Abstract
The introduction of imatinib mesylate (IM) has revolutionized the treatment of chronic myeloid leukemia (CML). Although experience is too limited to permit evidence-based evaluation of survival, the available data fully justify critical reassessment of CML management. The panel therefore reviewed treatment of CML since 1998. It confirmed the value of IM (400 mg/day) and of conventional allogeneic hematopoietic stem cell transplantation (alloHSCT). It recommended that the preferred initial treatment for most patients newly diagnosed in chronic phase should now be 400 mg IM daily. A dose increase of IM, alloHSCT, or investigational treatments were recommended in case of failure, and could be considered in case of suboptimal response. Failure was defined at 3 months (no hematologic response [HR]), 6 months (incomplete HR or no cytogenetic response [CgR]), 12 months (less than partial CgR [Philadelphia chromosome–positive (Ph+) > 35%]), 18 months (less than complete CgR), and in case of HR or CgR loss, or appearance of highly IM-resistant BCR-ABL mutations. Suboptimal response was defined at 3 months (incomplete HR), 6 months (less than partial CgR), 12 months (less than complete CgR), 18 months (less than major molecular response [MMolR]), and, in case of MMolR loss, other mutations or other chromosomal abnormalities. The importance of regular monitoring at experienced centers was highlighted.
Introduction
After the initial descriptions of chronic myeloid leukemia (CML) more than 150 years ago, little meaningful progress was made in its treatment for more than a century. Radiation therapy and busulfan contributed more to improving quality of life than to prolonging survival. Survival prolongation was first achieved with hydroxyurea (HU), much more with allogeneic hematopoietic stem cell transplantation (alloHSCT) and, later, in a minority of patients, with recombinant interferon-alpha (rIFNα).1 Understanding the pathogenesis of the disease began with the discovery of the Philadelphia (Ph) chromosome followed by appreciation of its molecular counterpart, the BCR-ABL fusion gene.2,3 Recognition of the tyrosine kinase (TK) activity of the Bcr-Abl proteins led to the discovery of a new series of compounds targeted against BCR-ABL–encoded proteins, which inhibited the TK activity, thus aborting the signals controlling the leukemic phenotype.4 One of the TK inhibitors, imatinib mesylate (IM), was found to have a high and relatively specific biochemical activity and an acceptable pharmacokinetic and toxicity profile, and was thus rapidly introduced into clinical practice.5-7 This resulted in a revolutionary step in the management of CML and by extension a shift in paradigm for the management of cancer in general.
The most recent comprehensive analysis of CML treatment was an evidence-based guideline developed in 1998 by an expert panel convened by the American Society of Hematology (ASH) covering conventional chemotherapy, rIFNα, and alloHSCT.8 TK inhibitors were not considered at that time but were subsequently the subjects of editorials and preliminary reviews.7,9-14 Although it is premature at this time to perform an evidence-based analysis of the effects of IM, the implications and consequences of the introduction of TK inhibitors are so important that it is not too early to review the available data and to discuss how the treatment of CML could be managed and further progress could be pursued based upon expert opinion. Therefore, the European LeukemiaNet appointed a panel of experts to review the current situation. This report constitutes its opinion.
Methods
Panel composition
The panel included 19 members with recognized clinical and research expertise in CML, of whom 10 came from the European Union countries (France, Germany, Italy, Spain, Sweden, and the United Kingdom), 1 from Switzerland, 7 from the United States, and 1 from Australia.
Scope of the review
The first step was to perform a comprehensive and critical review of the literature after 1998 (the date of the last ASH analysis). A computerized literature search of the Medline database was conducted in April 2005 and updated in November 2005. Relevant abstracts presented at the 2004 and 2005 meetings of ASH, the American Society of Clinical Oncology, the European Group for Blood and Marrow Transplantation (EBMT), the European Hematology Association, and the International Society for Experimental Hematology were also reviewed. Thereafter, the panel met several times to discuss definition, evaluation, and monitoring of the responses, as well as treatment policy. It was agreed that discussion and proposals should be limited to early chronic-phase (ECP) patients not only because the treatment of CML patients in a more advanced phase is less amenable to generalizations, but also to focus on the importance of a first-line treatment strategy, late therapeutic interventions being generally less effective.
Definitions
The criteria that we have used to distinguish CP from accelerated phase (AP) are those that have been used in the most recent treatment reports.15-22 These criteria are listed in Table 1, together with World Health Organization (WHO) criteria, which differs slightly.23 The relative risk (RR) of progression and death in ECP patients may be calculated by using either the Sokal24 or the Hasford25 formulations (Table 2).
Summary and update of rIFNα
The superiority of rIFNα-based regimens over conventional chemotherapy was reported previously in the ASH analysis8 and was confirmed in a subsequent study.26 A trial of rIFNα versus a combination of rIFNα and low-dose arabinosyl cytosine (LDAC)27 partially confirmed an earlier study28 reporting that the cytogenetic response (CgR) rate was higher with the combination, but that overall survival did not differ. A study testing 3 MIU of rIFNα 3 times a week versus 5 MIU/square meters body surface/day indicated that the low dose was as effective and better tolerated than the high dose.29 The last updates of the major rIFNα studies reported a 9- or 10-year overall survival (OS) ranging from 27% to 53%.30 In 1 study of 317 patients who had achieved a complete CgR (CCgR), 50% were still in CCgR and 70% were alive after 10 years, with a significant difference in OS between low and high Sokal risk patients (10-year OS, 90% vs 40%).31 Residual leukemia was detectable at the molecular level in almost all these patients. Several studies have provided some insights into the biologic and molecular bases of the therapeutic effects of rIFNα,30 but there have been no new or updated clinical studies.
Summary and update of allogeneic and autologous HSCT
The ASH panel reported that about 50% of the patients who received alloHSCT in first CP from a matched-related donor remained alive and leukemia-free after 5 years.8 Several subsequent reports confirmed the data and extended the follow-up to 10 years, with an OS of 60% and an event-free survival (EFS) of 50%,32,33 and to 15 years, with an OS of 47%34 and 52%.35 In a meta-analysis of 3 randomized studies of 316 patients in CP, 10-year survival estimates were 63% and 65%.36 The Center for International Blood and Marrow Transplant Research (CIBMTR) reported on 4513 patients, with a median age of 35 years, who received transplants between 1978 and 1997.37 OS at 18 years was 50% for 3372 first CP patients and 20% for 1141 non–first CP patients. The cumulative incidence of relapse at 18 years was 25% for CP patients and 37% for the others. Relapses were seen up to 21 years after treatment. The longest follow-up of patients who received transplants from a matched-related donor is that reported by the EBMT on 2628 patients given transplants between 1980 and 1990.38 OS at 20 years was 34% for all patients, 41% for patients who received transplants in first CP from an human leukocyte antigen (HLA)–identical sibling, and 49% for those who had an EBMT risk score of 0-1. In children, 10-year OS estimates were reported to be 65% to 70%.39
An EBMT survey analyzed 3142 patients submitted to conventional alloHSCT in any phase of CML and from any donor.40 This analysis led to the formulation of a prognostic score subsequently validated by 2 other analyses (Tables 3 and 4).41,42 Depending on the risk score, survival ranged from 72% to 11% in all patients and from 70% to 25% in the patients who were given transplants in ECP (Table 4).
Progress in molecular DNA typing of HLA alleles, in the management of opportunistic infections, and in supportive care, as well as modifications and improvement of conditioning regimes and immunosuppresive therapy, have contributed to improved results of alloHSCT, using both family members and unrelated donors.43 For patients with CML receiving conventional transplants, the use of peripheral blood stem cells has not been shown to be better than the use of marrow cells.44
Reduced intensity conditioning (RIC) is currently being evaluated for CML.45-48 The EBMT has reported on registry data of 187 patients (median age, 50 years) who were submitted to RIC-alloHSCT between 1994 and 2002, mainly from matched-related donors.49 Three-year OS was 70% for the patients with an EBMT score of 0 to 2, 50% for the patients with a score of 3 to 4, and about 30% for those with a score of 5 or higher. The use of RIC may permit transplantation also in older patients, but the long-term impact of these and other experimental procedures of alloHSCT on OS, EFS, and quality of life cannot yet be assessed.
The role of treatment intensification with autologous HSCT (autoHSCT) rescue has been the subject of a number of studies and reviews covering a period of more than 20 years.50 Several observations suggested that the procedure was useful in achieving more remissions and prolonging survival. Several randomized studies were initiated but none was completed. A meta-analysis of 6 such trials in which patients were randomly allocated to receive autoHSCT or a rIFNα-based regimen did not show an advantage for autoHSCT.51
Summary and update of IM data
IM versus rIFNα in ECP
The superiority of 400 mg IM daily over rIFNα and LDAC was established in a prospective randomized international study of 1106 ECP patients (International Randomized Study of Interferon and STI571 [IRIS]). IM was superior to rIFNα for efficacy, with a complete hematologic response (CHR) rate of 95% versus 55%, a CCgR rate of 76% versus 15% and progression-free survival (PFS; survival free from progression to AP/blast crisis [BC]) at 19 months of 97% versus 91% (P < .001). It was better also for compliance, toxicity, and quality of life.17,52 As expected, molecular response (MolR) rates were also significantly better, with an estimated major MolR (MMolR) rate at 12 months of 40% vs 2%.53 Since many patients who had been assigned to rIFNα and LDAC were crossed over to IM, it is difficult to meaningfully compare the long-term results of the 2 treatment arms. However, 2 independent retrospective analyses provided independent confirmation that IM was better than any other nontransplant treatment.54,55 Studies have shown that IM is a cost-effective first-line therapy compared with rIFNα.56
Follow-up clinical results in ECP
When IM was given at 400 mg daily for initial treatment of ECP patients, the CHR rate after 1 year was 95%, and the CCgR rate was 76%.17 Of those patients who had achieved a CCgR, a MMolR was achieved in 57% (40% of all the patients who had been assigned to IM).53 The proportion of MMolR patients was reported at 55% of all patients after 2 years.57 After 54 months of follow-up, PFS was 93%, OS was 90%, and survival freedom from progression to AP/BC as well as from hematologic or cytogenetic relapse was 84%.58 Currently, this survival outcome is better than for any other reported treatment. The annual rate of progression to AP/BC appeared to be fairly constant in the first 4 years of treatment, namely 1.5%, 2.8%, 1.6%, and 0.9%.58
Clinical results in late chronic phase, AP, and BC
Before IM was initially administered as first-line treatment for CML, it was given to patients who were in CP, but resistant or intolerant to rIFNα, or who had been treated with conventional chemotherapy. These patients are classified as “late CP” (LCP). Four international studies reported a CCgR rate ranging from 41% to 64% with a 5-year PFS of 69% and a 4-year OS of 86% to 88%.15,18,19,23,59-61 Moreover, 1 retrospective analysis found that survival of LCP IM-treated patients was superior to that of historical controls, even when a CCgR was not achieved.62
For AP patients the best results were achieved at a daily dose of 600 mg, with a CHR rate of 37%, a CCgR rate of 19%, and a 3-year PFS of 40%.17,63 In BC the rate of CHR was about 25%, and several responders also achieved a CCgR, but PFS was short, with a median of 10 months or less, and only 7% remained alive after 3 years.5,21-23,63,64
MolR
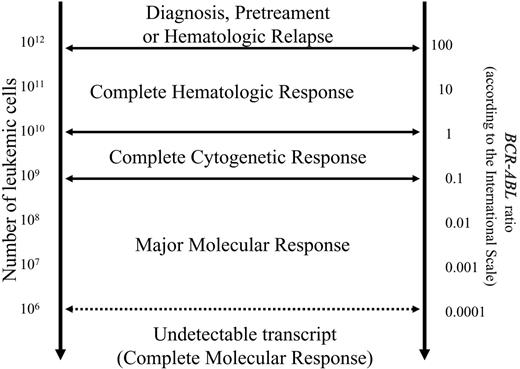
Since the frequency of CCgR is very high in IM-treated patients, it is necessary to measure the level of the BCR-ABL transcripts to determine minimal residual disease (MRD) (Figure 1). In about 50% of all patients, corresponding to about 70% of the patients who have achieved a CCgR, a substantial reduction, commonly referred to as a 3-log reduction from a standard baseline or MMolR, was reported in ECP,53,65-67 while in LCP the responses were consistently lower.19,20,67,68 The actual frequency with which no residual BCR-ABL transcripts can be detected by use of the most sensitive available methods, sometimes imprecisely referred to as “complete” MolR (CMolR), is very variable, and ranges from 4% to 34%.18,19,57,67,69 The rate at which the BCR-ABL transcript levels continue to fall reduces with time.57,70,71 This is consistent with the reports that Ph+ stem cells may be less sensitive to IM than later Ph+ progenitors.72-75 The question of whether the inability to detect BCR-ABL transcripts over the long term is consonant with “cure” cannot yet be answered. Some case reports suggest that the disease may recur shortly after IM discontinuation, so that until more information becomes available IM treatment should not be discontinued without reasons.76-80
Approximate relationship between response, the putative number of leukemic cells, and the level of BCR-ABL transcripts. When a complete cytogenetic response has been achieved, the (putative) number of residual Ph+ cells can be measured only with quantitative molecular methods. The figure highlights the importance of molecular methods in the evaluation of the response to treatment. However, the sensitivity of current methods may vary substantially and in any case, no method can detect the transcript at very low cellular levels. For this reason the term “complete molecular response” may be misleading, since it might erroneously be interpreted as an equivalent of complete disease eradication and cure. The term “undetectable BCR-ABL” may better describe the biologic situation.
Approximate relationship between response, the putative number of leukemic cells, and the level of BCR-ABL transcripts. When a complete cytogenetic response has been achieved, the (putative) number of residual Ph+ cells can be measured only with quantitative molecular methods. The figure highlights the importance of molecular methods in the evaluation of the response to treatment. However, the sensitivity of current methods may vary substantially and in any case, no method can detect the transcript at very low cellular levels. For this reason the term “complete molecular response” may be misleading, since it might erroneously be interpreted as an equivalent of complete disease eradication and cure. The term “undetectable BCR-ABL” may better describe the biologic situation.
Dose issues
The issue of the optimal dose of IM is not yet settled. In early studies for drug registration the maximum tolerated dose was not identified. A dose of 300 mg daily was sufficient to achieve a CHR in almost all LCP patients and at 400 mg daily the blood concentration of IM was consistently higher than that required to inhibit 50% of BCR-ABL TK activity in vitro.81,82 It was also found that a daily dose of 600 mg was likely to be more effective than 400 mg for AP/BC patients,16,21 and that increasing the IM dose to 600 or 800 mg could benefit a subgroup of patients with inadequate response or disease progression.83 Since at higher concentrations IM may inhibit more effectively unmutated BCR-ABL and some mutants, studies were initiated to test higher doses also in CP. In patients with both prior hematologic and cytogenetic resistance to 400 mg of IM daily, increasing the IM dose to 800 mg resulted in a CHR in 65% of patients and a CCgR in 18% of patients.84 In LCP patients who had not received prior IM, 66% achieved a CCgR.85 In ECP patients a CCgR was achieved in 90% of patients, with a 30% CMolR.86 In a multicenter Australian study of IM-naive ECP patients whose dose was escalated from 600 to 800 mg daily, the CCgR rate and the MMolR rate were 81% and 53%, respectively.70,87 These studies had no controls and the median follow-up was short (6 to 16 months). Thus, whether increased doses of IM, compared with the standard dose of IM, will achieve an increased overall number of CCgR and MMolR, or whether these effects will merely occur only earlier, remains to be determined. Answers are expected from prospective studies that are in progress.13,88,89
Combination with other drugs
Because rIFNα and AC are effective in the treatment of CML, and because their mechanisms of action differ, the combinations of IM with rIFNα and with AC were the first to be tested. In an exploratory study of 77 patients, the combination of 400 mg IM daily with pegylated rIFNα2b (PegIntron; Schering Plough, Kenilworth, NJ), 50 to 150 μg weekly, was administered.66 The compliance to the combination was limited, since the median tolerated dose of rIFNα was only 35 μg/week and 50% of patients discontinued rIFNα before the end of the first year of treatment; after 1 year the CCgR and the MMolR rates were 70% and 48%, respectively.66 The combination of 400 mg IM with LDAC has been investigated in 30 ECP patients;90 at 1 year the CCgR rate was 70%, with grades 3 and 4 hematologic toxicity in 53% of patients. Prospective randomized studies of IM alone versus IM in combination with rIFNα, LDAC, and high-dose AC are ongoing.13,89,91
Several drugs have been shown to overcome IM resistance or to synergize with IM in preclinical models, including leptomycin B, proteasome inhibitors, mTOR inhibitors, arsenic trioxide, mycophenolic acid, farnesyl-transferase inhibitors, bryostatin, decitabine, histone-deacetylase inhibitors, homoharringtonine, and phosphoinositol-dependent kinase-1 inhibitors,92-106 but results are still preliminary and limited.107-110
Relationship with alloHSCT
Treatment with IM prior to alloHSCT was not reported to be associated with an increase of transplantation-related morbidity and mortality.111-115 IM was also found to control leukemia in patients relapsing after alloHSCT.116,117 In a multicentric retrospective study of 128 patients, the CCgR rates were 58% in CP, 48% in AP, and 22% in BC, with molecular negativity in 37%, 33%, and 11% of cases, respectively.118 In patients treated in early molecular relapse after alloHSCT, molecular negativity was reinduced in 15/18 cases.78 A synergy of IM with donor lymphocyte infusion has been suggested.119
Factors affecting drug concentration in target cells
Several factors can influence IM concentration in target cells, including intestinal absorption, liver metabolism through cytochrome P450 isoenzyme-3A4, plasma binding to α1-acid-glycoprotein, and the transporters involved in multidrug resistance. P-glycoprotein (Pgp) was found to influence IM intracellular concentration in some studies,120-125 but not in others.126,127 Interestingly, some studies have suggested that Pgp inhibition restored IM sensitivity.120,124,125 IM does not cross the blood-brain barrier.128 Also, the expression of the organic cation transporter hOCT was reported to influence intracellular drug concentration.123,129
Resistance and mutations
Resistance may be multifactorial, including BCR-ABL mutations of the kinase domain interfering with IM binding, BCR-ABL amplification or overexpression, clonal evolution, and decreased IM biovailability or cell exposure.120,130-141 Clonal evolution and mutations (Table 5) are likely to be the most important factors and are related to each other.133,142 The frequency of BCR-ABL mutations in resistant patients was reported to range from 42%139 to 90%133 depending on the methodology of detection, the definition of resistance, and the phase of the disease. Mutations are found more frequently in AP/BC. In CP patients they are rarer and were identified more frequently in patients with more than 2-fold increase of the BCR-ABL transcript levels than in those with stable or decreasing levels.143 However, mutant Ph+ subclones may remain at low levels, may be transient or unstable, and may not be consistently associated with subsequent relapse.144,145 In many cases the mutations have been detected in samples that were collected during IM treatment, but in several cases the mutation was also traced back to samples collected before treatment, especially in cases of AP/BC.133,146,147 With more sensitive techniques, mutations were also found in some cases of IM-naive patients and in patients who were in CCgR.147-149 It is important to note that Ph+ primitive cells have been reported to be less sensitive to IM in vitro and in vivo, to harbor BCR-ABL mutations even prior to IM exposure, and to develop rapidly mutations under IM pressure.72,74,147,149-151 Not all mutations have the same biochemical and clinical properties (Table 5). The T315I mutation and some mutations affecting the so-called P-loop of BCR-ABL confer a greater level of resistance, whereas the biochemical resistance of other mutations can be overcome by a dose increase, and some mutations are functionally irrelevant.133,137-140,152-154 Thus, the detection of a kinase domain mutation must be interpreted within the clinical context.
ACAs in Ph+ cells (clonal evolution) and OCAs in Ph– cells
Within the Ph+ clone additional chromosome abnormalities (ACAs) can be found in a variable proportion of metaphases and in a variable number of patients. This phenomenon, also known and described as clonal evolution, is rare in ECP and becomes more frequent over time and with disease progression.23,134,155-160 A negative relationship of ACAs with IM response has been shown, including a lower CgR rate,157 a higher hematologic relapse rate (50% vs 9%),155 and a shorter OS (75% vs 90% at 2 years).156 Chromosome 9q+ deletions (del9q+) were reported to be associated with less CHR, less CgR, and a shorter PFS in LCP, AP, and BC patients in 1 study161 but not in another.162
Other chromosome abnormalities (OCAs) have been reported in the Ph– cells of about 5% of the patients who had achieved a CCgR with IM.163-170 Many of these patients were in LCP and had been pretreated with rIFNα-based regimens. OCA included trisomy 8 alone in about 50% of such cases, trisomy 8 with other abnormalities in about 10% of cases, a deletion of chromosome 7 alone or with other abnormalities in about 15% of cases, and other abnormalities in the remaining cases. The balance between the Ph+ clone and the Ph– clone with OCAs fluctuated depending on IM treatment, which suppressed Ph+ cells and allowed the Ph– clone with OCAs to expand. In some cases Ph– clones with ACAs were reported to be associated with a myelodysplastic syndrome, mainly in patients with a deletion of chromosome 7 and/or other complex abnormalities, but also in patients with isolated trisomy 8. It was also reported that many patients remained in complete cytogenetic and hematologic response after the detection of OCAs and that OCAs may be transient,165-167,169,170 but the follow-up is still short.
Prognostic factors
Two sets of prognostic factors can be considered, namely those that can be identified prior to treatment (baseline factors) and those that can be identified during the treatment (response-related factors). The main baseline factors are the phase of disease and the relative risk (RR). Although different definitions of AP and BC have been used (Table 1), the phase of the disease influences strongly the response, the duration of the response, and OS, with better results in CP than in AP and in AP than in BC. The RR, either by the Sokal24 or Hasford methods,25 predicts the cytogenetic response to 400 mg IM daily (Table 6).53,171,172 Moreover, the Sokal RR has been reported to predict also MolR and OS. In the IRIS study, the rate of 12-month MMolR among CCgRs was 66%, 45%, and 38% in low-, intermediate-, and high-risk patients, respectively (P = .007).53 The OS at 54 months was 94%, 88%, and 81% for low, intermediate, and high Sokal risk patients (P < .001).58 These risk definitions, which were derived from patients treated with conventional chemotherapy or rIFNα, are still useful, and should be used until further studies identify and confirm other factors of possible prognostic relevance, such as genomic profile,173-177 genetic polymorphisms,178,179 Wilms tumor gene expression,180 total phosphotyrosine levels in CD34+ cells,181 and the phosphorylation level of the adaptor protein Crkl.182 In addition, it has been reported that BCR-ABL expression levels affect the CgR to IM19 and determine the rate of development of resistance to IM.141 ACAs, including Ph duplication, and del9q+, are also candidate-adverse prognostic factors.
As data from IRIS study are continuously updated,58,172,183 early cytogenetic response seems to be the most important response-related prognostic factor (Table 7). If no CgR is achieved after 3 months, there is still a 50% chance of achieving a CCgR later on. If there is any (even minimal) CgR after 6 months of treatment, there is still a fair chance of achieving a CCgR later on, but if the 6-month karyotype remains more than 95% Ph+, the probability is only 15%. After 12 months of treatment, if the CgR is partial the probability of achieving a CCgR at 2 years is still 50%, but if the response is less than partial, this probability becomes less than 20%. The data reported in Table 7 also highlight the relationship between early CCgR and EFS.
The level of MolR was also found to be an important dynamic factor of prognosis. It was reported that transcript levels after 1 or 2 months of treatment predicted late responses,184,185 that a low level of residual disease was associated with continuous remission,68 and that a MMolR after 12 months of treatment was associated with a better EFS and PFS.53,58 A rise of BCR-ABL transcript level has been consistently associated with mutations or response loss.143,186
Defining and monitoring the response
HR and CgR
In almost all recent reports on the treatment of CML, HR and CgR were defined virtually the same way, and with only minor differences.15,17-19,26-29,66 We propose to use the definitions that are listed in Table 8. We recommend that HR be evaluated every 2 weeks until a CHR has been achieved and confirmed, and a conventional cytogenetic examination of marrow cells be performed before treatment, at least every 6 months until a CCgR has been achieved and confirmed, then every 12 months. Once an MMolR has been achieved and confirmed, conventional cytogenetic examination of marrow cells may be performed less frequently, depending on clinical, hematologic, and molecular findings.
Fluorescence in situ hybridization (FISH) on interphase cells has the potential advantage of evaluating many more cells and of using peripheral blood instead of marrow,187,188 but since the data obtained so far are all based on conventional cytogenetics, we recommend using FISH only before treatment to identify cases of Ph–, BCR-ABL+ CML, and those with variant translocations, Ph amplification, or del9q+.
MolR
The necessity for a quantitative definition of MolR has developed with the introduction of IM because with IM, most patients achieve a CCgR, so that molecular methods for measuring MRD are required (Figure 1). The IRIS trial provided evidence for the first time that a reduction of BCR-ABL transcripts by 3 or more logs below a standard baseline value correlated with PFS.53 The use of the “log reduction” terminology has led to some degree of confusion since it seems to imply that the value is a relative one. For this reason, at a consensus conference held in Bethesda under the auspices of the National Institutes of Health (NIH), it was proposed to move away from the term “log reduction” and to introduce a standardized numeric International Scale (IS) expressing the amount of BCR-ABL as a percentage of a control gene and anchored to 2 “absolute” values based on validated reference materials (plasmids, lyophilised cells or cell extracts) of known value.189 The first value will be designated 100% on the proposed IS and the second value will represent a 3-log reduction, ie 0.1%. A given laboratory will use the validated reference material to determine the local value that is equivalent to MMolR as determined in the IRIS trial. By comparing the value for a 3-log reduction with the value on the internationally agreed scale, each laboratory can derive a conversion factor which can then be used to express the results in any given patient on the IS.
In ECP patients, evaluating MRD with real-time quantitative polymerase chain reaction (RQ-PCR) does not require bone marrow cells. Blood is drawn (eg, 10 mL), which contains a sufficient amount of leukocytes for RNA extraction from the whole buffy coat. We propose RQ-PCR on peripheral blood cells be performed at regular intervals of 3 months, even after RQ-PCR becomes negative.
Assessing the molecular status of a patient is not limited to the evaluation of the level of the BCR-ABL transcripts. We propose performing a mutational analysis immediately in any case of treatment failure or suboptimal response, including a confirmed rise of BCR-ABL transcript level. We recognize, however, that there is currently no consensus regarding the degree of increase which should cause concern,189 and that there is at present only a limited number of laboratories worldwide currently performing these analyses.
Failure and suboptimal response
The goals of treatment, in order of time and importance, are CHR, CCgR, MMolR, and “complete” molecular response. Although the time to response may not always affect the prognosis, it is operationally useful to define at which timepoint a response may be satisfactory, thus encouraging continuation of current treatment, or if it is not satisfactory, thus requiring or suggesting a change in the therapeutic strategy. Based on the available information, as summarized in prior sections, we propose to define the response to the treatment at different timepoints as “failure” and “suboptimal.” In this context “failure” means that continuing IM treatment at the current dose is no longer appropriate for these patients, who would likely benefit more from other treatments. “Suboptimal response” means that the patient may still have a substantial benefit from continuing IM, but that the long-term outcome of the treatment would not likely be as favorable. Moreover, we propose that some factors should “warn” that standard-dose IM treatment may not be the best choice, and that patients with these factors require a more careful monitoring. The proposed criteria for failure, suboptimal response, and warning are listed in Table 9.
Treatment policy
Standard (noninvestigational) treatment of ECP Ph+ CML includes HU, rIFNα ± LDAC, 400 mg IM daily, and alloHSCT. The superiority of IFNα ± LDAC over HU was already demonstrated and confirmed.8,30 The superiority of 400 mg IM over IFNα ± LDAC has also been demonstrated.17,53 Standard alloHSCT is a recognized therapeutic procedure achieving long-lasting molecular remissions or cures in about 50% of the patients who are eligible for the procedure, with substantial differences among recognized risk groups.40,41 In countries where IM is available and standard alloHSCT is feasible, we are now in a rather privileged situation to have 2 potent strategies that are both established but are neither perfect nor mutually exclusive. IM is preferred as initial treatment. In a patient with a high disease risk and a low EBMT risk score the choice between IM and alloHSCT should be discussed, but there is little reason to deny such a patient a trial with IM since the early response to IM can either reinforce or weaken the indication for alloHSCT.
The motivations for treatments other than IM are intolerance or excess toxicity, failure, suboptimal response, and “warnings.”
In case of intolerance or excess toxicity, the choices are either alloHSCT or rIFNα ± LDAC, which must be weighed against investigational trials of new agents and should follow the principle of shared decision-making wherein the patient is informed of the risks and rewards of each treatment decision.
In case of failure (Table 9) we propose that the first choice be alloHSCT or dose-escalation of IM to 600 or 800 mg daily, provided that the patient tolerated 400 mg and that resistance to IM was not associated with a BCR-ABL mutation with a high level of insensitivity to IM.
In case of suboptimal response (Table 9) we propose that the first choice be dose escalation of IM to 600 or 800 mg daily, provided that the patient tolerated 400 mg. AlloHSCT could be offered to patients with a low or intermediate EBMT risk score and high RR or other warning features.
In patients presenting with “warning” features, standard treatment is still 400 mg IM, but any “warning” (Table 9) should alert that the patient might become eligible for IM dose escalation, alloHSCT, or, in selected cases, for investigational agents.
There are several other possible scenarios. The first is the patient in whom other treatment options are not available; in such case the choice would be between continuing IM treatment, if a CHR is maintained, or to resort to HU. The second scenario is the patient requiring IM dose reduction or frequent treatment discontinuations. We recommend that the treating physician advise the patient to adhere to the 400-mg dose insofar as possible; appropriate supportive care should be provided, including myeloid growth factors and erythropoietin; the response should be monitored frequently.
Monitoring of blood IM concentration is not required, but it would be desirable in case of failure, in patients who must take drugs interfering with cytochrome P450, and in those who experience a severe drug-related adverse event.
The proposals and recommendations discussed in this paper focus on ECP patients, but sometimes patients are first diagnosed when initially in AP or BC. There are few data pertaining to treatment results in these patients. We propose patients in early BC to be treated initially with IM or other TK inhibitors (based on mutational analysis) and then to proceed to alloHSCT. Since some temporal latitude exists after the diagnosis of AP, a more prolonged trial with IM is possible.
Conclusions
Progress in drug development, molecular and cellular biology, and HSCT obliges the medical community to maintain a critical attitude to the management of Ph+ CML. On the one hand it must be recognized that the introduction of IM has marked an important and hopefully revolutionary step, but the long-term outcome of this treatment cannot yet be assessed. On the other hand, alloHSCT holds the promise of cure, but with definite toxicity and mortality. At the same time, other TK inhibitors and targeted agents are already in preclinical and clinical evaluation.13,154,190-194 The proposals described in this report have been generated by a panel of experts to strike a balance between the magic freedom of research in progress and the practice of advising patients and managing treatment. The proposals concerning treatment policy may be provisional, in the absence of the evidence that will be provided only by longer follow-up of prospective studies; however, the recommendations concerning the methods that must be used to evaluate and to monitor the response are nonetheless cogent. Cytogenetic and molecular monitoring, including mutational analysis, is expensive and requires appropriate resources and sophisticated facilities. However, the cost of monitoring is negligible by comparison with the cost of treatment, whether it is a targeted agent or HSCT. Moreover, careful monitoring is required to ensure that an individual patient receives the proper treatment and to decide if and when a therapy should be changed. Finally, it should be realized that progress makes treatment more effective but not necessarily easier. Thus, the treatment of Ph+ CML should be provided under the guidance of an experienced center, offering and asking patients to be registered on investigational studies. This is necessary to ensure that all the data, clinical and biological, that are urgently required to answer the present questions, are collected and analyzed in an accurate and timely manner, for the benefit of the subsequent patients and for further progress in the treatment of leukemia.
Appendix
The panel identified in the title of this article comprises the authors of this article.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-02-005686.
A note regarding the members of the panel identified in the title appears in “Appendix.”
Supported by the European Union, Sixth Framework Programme, contract no. LSHC-CT-2004-503216 (European LeukemiaNet).
The scientific contributions of Prof Jörg Hasford and of many members of the European LeukemiaNet, Work Package 4, are acknowledged. The scientific and the technical assistance of Simona Soverini, PhD, Alessandra Dorigo, Chiara Ferri, and Katia Vecchi is also kindly acknowledged.