Abstract
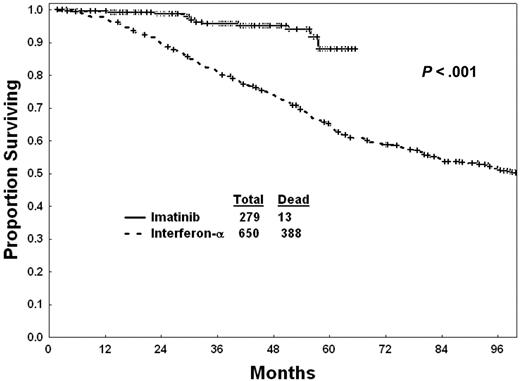
A survival benefit for imatinib mesylate versus interferon-α therapy could not be demonstrated in the randomized study in newly diagnosed Philadelphia chromosome (Ph)–positive chronic-phase chronic myelogenous leukemia (CML) due to the high rate of crossover (90%) from interferon-α to imatinib mesylate within a year of study entry. We compared survival in 279 patients with newly diagnosed CML treated with imatinib mesylate at our institution (2000-2004) to 650 patients treated with interferon-α (1982-1997). The complete cytogenetic response rates were 87% with imatinib mesylate and 28% with interferon-α (P < .001). The estimated 3-year survival rates were 96% with imatinib mesylate and 81% with interferon-α (P < .01). Survival rates with imatinib mesylate were significantly better than with interferon-α within each of the CML prognostic risks groups. By multivariate analysis, imatinib mesylate therapy was identified as an independent favorable prognostic factor, after accounting for the impact of pretreatment factors (hazard ratio, 0.44; P < .01). By landmark analysis at 12 months, survival within each cytogenetic response category was similar with imatinib mesylate or interferon-α, suggesting that the survival benefit of imatinib mesylate (versus interferon-α in newly diagnosed CML) is through improving cytogenetic response.
Introduction
Imatinib mesylate, a selective Bcr-Abl tyrosine kinase inhibitor, has revolutionized the treatment of Philadelphia chromosome (Ph)–positive chronic myelogenous leukemia (CML), and has become the standard of care for this disease.1,2 Imatinib mesylate induces complete hematologic response in 80% to 90% of patients with newly diagnosed chronic-phase CML, a complete cytogenetic response in 70% to 80% of patients, and a major molecular response (eg, 3-log reduction of BCR-ABL: BCR levels compared with a standardized pretreatment level by quantitative polymerase chain reaction [QPCR], or QPCR < 0.05%-0.1%) in 40% of patients. The International Randomized trial of Interferon versus STI571 (IRIS) did not show a survival advantage for imatinib mesylate, because 90% of patients on interferon-α crossed over to imatinib mesylate after a median of 9 months from start of study, thus benefiting from imatinib mesylate therapy early into their disease.2
Additional randomized studies of imatinib mesylate versus previous standard strategies are unlikely to be performed. A survival advantage for imatinib mesylate therapy can then be demonstrated only through careful comparative studies with precisely defined historical control populations treated with interferon-α–based regimens. This is the purpose of this study which confirms, with long-term follow-up, the survival advantage of imatinib mesylate therapy versus interferon-α in newly diagnosed CML.3
Patients, materials, and methods
Adults with newly diagnosed Ph-positive early chronic-phase CML (ie, within 6 months from diagnosis) treated at our institution from July 2000 until December 2004 with imatinib mesylate were analyzed. These patients were treated on frontline therapies with 400 mg daily imatinib mesylate orally (n = 73), 600 mg orally daily (n = 12), or 800 mg orally daily (400 mg orally twice daily, n = 194). Their survival by imatinib mesylate dose was identical, justifying their inclusion as 1 treatment group for comparative survival analysis. The minimum follow-up time of these patients was longer than 12 months. Their outcome was compared with 650 patients treated on interferon-α regimens from 1982 to 1997. Interferon-α regimens included interferon-α alone or with hydroxyurea (n = 270), interferon-α plus low-dose cytarabine (n = 285), and interferon-α plus homoharringtonine (n = 95).4-6 Survivals in different studies containing interferon-α were similar, justifying their inclusion as 1 treatment category for the purpose of comparative analysis with imatinib mesylate. The median follow-up times with imatinib mesylate regimens was 42 months (range, 12-66 months), and the median follow-up times with interferon-α regimens was 143 months (range, 4-270 months). All patients signed informed consents for the respective protocols according to institutional guidelines. Eligibility criteria were similar on all studies, and the details of the therapies were previously reported.3-6
Response criteria were as published.4 A complete cytogenetic response referred to disappearance of Ph-positive cells (0% Ph-positive) by routine cytogenetic analysis. A partial cytogenetic response referred to reduction of Ph-positive cells to 1% to 34%. A major cytogenetic response referred to reduction of Ph-positive cells to less than 35%. A major molecular response was defined as BCR-ABL/ABL transcript levels less than 0.05%. A complete molecular response was defined as undetectable BCR-ABL transcripts (QPCR levels below < 10–5).7,8
Differences among variables were evaluated by the chi-square test and Mann-Whitney U test for categoric and continuous variables, respectively. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test. Risk groups were defined by the Sokal and Hasford models.9,10 Survival was calculated from the date of start of therapy. Transformation-free survival was calculated from the date of start of therapy until progression to accelerated-blastic phase.11 Progression-free survival was calculated from the date of start of therapy, until cytogenetic or hematologic resistance or relapse, or progression to accelerated-blastic phase.12 Multivariate analyses to evaluate the independent prognostic variables used the Cox proportional hazards regression model for survival.13
Results
Two hundred seventy-nine patients treated with imatinib mesylate were compared with 650 patients who received interferon-α. The significant pretreatment characteristics of the imatinib mesylate and interferon-α groups are detailed in Table 1. Patients on imatinib mesylate therapy were older and had a higher incidence of marrow basophilia. Patients on interferon-α therapy had higher incidences of leukocytosis, and thrombocytosis, and a longer duration of CML disease (Table 1). One hundred forty-four patients on interferon-α therapy (22%) changed to imatinib mesylate therapy after a median CML duration of 84 months (range, 26-267 months) in chronic phase.
Response with imatinib mesylate versus interferon-α
The complete cytogenetic response rate was 87% with imatinib mesylate and 28% with interferon-α (Table 2). The incidence of complete cytogenetic response with imatinib mesylate or interferon-α within each risk group is shown in Table 3. With imatinib mesylate, 163 (61%) of 267 patients who had follow-up molecular tests performed achieved a major molecular response, and 85 (32%) patients had at least 1 negative QPCR test. There was no difference in survival in patients treated with imatinib mesylate by imatinib mesylate daily dose (P = .72).
Survival with imatinib mesylate versus interferon-α
Survival of patients with imatinib mesylate versus interferon-α is shown in Figure 1. Survival, censoring the 141 patients who changed from interferon-α to imatinib mesylate in chronic phase at the time of therapy change, was still significantly better with imatinib mesylate (estimated 5-year survival, 88% vs 63%; P = .001). Seven patients on imatinib mesylate (5 from related donors, 2 from unrelated donors) and 97 patients on interferon-α (63 from related donors, 30 from unrelated donors, 4 from donors unspecified) underwent allogeneic stem cell transplantation (SCT) in chronic phase. Five of the 7 patients who received transplants after imatinib mesylate remain alive without evidence of disease (NED) after a median follow-up of 17 months (range, 2-34 months). Thirty-five of the 97 patients who received transplants after interferon-α remain NED after a median follow-up of 126 months (range, 31-225 months). Survival with imatinib mesylate versus interferon-α censoring patients at the time they underwent allogeneic SCT in chronic phase demonstrates the persistent survival benefit with imatinib mesylate (estimated 5-year survival 90% versus 67%; P = .001). The estimated 2-year survival of patients after allogeneic stem cell transplantation (SCT) was 80% after imatinib mesylate and 55% after interferon-α (P = .52).
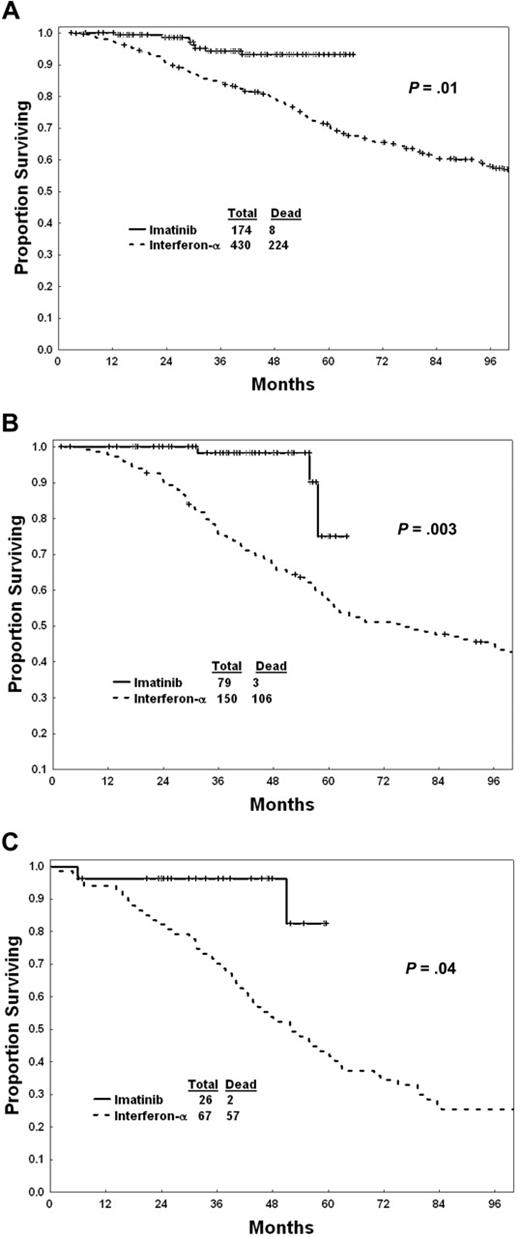
Survival of patients within each Sokal risk group is shown in Figure 2A-C. Within each risk group, imatinib mesylate was associated with a significantly better survival than interferon-α.
Since some characteristics of patients treated with imatinib mesylate versus interferon-α were different, we conducted univariate then multivariate analyses of pretreatment factors associated with survival in the total study group of 929 patients treated with either imatinib mesylate or interferon-α. The univariate analysis selected the following poor prognostic factors (P < .05): older age, splenomegaly, leukocytosis, higher blast percentage, higher basophil percentage, CML risk group, and longer duration of CML (Table 4). The multivariate analysis selected the following to be independent poor prognostic factors: older age (P = .01), higher peripheral blast percentage (P < .01), increased marrow basophils (P = .01), and longer CML disease duration (P < .01). Therapy (imatinib mesylate versus interferon-α), entered into the model after accounting for the effect of the independent pretreatment factors, remained a significant independent factor favoring imatinib mesylate therapy (hazard ratio, 0.44; P < .01).
Outcome with imatinib mesylate by response at 12 months into therapy
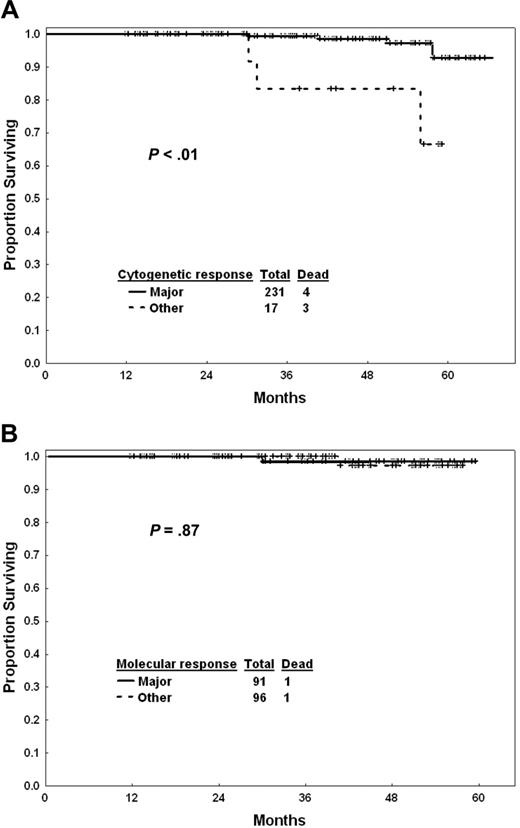
Response to imatinib mesylate therapy after 1 year of exposure may help define prognosis and the need for treatment modification. Figure 3A shows the outcome of patients by cytogenetic response at 12 months. Patients who did not achieve a major cytogenetic response by that time had a worse survival than others (estimated 3-year survival, 99% versus 84%; P < .001). The estimated 3-year survival rates were 98% for complete cytogenetic response (n = 210) and 100% for partial cytogenetic response (n = 21). The estimated 5-year survival rates were 94% in both cytogenetic subgroups. Survival of patients in complete cytogenetic response at 12 months was not different by whether they achieved a major molecular response or not (Figure 3B). Similarly, transformation-free survival was not different (estimated 3-year rates, 98% with major molecular response versus 95% for others; P = .28), nor was progression-free survival (estimated 3-year rates, 98% versus 94%; P = .14).
Survival with imatinib mesylate versus interferon-α within Sokal risk groups. (A) Low risk. (B) Intermediate risk. (C) High risk.
Survival with imatinib mesylate versus interferon-α within Sokal risk groups. (A) Low risk. (B) Intermediate risk. (C) High risk.
Survival with imatinib mesylate versus interferon within each cytogenetic response category
An important question is whether imatinib mesylate offers a survival advantage over interferon-α irrespective of cytogenetic response. The median time to a complete cytogenetic response was 3 months with imatinib mesylate versus 13.5 months with interferon-α. The median times to partial cytogenetic response were 3 and 9 months, respectively. To account for this time bias, we conducted a landmark analysis of the association of response at 12 months with survival among patients alive at 12 months and receiving imatinib mesylate or interferon-α. Within each cytogenetic group imatinib mesylate was associated with a similar survival as interferon-α (Table 5), suggesting that in newly diagnosed CML, imatinib mesylate improves survival through improving cytogenetic response (ie, a better control of CML disease).
Survival with imatinib mesylate by response. (A) Cytogenetic response at 12 months. (B) Molecular response at 12 months in patients in complete cytogenetic response.
Survival with imatinib mesylate by response. (A) Cytogenetic response at 12 months. (B) Molecular response at 12 months in patients in complete cytogenetic response.
Discussion
Imatinib mesylate therapy is presently the standard of care in Ph-positive CML. A survival advantage for imatinib mesylate versus interferon-α could not be demonstrated from the randomized IRIS trial because of the crossover design and the commercial availability of imatinib mesylate, resulting in 90% of patients on interferon-α changing to imatinib mesylate within a median of 9 months from start of therapy. The present analysis confirms the benefit of imatinib mesylate versus interferon-α for survival through comparison with a historical study group receiving interferon-α–based regimens. Imatinib mesylate was associated with a 56% reduction in mortality compared with interferon-α overall. The survival benefit was noted even in the low-risk patients.
Our results are similar to those of Roy et al,14 who compared survival with imatinib mesylate (551 patients from IRIS) to their past experience with interferon-α plus cytarabine (325 patients from CML 91). Imatinib mesylate was associated with higher complete cytogenetic response rates at 12 months (70% versus 14%) and at 36 months (87% versus 42%), with better 36-month rates of transformation-free survival (90% versus 82%; P = .005) and survival (92% versus 84%; P < .001).14 In contrast to the study of Roy et al, our study included patients on different imatinib mesylate daily doses, and patients on different interferon-α regimens. However, since survival by imatinib mesylate dose is similar (Table 4) and survival by different interferon-regimens also similar, the comparative analysis of the 2 treatment groups (imatinib mesylate versus interferon-α) is reasonable. Also, although 22% of patients on interferon-α crossed over to imatinib mesylate, this occurred quite late (median time to crossover, 84 months) and would not affect the comparative survival analysis in the first 3 to 5 years. Censoring these 22% of patients on interferon-α at the time of treatment crossover also yielded identical results. Thus, despite the differences between the study groups in the analysis of Roy et al14 and ours, the very similar results in the 2 studies further strengthen the conclusion that imatinib mesylate therapy offers a significant survival advantage over interferon-α therapy.
As in previous studies, the 12 month cytogenetic response to imatinib mesylate was predictive of prognosis.2,12 Patients who had achieved less than a major cytogenetic response had a worse prognosis (Figure 3A). However, among patients achieving a complete cytogenetic response, the degree of molecular response at 12 months was so far not associated with significant differences in survival, transformation-free survival, or progression-free survival (Figure 3B). This is in contrast to the recently updated IRIS results showing a difference in transformation-free survival by the degree of molecular response.11 This may be due to differences in the imatinib mesylate dose schedules, definitions of major molecular response and failure, QPCR methodologies, size of the study groups, and nature of the analysis. In particular, the IRIS study included a larger number of patients treated with imatinib mesylate (n = 551) compared with our study (n = 279), and the IRIS study was prospective in nature, while this analysis is a retrospective one.
An important issue is whether imatinib mesylate can improve survival independent of cytogenetic response. This study shows that the survival benefit in newly diagnosed CML (compared with interferon-α) with imatinib mesylate is through improving cytogenetic responses. This is different from the results of imatinib mesylate versus other therapies in late chronic-phase CML after interferon-α failure,15 which showed better survival with imatinib mesylate within each cytogenetic response category.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-02-004325.
Supported by Novartis Pharmaceutical Corp (H.K., M.T., F.G., and J.C.), Bristol-Myers Squibb (H.K., M.T., S.O., and J.C.), and the Betty Foster Leukemia Research Fund (H.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.