Abstract
Adenosine deaminase (ADA) deficiency is caused by a purine metabolic dysfunction, leading to severe combined immunodeficiency (SCID) and multiple organ damage. To investigate the efficacy of ex vivo gene therapy with self-inactivating lentiviral vectors (LVs) in correcting this complex phenotype, we used an ADA–/– mouse model characterized by early postnatal lethality. LV-mediated ADA gene transfer into bone marrow cells combined with low-dose irradiation rescued mice from lethality and restored their growth, as did transplantation of wild-type bone marrow. Mixed chimerism with multilineage engraftment of transduced cells was detected in the long term in animals that underwent transplantation. ADA activity was normalized in lymphocytes and partially corrected in red blood cells (RBCs), resulting in full metabolic detoxification and prevention of severe pulmonary insufficiency. Moreover, gene therapy restored normal lymphoid differentiation and immune functions, including antigen-specific antibody production. Similar degrees of detoxification and immune reconstitution were obtained in mice treated early after birth or after 1 month of enzyme-replacement therapy, mimicking 2 potential applications for ADA-SCID. Overall, this study demonstrates the efficacy of LV gene transfer in correcting both the immunological and metabolic phenotypes of ADA-SCID and supports the future clinical use of this approach.
Introduction
Genetic defects in the adenosine deaminase (ADA) gene are responsible for about 15% to 20% of severe combined immunodeficiency (SCID).1,2 The lack of ADA causes an accumulation of purine metabolites in the plasma, in lymphoid tissues, and red blood cells (RBCs). ADA-SCID patients suffer from lymphopenia, absent cellular and humoral immunity, failure to thrive, and recurrent infections.3,4 In addition, skeletal, hepatic, renal, lung, and neurologic abnormalities have been observed in some patients, indicating that the disease should be considered as a systemic metabolic disorder.2,5,6 Like other forms of SCID, bone marrow transplantation (BMT) from an human leukocyte antigen (HLA)–identical sibling donor is an effective treatment,7 but transplants from alternative donors are associated with high morbidity and mortality.8 Enzyme replacement therapy with bovine ADA conjugated to polyethylene glycol (PEG-ADA) provides adequate metabolic detoxification but often insufficient immune reconstitution, with the risk of developing neutralizing antibodies and autoimmunity.9
The strong rationale for somatic gene therapy (GT) and the necessity of an alternative treatment led to the development of multiple clinical protocols using autologous peripheral blood lymphocytes (PBLs) or hematopoietic stem/progenitor cells transduced with gammaretroviral vectors encoding for ADA.10-13 It is only recently that the therapeutic efficacy of GT has been investigated in the absence of enzyme replacement therapy.14 Results of this trial showed that GT with bone marrow (BM) CD34+ cells resulted in correction of both the immune and metabolic defects of ADA-SCID children pretreated with low-intensity conditioning.14 However, one patient displaying limited engraftment of gene-corrected stem/progenitor cells achieved partial immune recovery and systemic detoxification,14 indicating that the development of improved GT approaches remains important. In this respect, the use of superior vectors and transduction protocol may allow robust gene transfer efficiency and stable ADA expression into reconstituting hematopoietic stem cells. Moreover, despite the excellent safety record of all ADA-SCID gene transfer protocols, the adverse events occurred in one of the SCID-X1 GT trials15 have raised general concerns on the potential risks of gammaretroviral vectors. Self-inactivating (SIN) lentiviral vectors (LVs) based on human immunodeficiency virus (HIV)16 have an advanced safety profile over non-SIN gammaretroviral vectors and may thus reduce the risk of insertional oncogenesis. In addition, LVs have been shown to be superior to gammaretroviral vectors in infecting human candidate hematopoietic stem cells and maintaining sustained transgenes expression,17,18 particularly in short-term transduction protocols that induce minimal cell manipulation.
The development of lentiviral GT approaches into clinical use will require adequate preclinical assessment in animal disease models. ADA–/– mice retain many features associated with ADA deficiency in humans, such as SCID and a profound metabolic defect,19 and have been extensively used for studies of ADA-SCID pathogenesis.20,21 Elevated Ado nucleosides cause abnormal alveolar development and inflammation, leading ADA–/– mice to die postnatally within the first 3 weeks.22 Although the lung alterations do not represent the major phenotype of human ADA deficiency, it has been suggested that the purine metabolic disturbances may contribute to respiratory distress, chronic lung disease and asthma observed often in these patients.2,5,21 Accumulation of deoxyadenosine triphosphate induces inhibition of thymic development and T-cell lymphopenia,23 as well as impairment of germinal center maturation with defects in splenic B-cell proliferation and activation.24 Continuous treatment with PEG-ADA rescued mice from lethality, preventing pulmonary insufficiency and correcting the metabolic toxicity. However, high doses of PEG-ADA were required to partially restore the cellularity in lymphoid organs and the mice were followed only for 6 weeks of treatment.25
In the present study, we assessed the efficacy of ex vivo BM GT with LV, compared with transplantation of ADA+/+ BM cells, either as single therapy early after birth or after short-term PEG-ADA. We show here that LV-mediated ADA gene transfer rescues ADA–/– mice from lethality and corrects both their metabolic and immunologic defects, similar to BMT.
Materials and methods
Mice
ADA–/– mice were previously described by Blackburn et al.19 ADA+/– pairs for FVB;129-Adatm1MW-TgN(PLADA)4118Rkmb were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in a specific pathogen–free animal facility. Double-mutant mice for Adatm1MW or ADA+/+ mice were generated by intercrossing ADA+/– littermates, and the genotype of the progeny was identified by polymerase chain reaction (PCR) assay (www.jaxmice.jax.org). All procedures were performed according to protocols approved by the Committee for Animal Care and Use of San Raffaele Scientific Institute (IACUC 236).
LV production
The third-generation SIN vector pCCLsin.cPPT.hPGK.E-GFP.Wpre (phosphoglycerate kinase promoter–green fluorescent protein [PGK-GFP]) was described previously.26 For the construction of vector pCCLsin.cPPT. hPGK.hADA.Wpre (PGK-ADA), ADA cDNA was cloned into PGK-GFP plasmid by replacement of the enhanced GFP (E-GFP) cDNA fragment. Both plasmids were used to generate vescicular stomatitis virus–pseudotyped LV as previously described.26 Titer of concentrated virus was measured by infection of HeLa cells with serially diluted virus preparation by flow cytometry (PGK-GFP) or quantitative PCR (PGK-ADA) for LV (see “Quantification of donor cells and vector-positive cells”); virus concentration was estimated by measurement of HIV-1 Gag p24, using an immunocapture assay (Perkin Elmer, Shelton, CT). The titers of the concentrated PGK-ADA and PGK-GFP vector were 1.5 to 5 × 109 and 1 × 1010 transfer units (TU)/mL, respectively.
Transduction and transplantation of total BM cells
Total BM cells were harvested by flushing both tibias and femora of congenic 2-week-old male ADA–/– mice. After RBC lysis, cells were cultured (1 × 106/mL) for 24 hours in StemSpan serum-free expansion medium (SFEM) (StemCell Technologies, Vancouver, BC, Canada) in the presence of 50 ng/mL murine stem cell factor (SCF), 10 ng/mL human Flt3 ligand (Flt3-L), 20 ng/mL human interleukin-6 (IL-6), 10 ng/mL murine IL-3 (Peprotech, Rocky Hill, NJ), 20 ng/mL murine thrombopoietin (Tpo) (R&D Systems, Minneapolis, MN), 4 μg/mL polybrene, and PGK-ADA (0.4 μg of viral p24 per 106 cells, corresponding to a multiplicity of infection [MOI] of 4-12) or PGK-GFP LV with a MOI of 50. For adult mice GT, transduced BM cells (1.5 × 107 cells/mouse) were injected via the tail vein into 5-week-old irradiated (6 Gy) female ADA–/– mice previously treated with PEG-ADA (1000 U/kg/wk for 4 weeks intraperitoneally; Enzon, Piscataway, NJ). As control, a group of female ADA–/– mice treated with PEG-ADA, sublethally irradiated, was given transplants of 1.5 × 107 total BM cells freshly isolated from 8- to 10-week-old male congenic ADA+/+ mice. For the neonatal model, 5 to 7 × 106 BM cells, either from LV-transduced ADA–/– BM, freshly isolated ADA+/+ BM, or mocktransduced ADA+/+ BM, were infused by injection through the temporal vein of irradiated (3 Gy split dose) 5-day-old ADA–/– female mice.
Mouse follow-up
Mice were checked routinely for weight and general health status and followed periodically for blood cell counts, immune phenotype, purine metabolism, and donor and transduced cell engraftment. Mice were anesthetized and bled through the tail vein in EDTA-containing vials. Due to limitations in blood volume collection, not all mice were analyzed at every time point for all parameters. A group of 5 adult treated mice was killed at 6 months after BMT or GT for molecular, biochemical, immunologic, and histologic studies.
Western blot analysis
Total protein extracts from 1 × 106 5-day-cultured BM cells were prepared by standard method, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membrane. Anti–human ADA polyclonal rabbit antibody (H-300) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), which recognizes also the murine protein; mouse anti–glyceraldehyde-3-phosphate dehydrogenase monoclonal antibody (mAb) was from Chemicon (Temecula, CA). Human mononuclear cells were obtained from a healthy donor after informed consent and following a protocol approved by the San Raffaele Scientific Institute Review Board.
Quantification of donor cells and vector-positive cells
Genomic DNA was extracted by QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA). The frequency of male donor cells was determined by quantitative PCR for the Y chromosome and β-actin PCR was used to normalize samples for DNA content (Table S1, available on the Blood website; see the Supplemental Tables link at the top of the online article). Amplification reactions were performed following manufacturer's instructions in the 7700 Sequence Detector and analyzed using the GeneAmp Software (Applied Biosystems, Foster City, CA). The frequency of donor cells was calculated using a standard curve of male cells serially diluted (from 100% to 2.5%) into female cells. Integrated LV copies were detected by quantitative PCR using a standard curve containing known copies of the PGK-ADA plasmid diluted into irrelevant genomic DNA (Table S1). Results were expressed as LV copies per genome normalized for the percentage of donor cells.
Measurement of ADA activity and purine metabolites in RBCs
Intracellular ADA enzyme activity and deoxyadenosinemono-, di- and triphosphate (dAXP) levels were analyzed in lymphoid cells from spleen and lymph node, and in erythrocytes by an adenosine to inosine conversion assay followed by high-performance capillary electrophoresis (HPCE), as described.27
Histopathologic analysis
Tissues were collected at 6 months after treatment or at postnatal day 18 in untreated ADA–/– mice. Formalin-fixed, paraffin-embedded samples were cut at 3- to 5-μm-thick sections and stained with hematoxylin and eosin (H&E). Slides were analyzed in a double-blind fashion by at least 2 pathologists (M.P., F.S., C.D.) by bright-field Axioskop 2 plus microscope (Zeiss, Oberkochen, Germany) and images were automatically captured by using the AxioCam HRc system (Zeiss) with Axiovision 3.1 version 4.4. Images shown in Figures 6C and 7B were taken with a 5×/0.12 NA objective.
Hemogram analysis and flow cytometry
Total white blood cell (WBC) counts in the peripheral blood were measured by an automatic Sysmex KX-21N blood analyzer (DASIT, Cornaredo, Italy). The percentage of T cells (CD3+CD4+, CD3+CD8+), B cells (B220+IgM+), and natural killer (NK) cells in the peripheral blood was assessed by fluorescence-activated cell-sorter (FACS) analysis, and their absolute number calculated based on WBC counts. For flow cytometry, lysed peripheral blood and lymphoid organs (2 × 105 cells) were preincubated for 15 minutes at room temperature (RT) with murine immunoglobulins and stained for 20 minutes at 4°C with the following rat antimouse monoclonal antibodies, all from BD Pharmingen (San Diego, CA): fluorescein isothiocyanate (FITC)–conjugated anti-CD3ϵ (17A2) and anti-IgM (II/41); phycoerytrin (PE)–coniugated anti-CD4 (RM4-5), anti-CD45R/B220 (RA3-6B2), and anti-CD49b/pan NK cells (DX5); CyChrome5-conjugated anti-CD8a Ly-2 (53-6.7). After washing, cells were analyzed using a FACScan flow cytometer and CellQuest software (BD Pharmingen).
Lymphocyte proliferation and cytometric bead array
Splenocytes were collected, depleted of RBCs, and resuspended in RPMI 1640 containing 10% heat inactivated fetal calf serum (complete medium). For T-cell proliferation, 105 splenocytes were placed in round-bottomed 96-well plates in the presence of surface-bound anti–mouse CD3 mAb (0.2 and 2 μg/mL) alone, or combined with 50 IU/mL IL-2 (Proleukin; Chiron, Emeryville, CA). After 72 hours, cells were pulsed for 14 to 16 hours with 0.037 MBq (1 μCi) per well of [3H]-thymidine (Amersham Pharmacia Biotech, Piscataway, NJ). Cells were then harvested and counts per minute (cpm) measured in a scintillation counter. For cytometric bead array, cells were resuspended in complete medium and plated (2 × 105/well) in flat-bottomed 96-well plates in the presence of surface-bound anti-CD3 mAb (2 μg/mL) combined with 0.2 μg/mL anti–mouse CD28 mAb. Supernatant was harvested after 48 hours of stimulation, and cytokines were detected by the mouse T-helper 1 (Th1)/Th2-type cytokine kit according to the manufacturer's instructions (BD Pharmingen).
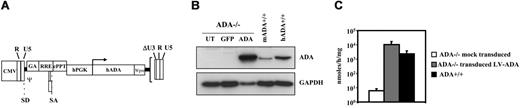
Lentiviral vectors transduced ADA null BM cells at high efficiency. (A) Schematic representation of the lentiviral vector construct used in this study. Expression of the human ADA cDNA was driven by the PGK promoter. R and U5 indicate long terminal repeat (LTR) regions; ΔU3, deletion of the 3 LTR; SD and SA, splice donor and acceptor sites; ψ encapsidation signal including the 5′ portion of the gag gene (GA); RRE, Rev-response element; cPPT, central polypurine tract; and Wpre, woodchuck hepatitis virus posttranscription regulatory element. (B) ADA expression level in ADA–/– BM cells following lentiviral gene delivery. Cells were transduced for 24 hours in the presence of cytokines with either PGK-ADA LV (p24, 0.4 μg/106 cells) (ADA) or PGK-GFP control vector (GFP) (MOI, 50). At 5 to 7 days after transduction, ADA expression was analyzed on protein extracts by Western blot. As controls, ADA expression in cultured murine ADA+/+ BM cells (mADA+/+) and human mononuclear cells (hADA+/+) is shown. GAPDH was included as loading control. UT indicates untransduced. (C) ADA activity was measured by HPCE-based analysis in 5- to 7-day cultured BM cells derived from ADA–/– mice, either mock-transduced or transduced with LV-ADA (n = 9), or from ADA+/+ mice (n = 7). Values are shown as mean ± standard deviation (SD).
Lentiviral vectors transduced ADA null BM cells at high efficiency. (A) Schematic representation of the lentiviral vector construct used in this study. Expression of the human ADA cDNA was driven by the PGK promoter. R and U5 indicate long terminal repeat (LTR) regions; ΔU3, deletion of the 3 LTR; SD and SA, splice donor and acceptor sites; ψ encapsidation signal including the 5′ portion of the gag gene (GA); RRE, Rev-response element; cPPT, central polypurine tract; and Wpre, woodchuck hepatitis virus posttranscription regulatory element. (B) ADA expression level in ADA–/– BM cells following lentiviral gene delivery. Cells were transduced for 24 hours in the presence of cytokines with either PGK-ADA LV (p24, 0.4 μg/106 cells) (ADA) or PGK-GFP control vector (GFP) (MOI, 50). At 5 to 7 days after transduction, ADA expression was analyzed on protein extracts by Western blot. As controls, ADA expression in cultured murine ADA+/+ BM cells (mADA+/+) and human mononuclear cells (hADA+/+) is shown. GAPDH was included as loading control. UT indicates untransduced. (C) ADA activity was measured by HPCE-based analysis in 5- to 7-day cultured BM cells derived from ADA–/– mice, either mock-transduced or transduced with LV-ADA (n = 9), or from ADA+/+ mice (n = 7). Values are shown as mean ± standard deviation (SD).
Serum immunoglobulin quantification and specific antibody production
Total IgM and IgG levels were quantified in sera collected from ADA–/– mice 17 weeks after treatment by enzyme-linked immunosorbent assay (ELISA) (Jackson Laboratories). To assess antigen-specific production, anesthetized mice were injected into footpads with 100 μg chicken egg albumin (Ova; Sigma-Aldrich, St Louis, MO) mixed in a 1:1.5 ratio with complete Freund adjuvant (Sigma-Aldrich). Blood was withdrawn weekly for 4 weeks and Ova-specific IgM, IgG1, IgG2a, and IgG2b serum levels were assessed by ELISA.
Statistical analysis
The statistical analysis was performed accounting for sample characteristics. Kaplan-Meier curves were used as nonparametric estimators of mice survival. Logrank chi-squared statistics were computed to compare the survival between groups. Whenever a parametric approach was proved as suitable tool to compare groups, both analysis of variance (ANOVA; Student t test in case of 2 groups only) and the Welch test were used to account of equality and diversity of variances, respectively. Nonparametric Kruksal Wallis and Mann-Whitney test statistics were computed when sample size and distribution did not satisfy the normality and independence assumptions underlying parametric tests. The results were considered to be significant when the P value was less than .05. All statistical analyses were performed using SPSS version 12.01 and R statistical packages (R Foundation; http://www.R-project.org).
Results
Efficient delivery of ADA gene by lentiviral vectors into BM progenitor cells of ADA–/– mice
We constructed a third-generation SIN LV in which expression of the human ADA cDNA was driven by the human PGK promoter (Figure 1A). The PGK-ADA LV transduced at high efficiency (91.3% ± 8%) BM clonogenic progenitor cells of ADA–/– mice, as assessed by quantitative PCR for vector DNA on single methylcellulose hematopoietic colonies. Western blot analysis confirmed ADA expression in BM cells infected with PGK-ADA LV, at higher level than that observed in control murine BM and human mononuclear cells from a healthy donor (Figure 1B). Since the anti–human ADA antibody may react differently with the murine protein, we measured enzymatic activity by an HPCE-based assay after 5 to 7 days of culture. As shown in Figure 1C, ADA-transduced BM cells from ADA–/– mice showed on average a 4-fold higher activity than ADA+/+ BM, indicating that ADA activity was restored by LV gene transfer.
Long-term engraftment of transduced cells in peripheral blood and lymphoid organs. (A) Quantitative PCR for donor cells (Y chromosome in sex-mismatched transplantation) and transduced cells (LV DNA) in peripheral blood–nucleated cells. Results obtained in different mice assessed at the indicated time are given as percentage of donor cells and vector copy per genome of donor cell, respectively. Horizontal bars represent the average values. Only 2 mice (GT) and 3 mice (BMT) were studied at 28 weeks because the remaining were killed 6 months after treatment for tissue analysis. (B) Donor cell engraftment and vector copy number in primary and secondary lymphoid organs studied at 6 months after GT and BMT. Results are given as the mean ± SD of the percentage of donor cells and vector copy per genome of donor cell in different mice, respectively. Analysis was performed in thymocytes (Thy), T cells (T), and B cells (B) from spleen and lymph nodes, as well as in B cells, myeloid cells (M), and erythroid cells (E) from the BM.
Long-term engraftment of transduced cells in peripheral blood and lymphoid organs. (A) Quantitative PCR for donor cells (Y chromosome in sex-mismatched transplantation) and transduced cells (LV DNA) in peripheral blood–nucleated cells. Results obtained in different mice assessed at the indicated time are given as percentage of donor cells and vector copy per genome of donor cell, respectively. Horizontal bars represent the average values. Only 2 mice (GT) and 3 mice (BMT) were studied at 28 weeks because the remaining were killed 6 months after treatment for tissue analysis. (B) Donor cell engraftment and vector copy number in primary and secondary lymphoid organs studied at 6 months after GT and BMT. Results are given as the mean ± SD of the percentage of donor cells and vector copy per genome of donor cell in different mice, respectively. Analysis was performed in thymocytes (Thy), T cells (T), and B cells (B) from spleen and lymph nodes, as well as in B cells, myeloid cells (M), and erythroid cells (E) from the BM.
Lentiviral vector transfer rescues ADA–/– mice lethality and restores normal growth
To assess the in vivo reconstitution and correction capacity of LV-transduced cells in comparison with BMT, we used 2 different strategies that differed by the age of treatment (adult vs neonatal) and the use of PEG-ADA before transplantation. In a first group of experiments, adult ADA–/– mice received transduced or wild-type cells following 4 weeks of PEG-ADA to initially rescue the animals from lethality. For this purpose, 1.5 × 107 BM cells from ADA–/– male mice were transduced with PGK-ADA LV and injected into 5-week-old female ADA–/– recipients after nonlethal irradiation (6 Gy; GT group, n = 17). In parallel, a group of ADA–/– mice underwent transplantation after irradiation with equal doses of freshly isolated, unmanipulated BM cells from ADA+/+ mice (BMT group, n = 12). In both groups of animals, PEG-ADA was discontinued 1 week before transplantation to evaluate the efficacy of the treatment without exogenous detoxification. As expected, all control ADA–/– mice that had been injected with ADA–/– BM cells transduced with a PGK-GFP control vector died within 3 weeks, as soon as PEG-ADA was cleared from the plasma (data not shown). In contrast, LV-ADA–transduced ADA–/– BM cells or wild-type cells rescued recipient ADA–/– mice long-term, with a similar proportion of mice surviving at 6 months of follow-up (41.2% vs 50% survival, respectively; P = .74). Importantly, the growth of surviving GT- or BMT-treated ADA–/– mice was indistinguishable from that of ADA+/+ control littermates (data not shown).
Long-term engraftment of vector-transduced cells
The presence of donor cells was evaluated in peripheral blood nucleated cells and lymphohematopoietic organs by quantitative PCR for Y chromosome. At 3 weeks after transplantation a mixed chimerism (range, 40%-80%) was detected in the peripheral blood of surviving ADA–/– mice treated with transduced ADA–/– or ADA+/+ BM cells (Figure 2A). As expected from the use of nonmyeloablative conditioning, the mixed chimerism persisted long-term in surviving animals, with donor cells representing 31.0% ± 10.7% and 50.8% ± 16.8% in the GT and BMT groups 21 weeks after transplantation, respectively. Transplantation of ADA+/+ BM into ADA+/– recipient after nonlethal conditioning resulted in long-term survival with mixed chimerism at similar levels (data not shown). Vector-positive cells in the peripheral blood increased in the first weeks after GT and then remained stable over time, with an average of 4.8 ± 2.7 copies/donor cells detected 21 weeks after treatment (Figure 2A). In contrast, a lower proportion of donor cells (14.7 ± 16.5) and of LV copies (0.11 ± 0.18 copies/donor cells) was found in GT-treated mice that died in the first month after transplantation, indicating that this early mortality resulted from the insufficient engraftment of ADA-expressing cells. The engraftment of transduced cells was multilineage, as shown by the presence of vector-positive cells in (1) myeloid, erythroid cells, and B lymphocytes from the BM; (2) thymocytes; and (3) mature T and B lymphocytes from the spleen and lymph nodes (Figure 2B). No significant differences were observed in vector copy number among different cell subsets. A higher donor engraftment was observed in mature lymphoid cells compared with myeloid and erythroid cells in the BM (P < .01) as well as in splenic B cells compared with BM B cells (P < .05), indicating a stronger selective advantage for mature gene-corrected lymphoid cells. However, the engraftment levels were lower in GT-treated mice compared with ADA–/– mice treated with fresh ADA+/+ BM cells (P < .05), particularly in the myeloid compartment.
Lentiviral vector–derived ADA prevents systemic metabolic toxicity
To prove that gene transfer into BM cells resulted in functional reconstitution of ADA, we analyzed ADA enzymatic activity in different lymphohematopoietic organs and in peripheral RBCs of GT-treated ADA–/– mice. ADA activity was present at normal levels in the total spleen and lymph nodes of both GT- and BMT-treated mice (Figure 3A) and confirmed by Western blot analysis (data not shown). RBC ADA activity was normal at 2 months after treatment in both groups but decreased at later time points in the GT group to levels measured in RBCs of ADA+/– control mice (P = .46). (Figure 3B). This finding is in agreement with the lower erythroid engraftment detected in ADA–/– mice given injections of transduced BM cells compared with that of ADA–/– mice that received BMT. Nevertheless, the overall production of ADA was sufficient to achieve full metabolic detoxification. Indeed, dAXP toxic metabolites, which are typically elevated in RBCs of untreated ADA–/– mice, were similarly reduced after GT or BMT, reaching the undetectable levels measured in ADA+/+ controls (Figure 3C).
ADA activity and metabolic detoxification. (A) ADA activity was measured in total cells (Tot) from spleen and lymph node (LN), as well as purified T cells (T) and B cells (B) from spleen 6 months after GT (–/– GT) or BMT (–/– BMT). No ADA activity was detected in lymphoid tissues from untreated ADA–/– mice (–/–). ADA activity was not significantly different among ADA+/+, ADA–/– GT, and ADA–/– BMT mice, with the exception of B splenocytes, in which it was higher in GT-treated mice (P < .01). (B) ADA activity was determined in peripheral RBCs at different months after treatments (–/– GT, n = 6; –/– BMT, n = 6). As controls, age-matched untreated ADA+/+ (+/+;n = 9), ADA+/– (+/–;n = 3) and 18-day-old ADA–/– mice (–/–;n = 9) are also shown. Values are given as means and SDs of the measurements in each mouse. (C) Measurement of dAXP toxic metabolites in RBCs of GT-treated (–/– GT; n = 6) and BMT-treated (–/– BMT; n = 5) mice at 2, 4, and 6 months after treatment. In the right side of panels B and C, control values of untreated ADA+/+ (+/+;n = 9), ADA+/– (+/–;n = 3) and 18-day-old ADA–/– mice (–/–;n = 9) are shown.
ADA activity and metabolic detoxification. (A) ADA activity was measured in total cells (Tot) from spleen and lymph node (LN), as well as purified T cells (T) and B cells (B) from spleen 6 months after GT (–/– GT) or BMT (–/– BMT). No ADA activity was detected in lymphoid tissues from untreated ADA–/– mice (–/–). ADA activity was not significantly different among ADA+/+, ADA–/– GT, and ADA–/– BMT mice, with the exception of B splenocytes, in which it was higher in GT-treated mice (P < .01). (B) ADA activity was determined in peripheral RBCs at different months after treatments (–/– GT, n = 6; –/– BMT, n = 6). As controls, age-matched untreated ADA+/+ (+/+;n = 9), ADA+/– (+/–;n = 3) and 18-day-old ADA–/– mice (–/–;n = 9) are also shown. Values are given as means and SDs of the measurements in each mouse. (C) Measurement of dAXP toxic metabolites in RBCs of GT-treated (–/– GT; n = 6) and BMT-treated (–/– BMT; n = 5) mice at 2, 4, and 6 months after treatment. In the right side of panels B and C, control values of untreated ADA+/+ (+/+;n = 9), ADA+/– (+/–;n = 3) and 18-day-old ADA–/– mice (–/–;n = 9) are shown.
Immunologic reconstitution following LV ADA gene transfer
In order to investigate whether restoration of ADA activity resulted in normal development of the immune system, we analyzed lymphoid cell subsets in the peripheral blood and lymphoid organs of treated ADA–/– mice. As expected, CD3+CD4+ and CD3+CD8+ cells were significantly decreased in untreated ADA–/– mice compared with age-matched ADA+/+ mice. Both GT and BMT restored normal numbers of peripheral blood CD3+CD4+ and CD3+CD8+ populations, IgM+ B cells, and NK cells in ADA–/– mice at 6 months after treatment (Table 1). In the thymi of ADA–/– mice given injections of gene-corrected cells, we found a normal distribution of double-negative (DN), double-positive (DP), CD4+ and CD8+ cells, similar to that of BMT-treated mice (Table 2). This result demonstrates that ADA expression driven by the LV overcomes the DN CD4–CD8– block observed in the thymus of ADA–/– mice. Moreover, the proportion of CD3+CD4+ and CD3+CD8+ cells as well as of IgM+ B cells in the spleens after GT was similar to age-matched ADA+/+ and controls that underwent transplantation (Table 2). Naive T cells were also detected at normal levels in the peripheral circulation and spleens of GT- and BMT-treated ADA–/– mice (data not shown). Overall, these data demonstrated that LV-mediated gene transfer reconstitutes normal lymphoid differentiation in primary and secondary organs of ADA–/– mice.
Correction of in vitro T-cell functions
We next investigated T-cell functions by analyzing in vitro proliferative responses and cytokine production after T-cell receptor (TCR) triggering of splenocytes obtained from untreated animals or 6 months after treatment with GT or BMT. ADA-deficient splenocytes showed severely depressed proliferative responses to anti-CD3 stimulation at 0.2 and 2.0 μg/mL, which were only partially improved by the addition of exogenous IL-2 (Figure 4A). Remarkably, GT resulted in full correction of T-cell–proliferative responses to both concentrations of anti-CD3, similar to that of ADA+/+ cells. As shown in Figure 4B, ADA–/– splenocytes were unable to produce cytokines in response to anti-CD3/anti-CD28 mAb stimulation. GT and BMT restored the normal ability to produce IL-2, IFNγ, TNFα, IL-4, and IL-5 upon TCR triggering, with no particular skewing toward Th1- and Th2-like cytokines.
Restoration of humoral immune response
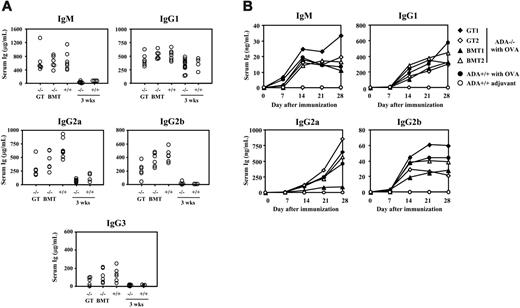
ADA-deficient B lymphocytes display a profound defect in proliferation, activation, and production of IgM.24 At 4 months after treatment, normal levels of IgM, IgG1, and IgG3 were detected in the serum of GT-treated mice, while IgG2a levels were comparable to those measured in BMT-treated ADA–/– mice, but lower than those in controls (Figure 5A). In order to evaluate the ability to mount immune response toward nominal antigens, ADA–/– mice were immunized with Ova 17 weeks after transplantation, and specific antibodies were evaluated weekly in the sera. We found that both GT and BMT ADA–/– mice produced normal levels of Ova-specific IgM and IgG antibodies belonging to different classes (Figure 5B). Moreover, splenic B cells from GT- and BMT-treated mice (6 months after transplantation) proliferated normally in response to anti-IgM mAb or LPS (data not shown).
Neonatal GT prevents onset of ADA phenotype
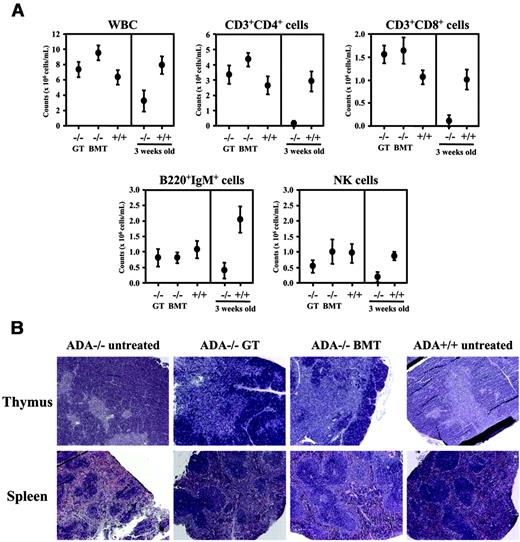
In order to assess the efficacy of GT and BMT without PEG-ADA pretreatment, we transplanted equal numbers (5-7 × 106) of sex-mismatched LV-transduced ADA–/– BM or wild-type BM cells into 5-day-old ADA–/– mice after nonlethal irradiation. The survival at 3 months was equivalent in mice that received GT (46.7%, n = 24), fresh ADA+/+ BM (47.5%, n = 38), or mock-transduced ADA+/+ BM (42.8%, n = 14). In surviving mice, donor cells were detected long-term in the peripheral circulation, but engraftment levels and ADA expression were lower in the GT group compared with both BMT groups (Figure 6A-B). Nevertheless, similar to adult treated mice, full systemic metabolic detoxification was achieved in GT- and BMT-treated ADA–/– mice (Figure 6B). To evaluate the effects of ADA expression and metabolic restoration on pulmonary alveogenesis and inflammation, we analyzed the lung tissues 6 months after treatment. Histopathologic analysis revealed a near-to-complete reconstitution ad integrum of the morphology and architecture in GT- and BMT-treated mice compared with untreated ADA–/–, associated with a dramatic reduction of the typical inflammation and thickening observed in ADA-deficient mice19 (Figure 6C). Furthermore, no histologic signs of liver toxicity or inflammation28 were observed in the treated mice. Hematologic studies performed 5 months after treatment showed normalization of absolute counts of WBCs and CD3+CD4+ and CD3+CD8+ cells, as well as B220+IgM+ B cells in the peripheral blood (Figure 7A), with improvement in NK cell number. Moreover, a normal thymic architecture with correct representation of cortex and medulla was restored after GT and BMT (Figure 7B). Histopathologic analysis of the spleen revealed a normal amount of white pulp and regular formation of lymphoid follicles after both treatments, while untreated ADA–/– mice showed white pulp hypoplasia with relative red pulp expansion (Figure 7B). Taken together, these data demonstrate that GT as single therapy is effective in preventing the metabolic and immunologic alterations associated with ADA-SCID in the murine model.
In vitro T-cell functions. (A) Proliferation of splenic T cells in response to TCR stimulation 6 months after treatment. Values are expressed as mean ± SD of stimulation index (calculated dividing cpm numbers of stimulated and unstimulated conditions), obtained from 3H-thymidine incorporation. (B) Cytokine production of splenic T cells. Total splenocytes from ADA–/– mice at 6 months after GT (ADA–/– GT; n = 3) or after BMT (–/– BMT; n = 5) were stimulated with anti-CD3 and anti-CD28 mAbs for 48 hours. Cytokines were measured in culture supernatants by cytometric bead array in triplicate. The values shown are the means and SD of the average of the measurements in the individual mice. As controls, age-matched untreated ADA+/+ mice (n = 7) and 18-day-old ADA–/– mice (n = 4) are presented. There was no significant difference among ADA–/– treated and ADA+/+ mice.
In vitro T-cell functions. (A) Proliferation of splenic T cells in response to TCR stimulation 6 months after treatment. Values are expressed as mean ± SD of stimulation index (calculated dividing cpm numbers of stimulated and unstimulated conditions), obtained from 3H-thymidine incorporation. (B) Cytokine production of splenic T cells. Total splenocytes from ADA–/– mice at 6 months after GT (ADA–/– GT; n = 3) or after BMT (–/– BMT; n = 5) were stimulated with anti-CD3 and anti-CD28 mAbs for 48 hours. Cytokines were measured in culture supernatants by cytometric bead array in triplicate. The values shown are the means and SD of the average of the measurements in the individual mice. As controls, age-matched untreated ADA+/+ mice (n = 7) and 18-day-old ADA–/– mice (n = 4) are presented. There was no significant difference among ADA–/– treated and ADA+/+ mice.
Lymphomas develop in irradiated animals but are not increased in frequency after GT
In addition to the lethality occurring in the early phases, we observed the development of lymphomas in a significant proportion of treated animals starting 3 months after treatment (Table 3). Tumors occurred in one BMT-treated mouse and one GT-treated adult mouse as well as in 10 BMT- and 2 GT-treated animals from the neonatal group. Tumors were typically lymphomas with enlarged thymic mass, CD4+/CD8+ phenotype, and involvement of multiple organs. These lymphomas were also observed in a significant fraction (6%-33%) of ADA+/+ mice treated with irradiation, indicating a high susceptibility of the strain to irradiation-induced lymphomas (Table 3). Of the tumors occurring in LV-transduced mice, 2 were of donor origin, containing 2 and 8 LV copies per cell by quantitative PCR, respectively. Seven integrations could be unequivocally mapped on the mouse genome by linear amplification-mediated PCR29 (Table S2), and none of them was located inside or in the proximity (< 100 kb) of known proto-oncogenes or mouse common integration sites (CISs).30
Serum immunoglobulins and in vivo humoral responses. (A) IgM and subclasses of IgG immunoglobulins (Ig) were quantified in the serum collected from ADA–/– mice that received GT (–/– GT; n = 6) or BMT (–/– BMT; n = 6), 17 weeks after treatment. Serum Ig from untreated age-matched ADA+/+ mice (n = 7), 18-day-old ADA–/– mice (n = 13), or ADA+/+ mice (n = 4) are shown as controls. Each dot represents values obtained in a single mouse. Serum IgM, IgG1, and IgG3 were normal in ADA–/– GT and ADA–/– BMT mice, while IgG2a levels were not different between GT and BMT. (B) Ova-specific Ig production. ADA–/– mice treated with GT (n = 2) or BMT (n = 2) and age-matched ADA+/+ mice were challenged with Ova or adjuvant only. Ova-specific Ig belonging to different classes were measured weekly in the serum for 1 month.
Serum immunoglobulins and in vivo humoral responses. (A) IgM and subclasses of IgG immunoglobulins (Ig) were quantified in the serum collected from ADA–/– mice that received GT (–/– GT; n = 6) or BMT (–/– BMT; n = 6), 17 weeks after treatment. Serum Ig from untreated age-matched ADA+/+ mice (n = 7), 18-day-old ADA–/– mice (n = 13), or ADA+/+ mice (n = 4) are shown as controls. Each dot represents values obtained in a single mouse. Serum IgM, IgG1, and IgG3 were normal in ADA–/– GT and ADA–/– BMT mice, while IgG2a levels were not different between GT and BMT. (B) Ova-specific Ig production. ADA–/– mice treated with GT (n = 2) or BMT (n = 2) and age-matched ADA+/+ mice were challenged with Ova or adjuvant only. Ova-specific Ig belonging to different classes were measured weekly in the serum for 1 month.
Long-term engraftment of donor cells, correction of systemic detoxification and lung alterations after neonatal treatment. (A) The percentage of donor cells was evaluated in peripheral blood–nucleated cells from ADA–/– mice treated with fresh (ADA–/– BMT fresh; n = 8) or cultured BM (ADA–/– BMT cultured; n = 5), and GT (ADA–/– GT; n = 5) at the indicated time by quantitative PCR for donor cells. ND indicates not done. (B) ADA activity (top) and dAXP levels (bottom) were measured in RBCsof ADA–/– mice treated with fresh (ADA–/– BMT fresh) or ex vivo cultured BM (ADA–/– BMT cultured), as well as with GT (ADA–/– GT) at 7, 13, and 17 weeks after treatment. Values obtained from untreated age-matched ADA+/+ mice (+/+; n = 9) and 18-day-old ADA–/– mice (–/–; n = 10) are also shown. ND indicates not done. Values in panels A and B are shown as mean ± SD. (C) Histopathologic analysis (×5 magnification) of H&E-stained sections from lungs of untreated ADA+/+ (top left panel) and ADA–/– (top right panel), and ADA–/– mice given transplants of LV-transduced BM cells (–/– GT; bottom left panel) or ADA+/+ BM cells (–/– BMT; bottom right panel). Tissues from ADA+/+, GT, and BMT mice were collected at 6 months after treatment, and from untreated ADA–/– mice at 18 days after birth.
Long-term engraftment of donor cells, correction of systemic detoxification and lung alterations after neonatal treatment. (A) The percentage of donor cells was evaluated in peripheral blood–nucleated cells from ADA–/– mice treated with fresh (ADA–/– BMT fresh; n = 8) or cultured BM (ADA–/– BMT cultured; n = 5), and GT (ADA–/– GT; n = 5) at the indicated time by quantitative PCR for donor cells. ND indicates not done. (B) ADA activity (top) and dAXP levels (bottom) were measured in RBCsof ADA–/– mice treated with fresh (ADA–/– BMT fresh) or ex vivo cultured BM (ADA–/– BMT cultured), as well as with GT (ADA–/– GT) at 7, 13, and 17 weeks after treatment. Values obtained from untreated age-matched ADA+/+ mice (+/+; n = 9) and 18-day-old ADA–/– mice (–/–; n = 10) are also shown. ND indicates not done. Values in panels A and B are shown as mean ± SD. (C) Histopathologic analysis (×5 magnification) of H&E-stained sections from lungs of untreated ADA+/+ (top left panel) and ADA–/– (top right panel), and ADA–/– mice given transplants of LV-transduced BM cells (–/– GT; bottom left panel) or ADA+/+ BM cells (–/– BMT; bottom right panel). Tissues from ADA+/+, GT, and BMT mice were collected at 6 months after treatment, and from untreated ADA–/– mice at 18 days after birth.
Discussion
Lentivirus-mediated gene transfer into hematopoietic cells is a promising approach for the treatment of inherited blood borne disorders. Here, we describe the use of ADA–/– mice as a preclinical model to investigate the ability of ex vivo LV-mediated ADA gene transfer to rescue the ADA-SCID metabolic and immune phenotype. We show that GT combined to nonmyeloablative conditioning rescued mice from lethality and restored their normal growth, equally to transplant of wild-type BM cells. LV gene transfer led to long-term expression of ADA in hematopoietic cells, resulting in complete systemic detoxification and alleviation of the severe pulmonary insufficiency observed in ADA–/– mice. GT also resulted in normal lymphoid development and restoration of mature T-cell and B-cell functions, as shown by proliferative responses and cytokine production to TCR engagement and normal antibody production after immunization with Ova.
Lymphoid reconstitution after neonatal treatment. (A) Number of mature T and B cells in the peripheral circulation of GT (–/– GT) and transplant-recipient ADA–/– mice (–/– BMT) treated at neonatal age. As control, untreated age-matched ADA+/+ mice (+/+; n = 6) are reported. FACS analysis was used to determine the absolute number, represented as mean ± SD, of white blood cells, CD3+CD4+ and CD3+CD8+ T cells, B220+IgM+ B cells, and NK cells evaluated 5 months after GT (n = 5) or BMT (n = 8). Cell counts from untreated 18-day-old ADA–/– (–/–; n = 14) and ADA+/+ (+/+; n = 8) mice are shown on the right. (B) H&E stainings of thymus and spleen sections (× 5 magnification) in ADA–/– mice (untreated, treated with GT or BMT) and ADA+/+ mice. Tissues from ADA–/– untreated controls were collected postnatally at day 18, while all other mice were analyzed 6 months after treatment.
Lymphoid reconstitution after neonatal treatment. (A) Number of mature T and B cells in the peripheral circulation of GT (–/– GT) and transplant-recipient ADA–/– mice (–/– BMT) treated at neonatal age. As control, untreated age-matched ADA+/+ mice (+/+; n = 6) are reported. FACS analysis was used to determine the absolute number, represented as mean ± SD, of white blood cells, CD3+CD4+ and CD3+CD8+ T cells, B220+IgM+ B cells, and NK cells evaluated 5 months after GT (n = 5) or BMT (n = 8). Cell counts from untreated 18-day-old ADA–/– (–/–; n = 14) and ADA+/+ (+/+; n = 8) mice are shown on the right. (B) H&E stainings of thymus and spleen sections (× 5 magnification) in ADA–/– mice (untreated, treated with GT or BMT) and ADA+/+ mice. Tissues from ADA–/– untreated controls were collected postnatally at day 18, while all other mice were analyzed 6 months after treatment.
Previous GT preclinical studies for ADA-SCID were carried out in normal mice or in SCID mice given injections of human PBLs or CD34+ cells.31-33 Our results establish the ADA–/– model as a valid tool to study novel therapeutic approaches for ADA-SCID. In addition, they provide the demonstration that an animal genetically deficient in a metabolic pathway resulting in an early lethal phenotype can be rescued by GT. LV-mediated gene transfer in BM cells was recently shown to restore normal metabolic and neurological functions in mice deficient for arylsulfatase A, but the model is characterized by a relatively milder phenotype.34 LV were previously shown to rescue a severe model of thalassemic mice, but the protocol required high-dose irradiation and the lethal phenotype appeared at 7 to 8 weeks after transplantation of beta-globin null cells, in contrast to the shorter time observed in ADA–/– mice.35
The use of conditioning regimens that permit engraftment of clinically relevant numbers of transduced autologous cells with limited toxicity is highly desirable for the application of GT for genetic diseases. Gene marking studies in mice or large animal models have previously shown that nonmyeloablative regimens were associated with sustained marking levels.36-38 In ADA-SCID, significant engraftment of transduced stem/progenitor cells was obtained in a clinical GT trial that included nonmyeloablative conditioning.14 Our results show that pretransplant irradiation was crucial for long-term survival of ADA–/– mice, since animals given transplants of ADA+/+ BM cells without irradiation died 2 weeks after transplantation because of poor engraftment (data not shown). The nonlethal conditioning regimen used in the present study was effective in permitting long-term donor-recipient mixed chimerism in the peripheral circulation and in lymphoid organs of GT- and BMT-treated mice. Due to the short time available for cell engraftment and expansion before the appearance of the lethal phenotype, we designed experiments based on transplantation of transduced total BM cells rather than purified stem cells to rapidly provide an adequate systemic detoxification. We found that the mass of ADA expressing hematopoietic cells engrafted during the early phase after transplantation was a crucial factor in determining mice survival, since initial engraftment levels above 40% with more than 0.7 LV copy/donor cell were required to rescue the mice.
Attempts to intensify or reduce the irradiation dose did not improve the long-term survival of mice, as a result of irradiation toxicity and poorer engraftment, respectively (data not shown). Future studies will be important to determine whether the administration of higher cell doses or the use of chemotherapy-based conditioning regimen will enable to increase donor chimerism and the overall survival of these animals. On the other hand, preliminary results suggest that transplantation of transduced purified BM lineage-negative cells is sufficient to engraft and rescue the animals (A.M., A.A., unpublished data, October 2005).
Consistent with the clinical observation that ADA-expressing lymphocytes carry a selective advantage over defective lymphocytes,39-41 we observed a higher degree of engraftment of genetically corrected cells in the mature lymphoid compartment as compared to lymphoid precursors or BM myeloid cells in mice transduced with LV. This finding is in agreement with the preferential development of gene-corrected mature B and T cells observed in ADA-SCID patients treated with gammaretrovirally transduced CD34+ cells.14
The decrease in donor cells observed after both BMT and GT was expected from nonmyeloablative conditioning, leading to recovery of autologous BM cells. However, we found that mice given transplants of transduced BM displayed a significantly lower proportion of engraftment in the myeloid and precursor compartment in comparison to BMT. Since the mice received graft containing a similar proportion of Lin-negative/Sca-1+ progenitor cells (0.8%-1.0% of total BM), and no differences were observed between transplant of unmanipulated or mock-transduced ADA+/+ BM cells, it is unlikely that ex vivo culture affected the BM repopulating ability. Nevertheless, it is possible that in vitro exposure to the virus42 or overexpression of the ADA transgene in cells carrying multiple copies may compromise the repopulating activity of transduced ADA–/– stem cells. Donor BM cells were isolated from sex-mismatched littermates of an inbred strain both for BMT and GT: BMT donors (ADA+/+) were congenic for ADA while GT donors (ADA–/–) contained T cells which were reduced and nonfunctional. Thus, we cannot exclude that wild type BM T cells might have played a facilitating role for transplant by secretion of growth factors or response toward host BM (minor or sex-mismatched antigens), although graft-versus host disease was not observed in this setting.
Similar degrees of metabolic and immunological correction were obtained by treating mice either short-term after birth, or after an initial rescue with PEG-ADA. These approaches mimic the clinical intervention on patients directly enrolled at disease onset or previously treated with PEG-ADA. In agreement with the severity of the condition, all untreated animals and animals given transplantations of nontherapeutic vectors died within 3 weeks if PEG-ADA was not administered, confirming the severity of the pulmonary distress in ADA–/– mice. It is important to note that both GT approaches allowed normal alveogenesis and prevented lung inflammation, equally to transplant of wild-type BM cells (Figure 6C and data not shown). Similar correction of the lung pathology in ADA–/– mice was reported after weekly injection of PEG-ADA at doses much higher compared to those used in humans.25 Our data demonstrate that intracellular ADA produced by LV-transduced cells is equivalent to the extracellular detoxification provided by plasmatic ADA activity in preventing the pulmonary phenotype.
It is well established that SCID mice with defects in recombinase system have a high incidence of thymic lymphomas which is dramatically increased by irradiation,43,44 but no information is available in ADA–/– mice due to their short life span. Our results suggest that in the context of nonlethal irradiation and BMT also ADA–/– mice show a high risk of developing lymphomas. However, the development of tumors in irradiated ADA+/+ reveals an intrinsic susceptibility of the strain to nonlethal conditioning. The incidence of tumors was similar in irradiated adult ADA–/– mice treated with GT and BMT, and increased in the BMT group when animals were treated at neonatal age. Tumors of host origin may develop as the consequence of exposure of thymocytes or BM progenitors to ionizing radiation, as described for SCID mice.43 On the other hand, tumors of donor origin may result from an intrinsic susceptibility of ADA-deficient cells or an ineffective immunologic surveillance, as hypothesized for lymphomas developing in ADA-SCID patients receiving PEG-ADA.2,45 Importantly, we found no evidence of insertional mutagenesis induced by LV integrated at high copies in these tumors. Nevertheless, in-depth studies in a larger group of animals will be required to specifically address the safety of LV gene transfer in the ADA-deficient mouse model. To avoid the potential deleterious effect of irradiation, transplants could be performed with a chemotherapy-based conditioning or using recipient mice with a different background.
In summary, we report the successful rescue of ADA–/– mice with LV-mediated gene transfer in BM cells combined to nonmyeloablative conditioning. Our results indicate that restoring the expression of an ubiquitous enzyme in human hematopoietic cells is sufficient to allow adequate systemic detoxification and correct a severe organ phenotype. Finally, they provide evidence that the ADA-deficient mouse is a valuable preclinical model for exploring new gene transfer and transplantation approaches for this disease to further improve its treatment.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-05-023507.
A.M. designed and performed most research, analyzed data, and wrote the paper; R.J.H. and M.M.G. contributed to the development of the model and performed animal experiments; F.C. and A.T. conducted the biochemical studies; M.P., F.S., and C.D. performed the histopathology studies; C.D.S. performed the statistical analysis; L.B. conducted the studies on vector integrations; A.F. contributed to the generation of the lentiviral vector; L.N. and C.B. contributed to the study design and revised the paper; M.G.R. contributed to the study design, supervised A.M., and revised the paper; A.A. designed the research, analyzed the data, and wrote the paper; and all authors checked the final version of the manuscript.
Supported by grants from Italian Telethon Foundation, AFM/Telethon (GAT0205), Associazione Italiano per la Ricerca sul Cancro (AIRC), the European Community (project CONSERT LSBH-CT-2004-005242), Istituto Superiore di Sanità (Cellule Staminali), and Ministero dell' Università e della Ricerca (Fondo per gli investimenti della ricerca di base [MUIR FIRB]).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sara Deola and Lucia Sergi Sergi for help in preparation of GFP- and ADA-encoding LV. We are grateful to Francesco Marangoni for his precious help in the setting up of hemogram analysis, to Martina Rocchi for processing histopathological specimens, to Alessandro Ambrosi for statistical analysis, to Eugenio Montini for help in setting up the integration analysis, and to Loïc Dupré for helpful discussions during the course of this work. We acknowledge Rose Zamoyska for the external supervision of Alessandra Mortellaro.