region). PCR with primers 3 and 4 followed by Bsu36I digestion was used to distinguish βS and βA alleles. (C) 5′ PCR (primers 1 and 2) and 3′ PCR (primers 5 and 6) from 2 positive homologous recombinant ES cell lines (clones 1 and 2). In clone 1, recombination at the 5′ end occurred downstream of the –383 γ sequence; therefore, the replacement allele maintained the –1400 γ promoter (2.8-kb PCR product). In clone 2, recombination at the 5′ end occurred upstream of the –383 γ sequence; therefore, the replacement allele contained the –383 Aγ promoter (1.8-kb PCR product). Bsu36I digestion of PCR fragments derived with primers 3 and 4 is presented in the second panel of panel C. βA fragments are digested, but βS fragments are resistant to digestion.
Replacement of the βS-globin gene with a βA-globin gene in knock-in sickle ES cells. (A) Schematic representation of the human β-globin locus, mouse β-globin locus, and the human –1400 Aγ-βS knock-in locus in ES cells derived for this study. Arrows indicate DNase I hypersensitive sites (HSs) that mark the locus control region (LCR). Red and yellow boxes represent functional human and mouse genes, respectively. White boxes represent pseudogenes. Black circles indicate loxP sites. (B) Schematic representation of gene replacement in knock-in sickle ES cells. The 24-kb replacement vector contains a 2.1-kb PGK/TK marker gene, 1.7 kb of mouse 5′ flanking sequence, a –383 γ-βA fragment (8.7 kb), a 1.8-kb floxed PGK/Hygro gene, and 7 kb of mouse 3′ flanking sequence. Homologous recombinants were identified by PCR with primers 1 and 2 to identify correct 5′ sequences (primer 1 is outside of the vector homology region) and with primers 5 and 6 to identify correct 3′ sequences (primer 6 is outside of the vector homology region). PCR with primers 3 and 4 followed by Bsu36I digestion was used to distinguish βS and βA alleles. (C) 5′ PCR (primers 1 and 2) and 3′ PCR (primers 5 and 6) from 2 positive homologous recombinant ES cell lines (clones 1 and 2). In clone 1, recombination at the 5′ end occurred downstream of the –383 γ sequence; therefore, the replacement allele maintained the –1400 γ promoter (2.8-kb PCR product). In clone 2, recombination at the 5′ end occurred upstream of the –383 γ sequence; therefore, the replacement allele contained the –383 Aγ promoter (1.8-kb PCR product). Bsu36I digestion of PCR fragments derived with primers 3 and 4 is presented in the second panel of panel C. βA fragments are digested, but βS fragments are resistant to digestion.
Mice derived from corrected sickle ES cells express high levels of human HbA
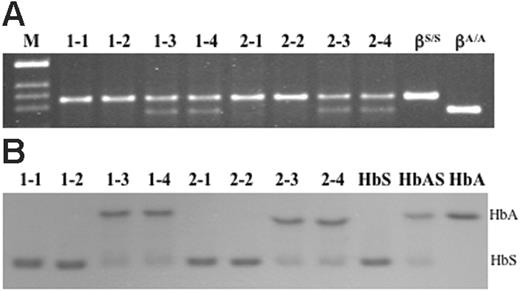
After removal of the marker, –1400 γ-βA and –383 γ-βA ES cell lines were injected into C57BL/6 blastocysts. Chimeric males obtained from these blastocysts were mated with females that were homozygous for human α-globin gene knock-in and heterozygous for human –1400 γ-βS knock-in (hα/hα, –1400 γ-βS/mβ), and offspring were screened for the corrected genotypes (–383 γ-βA/–1400 γ-βS and –1400 γ-βA/–1400 γ-βS) by PCR of tail DNA and Bsu36I digestion. Figure 2A demonstrates that animals with corrected genotypes were obtained from both cell lines. Animals 1-3 and 1-4 are –1400 γ-βA/–1400 γ-βS and animals 2-3 and 2-4 are –383 γ-βA/–1400 γ-βS. The 1-1, 1-2, 2-1, and 2-2 animals are homozygous sickle mice derived from the same matings. The last 2 lanes are controls obtained from a human sickle patient (βS/βS) and an unaffected individual (βA/βA).
Blood samples were obtained from the animals, and hemolysates were analyzed on IEF gels. The results are illustrated in Figure 2B. The last 3 lanes are control hemolysates from a sickle patient, an individual with sickle trait, and an unaffected individual. The results in Figure 2B demonstrate that high levels of human HbA are synthesized in red blood cells from 1-3, 1-4, 2-3, and 2-4 animals. Of interest, all 4 of these animals mimic the HbA to HbS ratio in humans with sickle trait. Individuals with sickle trait present little if any pathology; therefore, these results suggest that the level of HbA achieved by gene replacement is sufficient to correct the disease.
Genomic DNA and hemoglobin analysis of mice derived from targeted ES cell lines 1 and 2. (A) After removal of the PGK/Hygro marker from corrected ES cell clone 1 (–1400 γ-βA/–1400 γ-βS) and clone 2 (–383 γ-βA/–1400 γ-βS), cells were injected into C57BL/6 blastocysts. Chimeric males obtained from these blastocysts were mated with hα/hα, –1400 γ-βS/mβ females and offspring were screened for the corrected genotypes (–1400 γ-βA/–1400 γ-βS and –383 γ-βA/–1400 γ-βS) by PCR of tail DNA and Bsu36I digestion. (B) IEF gel of hemolysates from mice identified as sickle and corrected animals in panel A. The last 3 lanes are human control hemolysates from a sickle patient, an individual with sickle trait, and an unaffected individual. Of interest, the ratio of HbA to HbS in corrected animals mimics the ratio in humans with sickle trait.
Genomic DNA and hemoglobin analysis of mice derived from targeted ES cell lines 1 and 2. (A) After removal of the PGK/Hygro marker from corrected ES cell clone 1 (–1400 γ-βA/–1400 γ-βS) and clone 2 (–383 γ-βA/–1400 γ-βS), cells were injected into C57BL/6 blastocysts. Chimeric males obtained from these blastocysts were mated with hα/hα, –1400 γ-βS/mβ females and offspring were screened for the corrected genotypes (–1400 γ-βA/–1400 γ-βS and –383 γ-βA/–1400 γ-βS) by PCR of tail DNA and Bsu36I digestion. (B) IEF gel of hemolysates from mice identified as sickle and corrected animals in panel A. The last 3 lanes are human control hemolysates from a sickle patient, an individual with sickle trait, and an unaffected individual. Of interest, the ratio of HbA to HbS in corrected animals mimics the ratio in humans with sickle trait.
Correction of abnormal RBC morphology in –383 γ-βA–corrected mice. (A) Blood smear of an hβA/hβA control. (B) Blood smear of an hβS/hβS sickle animal with characteristic sickled erythrocytes and a pronounced reticulocytosis. (C) Blood smear of an hβA/hβS-corrected mouse. No sickled cells were observed in any field examined. Blood smears were stained with Wright-Giemsa and the magnification is 100×/1.40 NA oil objective (Nikon Eclipse E800 inverted microscope [Nikon, Tokyo, Japan], Hamamatsu C5810 Color Chilled 3CCD camera [Hamamatsu City, Japan]; Adobe Photoshop CS version 8.0 imaging software [San Jose, CA]). Data from –1400 γ-βA–corrected mice are identical to –383 γ-βA–corrected animals.
Correction of abnormal RBC morphology in –383 γ-βA–corrected mice. (A) Blood smear of an hβA/hβA control. (B) Blood smear of an hβS/hβS sickle animal with characteristic sickled erythrocytes and a pronounced reticulocytosis. (C) Blood smear of an hβA/hβS-corrected mouse. No sickled cells were observed in any field examined. Blood smears were stained with Wright-Giemsa and the magnification is 100×/1.40 NA oil objective (Nikon Eclipse E800 inverted microscope [Nikon, Tokyo, Japan], Hamamatsu C5810 Color Chilled 3CCD camera [Hamamatsu City, Japan]; Adobe Photoshop CS version 8.0 imaging software [San Jose, CA]). Data from –1400 γ-βA–corrected mice are identical to –383 γ-βA–corrected animals.
Correction of abnormal RBC morphology and hematologic parameters in mice derived from targeted ES cells
Blood smears from knock-in sickle (βS/βS), corrected (βA/βS), and control (βA/βA) animals are illustrated in Figure 3. Many rigid, elongated cells are observed in the sickle mice (Figure 3B); however, no sickled cells are observed in βA/βS-corrected mice or in βA/βA controls (compare 3A and 3C). The blood smears of corrected animals also lack the anisocytosis, poikilocytosis, and polychromasia characteristics of erythrocytes in the sickle mice. The red cells in corrected mice are nearly identical to the cells in control animals, and this phenotype is similar to human red cells from individuals with sickle trait.
We also measured hematologic values in the corrected mice and compared these values to the numbers obtained for sickle and control animals. These data are presented in Table 1. Compared with sickle animals, the corrected mice have marked increases in RBC counts (6.7 ± 1.1 to 11.3 ± 1.0 × 1012/L [6.7 ± 1.1 to 11.3 ± 1.0 × 106/μL]), Hb levels (71 ± 9 to 108 ± 10 g/L [7.1 ± 0.9 to 10.8 ± 1.0 g/dL]), and hematocrit levels (.351 ± .033 to .417 ± .025 as a proportion of 1 [35.1% ± 3.3% to 41.7% ± 2.5%]) and a significant reduction in reticulocyte counts (.754 ± .039 to .084 ± .026 as a proportion of RBCs [75.4% ± 3.9% to 8.4% ± 2.6%]). All of the values in the corrected mice (βA/βS) are similar to values in controls (βA/βA). These data demonstrate that the hematologic defects in sickle cell disease can be corrected by replacing one βS allele with a βA allele in ES cells.
Amelioration of spleen, liver, and kidney pathology, and restoration of kidney function in mice derived from corrected ES cells
βA/βS-corrected mice developed little spleen, liver, and kidney pathology compared with knock-in βS/βS sickle mice. Histologic sections of control (βA/βA), sickle (βS/βS), and corrected (βA/βS) animals are presented in Figure 4A. The spleens of knock-in sickle mice are characterized by a massive expansion of red pulp, dramatic pooling of sinusoidal erythrocytes, vasoocclusion, and a complete loss of lymphoid follicular structure. In corrected mice, normal splenic red and white pulp is observed, and virtually no pools of sickle erythrocytes or infarcts are evident. In addition, splenomegaly is substantially diminished in corrected mice (Figure 4B). The spleens of βA/βS mice weigh approximately 7-fold less than the spleens of βS/βS animals and are almost the same size as spleens of βA/βA control animals.
The livers of knock-in sickle mice are characterized by focal areas of necrosis and pronounced congestion of the intrahepatic vasculature with aggregates of sickled RBCs. Erythroid progenitors are evident in the sinusoids, and this extramedullary hematopoiesis is indicative of severe anemia. There is also abundant hemosiderin deposition subsequent to Kupffer cell erythrophagocytosis. In βA/βS-corrected animals, focal areas of necrosis and aggregation of sickled erythrocytes are not observed. In addition, extramedullary hematopoiesis and hemosiderin deposition are absent. These results demonstrate that organ pathology is significantly ameliorated in mice derived from corrected ES cells.
In the kidneys of knock-in sickle mice, engorgement and occlusion of blood vessels with sickled erythrocytes results in vascular, tubular, and glomerular changes. Sequestration and occlusion are most obvious at the corticomedullary junctions where dilated capillaries are easily observed in this region of reduced oxygen tension. Reduced medullary blood flow in HbS patients causes extensive tubular damage that results in hyposthenuria, and this same loss of urine-concentrating ability is observed in the knock-in βS/βS sickle mice. In contrast, the kidneys of βA/βS-corrected mice appear normal and free of the disruptive vascular RBC pooling and hemosiderin deposits observed in the sickle mice. Most importantly, urine-concentrating ability is completely restored in βA/βS-corrected mice (Table 1). Urine concentrations are increased from 768 ± 102 mOsm in βS/βS animals to 2388 ± 397 mOsm in βA/βS mice; urine concentrations in βA/βA controls are 2129 ± 281. These data demonstrate that kidney function is restored in mice derived from corrected ES cells.
Amelioration of spleen, liver, and kidney pathology in –383 γ-βA– corrected mice. (A) Spleen, liver, and kidney sections were analyzed at low (10×/0.45 NA objective for spleen and kidney, 40×/0.75 NA objective for liver) and high (100×/1.40 NA oil objective) magnification. In –383 γ-βA–corrected mice, normal splenic red and white pulp is observed, and virtually no pools of sickle erythrocytes or infarcts are evident. In livers of –383 γ-βA animals, focal areas of necrosis and aggregation of sickled erythrocytes are not observed; also, extramedullary hematopoiesis and hemosiderin deposition are absent. Kidneys of –383 γ-βA mice appear normal and free of the disruptive vascular RBC pooling. All sections were stained with hematoxylin-eosin. (B) Correction of splenomegaly in –383 γ-βA–corrected mice. Data from –1400 γ-βA–corrected mice are identical to –383 γ-βA–corrected animals.
Amelioration of spleen, liver, and kidney pathology in –383 γ-βA– corrected mice. (A) Spleen, liver, and kidney sections were analyzed at low (10×/0.45 NA objective for spleen and kidney, 40×/0.75 NA objective for liver) and high (100×/1.40 NA oil objective) magnification. In –383 γ-βA–corrected mice, normal splenic red and white pulp is observed, and virtually no pools of sickle erythrocytes or infarcts are evident. In livers of –383 γ-βA animals, focal areas of necrosis and aggregation of sickled erythrocytes are not observed; also, extramedullary hematopoiesis and hemosiderin deposition are absent. Kidneys of –383 γ-βA mice appear normal and free of the disruptive vascular RBC pooling. All sections were stained with hematoxylin-eosin. (B) Correction of splenomegaly in –383 γ-βA–corrected mice. Data from –1400 γ-βA–corrected mice are identical to –383 γ-βA–corrected animals.
Discussion
We have recently produced a new mouse model of sickle cell disease by replacing the mouse β-globin genes with a 9.7-kb DNA fragment containing the human Aγ-globin gene (5.6 kb) and a human βS-globin gene (4.1 kb). The γ- and βS-globin genes contain 1400 bp and 815 bp of 5′ flanking sequence, respectively. Mouse α-globin genes were also replaced with a human α-globin gene. At birth, these knock-in sickle mice continue to synthesize significant amounts of human HbF. During the first week after birth, the animals switch to more than 99% HbS and develop severe disease. We linked the human γ- and βS-globin genes in a relatively small fragment because we previously found that this design resulted in an HbF to HbS switch that mimicked the switch in human sickle patients.11-13 Larger fragments derived from cosmids, YACs, and BACs switch hemoglobins in utero,14-19 and this early switch from HbF to high levels of HbS results in a high rate of perinatal mortality. The γ-βS fragment in the knock-in sickle mice is identical to the configuration of the transgene in our previous knock-out/transgenic sickle mice, and globin gene switching is similar in these 2 models.
We derived ES cells from these animals and tested a gene replacement protocol for correcting the disease. This approach avoids random insertions that are characteristic of viral gene therapy approaches. Although relatively few insertional mutations have been observed in viral gene therapy studies, the SCID gene therapy trials in France demonstrated that leukemic cell clones can arise from insertional activation of LMO-2,24 and experiments in nonhuman primates have suggested that insertional inactivation of BCL-2A1 can result in acute myeloid leukemia.26 The risk of mutagenesis is a consequence of random insertion of one or more copies of the viral vector in a large number of cells. If 2 to 3 million CD34+ cells per kilogram of body weight are transduced and transplanted, a 50-kg patient would receive 100 million cells and, potentially, 100 million different viral insertions. Although the number of reported insertional mutations after viral gene therapy has been low, the large number of insertion sites remains a concern.
The gene replacement strategy described in this paper avoids the risk of insertional mutagenesis. The defective β-globin gene (βS) was replaced with a normal copy of the gene (βA) in ES cells, and cells derived from a single corrected clone were fully characterized before transplantation. In the present study, the cells were transplanted into blastocysts, and hematopoietic stem cells (HSCs) derived from these cells in vivo produced corrected red blood cells that did not sickle in recipients; consequently, the anemia and organ pathology of the disease was cured. Of course, translation of this approach to human patients would involve in vitro derivation of HSCs27-29 from corrected, patient-specific ES cells. This approach would provide corrected, fully characterized, syngeneic HSCs for intravenous transplantation, and red blood cells derived from these HSCs in vivo would not sickle.
The application of this approach to patients with sickle cell disease requires successful derivation of patient-specific ES cells by somatic cell nuclear transfer (SCNT). The recent retractions of 2 papers30,31 describing the derivation of human ES cells by this technique are a significant setback for the field. However, the feasibility of the approach has been demonstrated in animal models.32,33 Therefore, with additional basic research, there is a reasonable expectation that patient-specific, nuclear transfer ES (ntES) cells can be derived in the future. Correction of the sickle mutation by gene replacement in ntES cells derived from skin fibroblasts (or in skin fibroblasts before nuclear transfer) should provide a means to produce corrected cells that can be differentiated into HSCs in vitro and transplanted into patients. Red blood cells derived from these HSCs in vivo will not sickle, and this treatment should cure the disease.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2006-02-004812.
Supported by grants from the National Heart, Lung, and Blood Institute (T.M.T. and T.M.R.).
L.-C.W., C.-W.S., and T.M.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Correction of abnormal RBC morphology in –383 γ-βA–corrected mice. (A) Blood smear of an hβA/hβA control. (B) Blood smear of an hβS/hβS sickle animal with characteristic sickled erythrocytes and a pronounced reticulocytosis. (C) Blood smear of an hβA/hβS-corrected mouse. No sickled cells were observed in any field examined. Blood smears were stained with Wright-Giemsa and the magnification is 100×/1.40 NA oil objective (Nikon Eclipse E800 inverted microscope [Nikon, Tokyo, Japan], Hamamatsu C5810 Color Chilled 3CCD camera [Hamamatsu City, Japan]; Adobe Photoshop CS version 8.0 imaging software [San Jose, CA]). Data from –1400 γ-βA–corrected mice are identical to –383 γ-βA–corrected animals.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/4/10.1182_blood-2006-02-004812/3/m_zh80160699820003.jpeg?Expires=1768147522&Signature=e7iYyzbH0KP2AqfeTg1OTo-hNqczkSAnGXIQklb4JM4wW~9ICU69bKHWlVrMdLovhNxGz8iTFKObvlVI1Ens0snbv1KCH1PZ9pXjHh8A9CfbrdiMsm2WWmPyQ~AmWO9c2tK6V0pH7hSQUqtDPbCfZ~jz9qmJGMlAbZqDjgNapwigkusqsEpVFgO0olvKjY6S32cYxKLtmZdXUEfl-xCny6pYPvHiYrRt8AU2bclCD-1OVRe86Ylw2pXacP-MWM-zYOBRPmrzXo2l~fzfrbb2DCWTHJxB8DTQsHQfAjlCKTsH~93T5y-yoQqEsUuHvkz5jWErAz0VeYP2ntCB~meWJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

