Abstract
Aberrant micro RNA (miRNA) expression has been described in human malignancies including B-cell lymphomas. We here report BCR-ABL– and c-MYC–dependent regulation of miRNA expression in chronic myeloid leukemia (CML) using microarray analysis (miCHIP) and miRNA-specific quantitative real-time reverse transcriptase–polymerase chain reaction (miR-qRT-PCR). In 3 bcr-abl–positive cell lines, expression of miRNAs encoded within the polycistronic miR-17-92 cluster is specifically down-regulated (2- to 5-fold) by both imatinib treatment and anti–BCR-ABL RNA interference (RNAi). In addition, anti–c-MYC RNAi reduces miR-17-92 expression in K562 cells in which miRNAs can specifically repress reporter gene expression, as demonstrated by specific miRNA inhibition with antagomirs. Furthermore, lentivirus-mediated overexpression of polycistronic miRNAs in K562 cells confers increased proliferation, partial resistance against anti–c-MYC RNAi, and enhanced sensitivity to imatinib-induced cell death. Finally, we determined miR-17-92 expression in purified normal (n = 4), early chronic-phase (CP) (n = 24), and blast-crisis (BC) (n = 7) CML CD34+ cells and found up-regulation of polycistronic pri-miRNA transcripts in CML and mature miRNAs in CP but not in BC CML. These data are in accordance with a BCR-ABL–c-MYC–miR-17-92 pathway that mediates enhanced miRNA expression in CP but not BC CML CD34+ cells. Altered miRNA expression may contribute to the pathophysiology of the disease and may provide potential targets for therapeutic intervention.
Introduction
Micro RNAs (miRNAs) are small noncoding RNAs regulating gene expression by hybridization to complementary sequences usually located in the 3′-untranslated region (UTR) of coding transcripts. Subsequent recruitment of an RNA-multiprotein complex (RISC-RNA–induced silencing complex) results either in inhibition of mRNA translation or reduced mRNA stability.1 miRNAs are processed in a regulated multi-step process from primary transcripts (pri-miRNA) into mature miRNAs by cellular components also involved in the process of RNA interference (RNAi).2–4 During development, miRNA expression is tightly regulated in a cell- and tissue-specific manner. Interestingly, aberrant expression of specific miRNAs has recently been described for a variety of human malignancies including chronic lymphocytic leukemia (CLL) and other B-cell lymphomas5–7 and lung cancer.8,9 Accordingly, miRNA expression profiles can be used to classify human cancer.10
miRNAs may be encoded in (paralogue) clusters transcribed as polycistronic pri-miRNA transcripts. For example, 7 miRNAs (miR-17-5p, miR-17-3p, miR-18a, miR-19a, miR-20a, miR-19b, miR-92-1) are annotated to and transcribed from an intron of the C13-25orf locus at 13q31-q3211 (Figure 1A). This miR-17-92 polycistron is amplified and overexpressed in different types of B-cell lymphoma,13 promotes lymphomagenesis in a murine stem cell transplantation model,14 and is transcriptionally regulated by c-MYC.15 Interestingly, oncogenic ABL variants induce expression of c-MYC, and c-MYC is required for and cooperates with BCR-ABL in transformation of hematopoiesis in chronic myeloid leukemia (CML).16
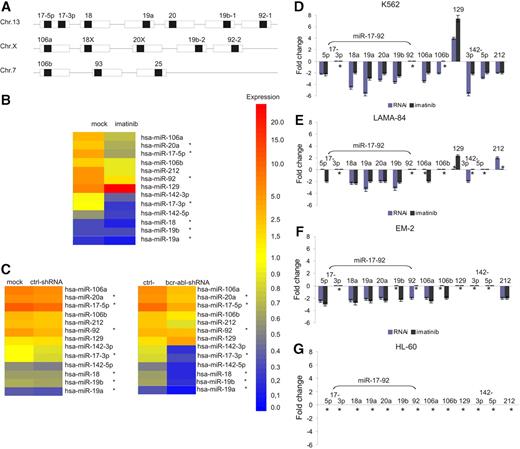
BCR-ABL–dependent expression of the miR-17-92 cluster in bcr-abl+ cells. (A) Genomic organization of 3 paralogous miR-17 miRNA clusters (▪ indicates mature miRNAs embedded in precursor miRNAs; □, pre-miRNAs) according to Tanzer and Stadler.11 The role of 17-3p expression as a unique miRNA or a reverse-complement strand is not yet clearly defined. (B) Heat map of miRNA expression profiling from total RNA of K562 cells treated with imatinib, using the miCHIP microarray platform. For each capture probe, the median of 4 background corrected replicas ± SD was calculated. Every experiment was normalized to the average of total signal intensity on each array. For comparative analysis, technical and biologic replicas were averaged after normalization. The left column shows parental K562 cells, and the right column shows imatinib-treated K562 cells. *miRNAs of the miR-17-92 cluster. (C) Heat map of miRNA expression profiling from total RNA of K562 cells treated with lentivirally expressed anti–bcr-abl shRNAs using the miCHIP microarray platform. The left side shows the comparison between parental and control shRNA20 transduced K562 cells, and the right side that between control shRNAs20 and anti–bcr-abl shRNAs. miRNA expression shown in panels B and C was regulated at least 2-fold in at least 1 treatment condition. (D) miRNA-specific quantitative RT-PCR of miRNAs regulated by BCR-ABL as determined by miCHIP. miRNA expression in K562 cells treated as described above with imatinib (dark gray bars) and lentivirally expressed anti–bcr-abl shRNA (blue bars) was compared with the appropriate controls as described in panels B and C. Mean and SD of 2 independent experiments. *No change in miRNA expression. miR-16 served as an endogenous control. (E-F) miRNA-specific quantitative RT-PCR from LAMA-84 (E) and EM-2 (F) cells treated as described for K562 cells. miR-16 served as an endogenous control. Mean and SD of 2 independent experiments. *No change in miRNA expression. (G) miRNA-specific quantitative RT-PCR after treatment of BCR-ABL–negative HL-60 cells with imatinib. miR-16 served as an endogenous control. *No change in miRNA expression.
BCR-ABL–dependent expression of the miR-17-92 cluster in bcr-abl+ cells. (A) Genomic organization of 3 paralogous miR-17 miRNA clusters (▪ indicates mature miRNAs embedded in precursor miRNAs; □, pre-miRNAs) according to Tanzer and Stadler.11 The role of 17-3p expression as a unique miRNA or a reverse-complement strand is not yet clearly defined. (B) Heat map of miRNA expression profiling from total RNA of K562 cells treated with imatinib, using the miCHIP microarray platform. For each capture probe, the median of 4 background corrected replicas ± SD was calculated. Every experiment was normalized to the average of total signal intensity on each array. For comparative analysis, technical and biologic replicas were averaged after normalization. The left column shows parental K562 cells, and the right column shows imatinib-treated K562 cells. *miRNAs of the miR-17-92 cluster. (C) Heat map of miRNA expression profiling from total RNA of K562 cells treated with lentivirally expressed anti–bcr-abl shRNAs using the miCHIP microarray platform. The left side shows the comparison between parental and control shRNA20 transduced K562 cells, and the right side that between control shRNAs20 and anti–bcr-abl shRNAs. miRNA expression shown in panels B and C was regulated at least 2-fold in at least 1 treatment condition. (D) miRNA-specific quantitative RT-PCR of miRNAs regulated by BCR-ABL as determined by miCHIP. miRNA expression in K562 cells treated as described above with imatinib (dark gray bars) and lentivirally expressed anti–bcr-abl shRNA (blue bars) was compared with the appropriate controls as described in panels B and C. Mean and SD of 2 independent experiments. *No change in miRNA expression. miR-16 served as an endogenous control. (E-F) miRNA-specific quantitative RT-PCR from LAMA-84 (E) and EM-2 (F) cells treated as described for K562 cells. miR-16 served as an endogenous control. Mean and SD of 2 independent experiments. *No change in miRNA expression. (G) miRNA-specific quantitative RT-PCR after treatment of BCR-ABL–negative HL-60 cells with imatinib. miR-16 served as an endogenous control. *No change in miRNA expression.
To screen for BCR-ABL–dependent miRNA expression, we initially performed microarray analysis (miCHIP) for 210 human miRNAs in K562 cells following treatment with either imatinib (to inhibit BCR-ABL kinase activity) or specific anti–BCR-ABL shRNA (to knock-down BCR-ABL gene expression). We found BCR-ABL–mediated induction of the miR-17-92 cluster and confirmed its BCR-ABL–dependent expression by miRNA-specific quantitative real-time reverse transcriptase–polymerase chain reaction (miR–qRT-PCR) in 2 additional CML cell lines. In addition to BCR-ABL, miR-17-92 expression also depends on c-MYC as demonstrated by c-myc–specific RNAi. miRNAs specifically repress reporter gene activity in K562 cells, and overexpression of an miR-17-92 variant (miR-17-19b-1) induces proliferation, confers partial resistance against inhibition of c-MYC expression, and sensitizes to imatinib-induced cell death. Finally, miR–qRT-PCR reveals that miR-17-92 miRNAs are overexpressed in primary CML CD34+ cells in chronic phase CP but not in blast crisis BC compared with normal CD34+ cells, suggesting a potential contribution of a BCR-ABL–c-MYC–miR-17-92 pathway to the pathophysiology of CML.
Patients, materials, and methods
Approval was obtained from the Ethical Committee of Hannover Medical School for these studies, and informed consent was obtained in accordance with the Declaration of Helsinki.
RNA isolation and miCHIP, miR–qRT-PCR, and pri-miRNA quantification
Total RNA from human K562, LAMA-84, EM-2 (all chronic myeloid leukemia), and HL-60 cells (acute myeloid leukemia); from purified normal peripheral blood CD34+ (purity > 90%); from peripheral blood CD34+ cells from newly diagnosed CP CML (purity > 98%); and from patients in BC (purity > 90%) was prepared using Trizol (Invitrogen, Carlsbad, CA) and analyzed using an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). Total RNA (5 μg) was labeled with a Cy3-conjugated RNA linker and hybridized to miCHIP as described.17 miCHIP is based on locked nucleic acid (LNA) technology, whereby LNA-modified, Tm-normalized capture probes (miRCURY; Exiqon, Woburn, MA) are printed onto Codelink (GE Healthcare, Slough, United Kingdom) slides. Images were acquired using an Axon scanner (4000B) with identical photomultiplier settings and processed and analyzed with Genepix 6 (Axon Instruments, Sunnyvale, CA) and Excel software.
Expression of mature miRNAs was determined using miR–qRT-PCR (Applied Biosystems, Foster City, CA) and was normalized using the 2−ΔΔCT method18 relative to miR-16 miRNA, which was nearly equally expressed in normal CD34+ cells (ie, < 1.3-fold change in CD34+ and K562 cells in all conditions tested; data not shown). PCR was performed in duplicate using an ABI7500 cycler, and miRNA expression of CML samples was normalized to the averaged ratio of 4 normal CD34+ samples.
miR-17-92 pri-miRNA expression was quantified by TaqMan RT-PCR (forward primer [FP]: 5′-CAGTAAAGGTAAGGAGAGCTCAATCTG-3′, reverse primer [RP]: 5′-CATACAACCACTAAGCTAAAGAATAATCTGA-3′, TaqMan-probe: 6-FAM-TGGAAATAAGATCATCATGCCCACTTGAGAC-TAMRA) and normalized to GAPDH expression (Applied Biosystems). c-MYC mRNA expression was quantified by TaqMan RT-PCR using primers/probe purchased from Applied Biosystems and normalized to GAPDH expression. TaqMan PCR was performed in triplicate, and CML samples were normalized to the averaged ratio of 4 normal CD34+ samples.
Antagomirs, sensor plasmids, and luciferase assays
Antagomirs (AmiR-17.5p: 5′-ACUACCUGCACUGUAAGCACUUUG-3′, AmiR-20a: 5′-CUACCUGCACUAUAAGCACUUUA-3′, and scramble-AmiR: 5′-aaaaccuuuugaccgagcguguu-3′) were chemically synthesized as 2′-O-methyloligoribonucleotides by BioSpring (Frankfurt, Germany). The sensor and control luciferase plasmids pGL3–miR-17.5p sensor and control as well as pGL3–miR-20a sensor and control were kindly provided by Joshua Mendell.15 Each sensor plasmid has 2 sites complementary to miR-17.5p or miR-20a and the control plasmids have the insert in reverse complement orientation.
K562 cells (1 × 106) were electroporated (330V, 10 ms) in 100 μL RPMI 1640 containing either 1 μg pGL3–miR-17.5p sensor, pGL3–miR-17.5p control, miR-20a sensor, or miR-20a control plasmid and 0.1 μg pBabe-Puro plasmid in a 4-mm electroporation cuvette using an EPI 2500 gene pulser (Fischer, Heidelberg, Germany). The cells were selected with 2 μg/mL puromycin to generate stable clones of K562-17.5p sensor, K562-17.5p control, K562-20a sensor, or K562-20a control cells.
The transfection of the antagomirs into K562 cells stably expressing the 17.5p sensor, 17.5p control, 20a control, or 20a sensor cassette was carried out by electroporation. Cells (1 × 106) were electroporated (330V and 10 ms) in a volume of 100 μL medium containing 2 μg antagomirs and 0.25 μg pRLSV40 (Promega, Madison, WI). Luciferase assays were performed 24 hours after transfection using the Dual Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity for each reaction.
Lentiviral overexpression of the miR-17-19b cluster and anti–c-myc RNAi
The retroviral vector MSCV-PIG-17-19b containing miR-17-19b-114 was kindly provided by Gregory Hannon. To construct the lentiviral vector S-17-19b-IEW, the plasmid pHR'-SIN-SIEW-SnaBI was digested with BamHI followed by a DNA polymerase I fill-in reaction and gel purification. The miR-17-19b-1 cassette was excised from MSCV-PIG-17-19b plasmid with XhoI/EcoRI, and the cohesive ends were filled in using Klenow. The approximately 790-nucleotide (nt) blunt-end fragment was ligated with the blunted BamHI vector fragment, placing 17-19b-1 downstream of the SFFV promoter (S-17-19b-IEW).
Anti–c-MYC shRNAs corresponding to position 817 to 835 of the human c-MYC gene19 (GenBank accession no. NM_002467), lentiviral transgene plasmids pdc-SR, and shRNA controls were cloned as described.20 Lentiviral vector particles were produced by transient calcium phosphate cotransfection of 293T cells in a T175 flask with 60 μg lentiviral vector DNA together with 45 μg of packaging plasmid DNA and 30 μg of VSV.G plasmid.20 Viral supernatants were harvested 32 and 48 hours after transfection, concentrated by low-speed centrifugation at 10 500g for 16 hours at 10°C, and titered as described.12 The titers were averaged and typically ranged between 1 × 107 and 10 × 107 IU/mL. Parental K562 cells and K562 cells stably expressing SIEW or S-17-19b-IEW were transduced with an anti-cMyc shRNA at a multiplicity of infection (MOI) of approximately 1 as described,20 were sorted for GFP expression using DakoCytomation MoFlo (Glostrup, Denmark), and individual clones were isolated.
Cell proliferation of transduced K562 cells
Transduced K562 cells (5 × 104/mL) were cultured in 24-well plates and viable cells were counted from 1 to 7 days by trypan blue exclusion.
Results
miRNA expression in CML cell lines
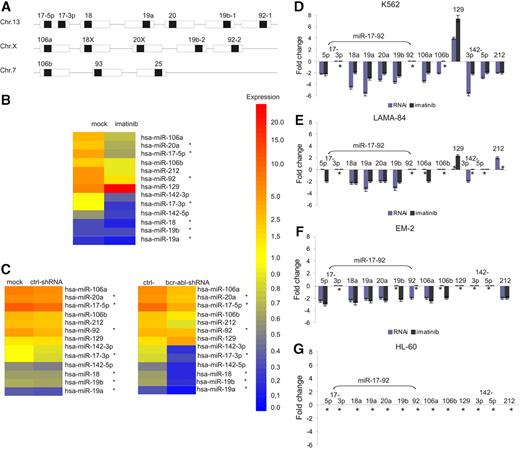
To search for BCR-ABL–dependent miRNA expression, K562 cells were initially treated with either imatinib (1 μM for 24 h) to inhibit BCR-ABL tyrosine kinase activity or lentivirally expressed anti–BCR-ABL shRNA (for 96 h after transduction) to silence BCR-ABL gene expression.21 An miRNA-based microarray chip assay (miCHIP) was used to screen for differentially expressed miRNAs. Among 210 mature miRNAs analyzed, 9 were found to be regulated more than 2-fold by both imatinib and/or anti–BCR-ABL–RNAi treatment compared with the appropriate controls; miR-17-5p, miR-17-3p, miR-20a, miR-92, miR-106a, miR-106b, miR-142-3p, and miR-212 were down-regulated and miR-129 was up-regulated, respectively (Figure 1B-C). Interestingly, the first 4 of these miRNAs belong to the miR-17-92 polycistronic cluster, and miR-106a and miR-106b are located in paralogue clusters of miR-17-9211 (Figure 1A).
To validate miRNA expression as determined by miCHIP, miR–qRT-PCR was performed on RNA isolated from K562 cells treated as described above. As shown in Figure 1D, miR–qRT-PCR confirmed BCR-ABL–dependent miRNA expression for both imatinib and anti–BCR-ABL RNAi for most miRNAs identified by miCHIP. However, no regulation was observed for miR-17-3p or miR-92 under both treatment conditions and miR-106b in the case of imatinib treatment.
To analyze whether BCR-ABL–dependent regulation of miRNA expression is CML specific and not restricted to the K562 cell line, 2 additional BCR-ABL–positive myeloid CML cell lines (LAMA-84 and EM-2) and BCR-ABL–negative HL-60 cells were analyzed by miR–qRT-PCR. As shown in Figure 1E-G, BCR-ABL–dependent expression of the miR-17-92 cluster in LAMA-84 and EM-2 cells was very similar to that observed in K562 cells, whereas expression of miR-106b, miR-129, miR-142-3p, miR-142-5p, and miR-212 was more heterogeneous. In addition, imatinib treatment did not effect miRNA expression in HL-60 cells (Figure 1G). We therefore decided to analyze miR-17-92 expression in greater detail.
c-MYC–dependent expression of miR-17-92 in K562 cells
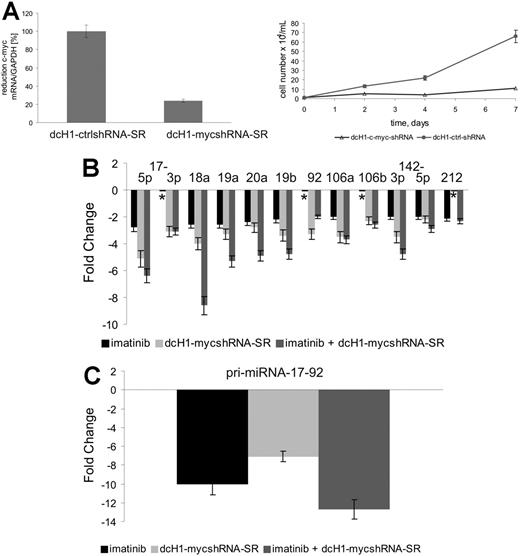
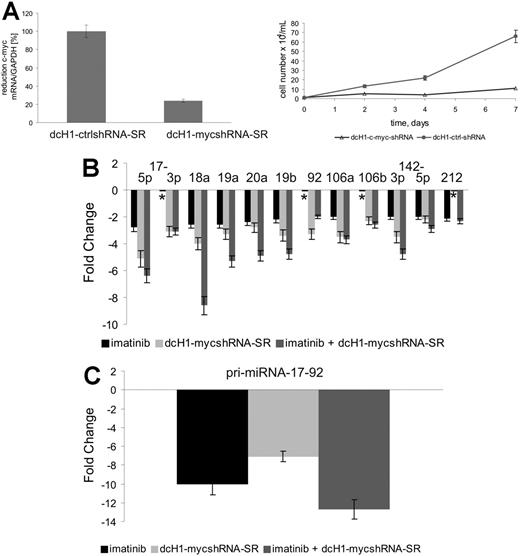
Since c-Myc is known to activate miR-17-92 transcription,15 we analyzed its impact on expression of BCR-ABL–regulated miRNAs in K562 cells. Anti–c-myc–specific shRNAs were expressed from lentiviral vectors used to transduce K562 cells. As shown in Figure 2Ai, anti–c-myc–specific, but not control, shRNAs reduce c-myc mRNA levels by about 75%. In addition, inhibition of c-MYC expression in K562 cells induces a strong, almost complete inhibition of cell proliferation (Figure 2Aii). We next analyzed the expression of miR-17-92 pri-miRNA and the mature miRNAs by real-time RT-PCR and miR–qRT-PCR, respectively. As shown in Figure 2B-C, both imatinib and anti–c-MYC RNAi reduced pri-miRNA and miRNA expression to similar extents, with enhanced repression upon combination treatment. Interestingly, expression of miR-17-3p and miR-92 was reduced by anti–c-MYC RNAi but not imatinib as seen before, and miR-212 levels were reduced by imatinib but not anti–c-MYC shRNAs.
miR-17-92 expression in K562 cells. (A) c-MYC mRNA levels (i) were measured by real-time qRT-PCR 4 days after lentiviral transduction and normalized in comparison to GAPDH expression. c-MYC mRNA expression of control dcH1-ctrl-SR–transduced cells was set to 100% (average of 3 independent experiments). Effects of lentiviral-mediated c-MYC shRNA expression compared with control shRNAs on cell proliferation (ii). Viable cells were counted by trypan blue exclusion. Mean and SD of 2 independent experiments are shown. (B) miR-17-92 expression after treatment with imatinib (black bars), lentivirally expressed anti–c-MYC shRNA (light gray bars), or simultaneous treatment with imatinib and anti–c-MYC shRNA (gray bars) as determined by miR–qRT-PCR. Mean and SD of 2 independent experiments. *No change in miRNA expression. (C) Expression of miR-17-92 pri-miRNA transcripts after treatment with imatinib (dark gray bar), lentivirally expressed anti–c-myc shRNA (light gray bar), or simultaneous treatment with imatinib and c-myc RNAi as determined by real-time RT-PCR. Mean and SD of 2 independent experiments.
miR-17-92 expression in K562 cells. (A) c-MYC mRNA levels (i) were measured by real-time qRT-PCR 4 days after lentiviral transduction and normalized in comparison to GAPDH expression. c-MYC mRNA expression of control dcH1-ctrl-SR–transduced cells was set to 100% (average of 3 independent experiments). Effects of lentiviral-mediated c-MYC shRNA expression compared with control shRNAs on cell proliferation (ii). Viable cells were counted by trypan blue exclusion. Mean and SD of 2 independent experiments are shown. (B) miR-17-92 expression after treatment with imatinib (black bars), lentivirally expressed anti–c-MYC shRNA (light gray bars), or simultaneous treatment with imatinib and anti–c-MYC shRNA (gray bars) as determined by miR–qRT-PCR. Mean and SD of 2 independent experiments. *No change in miRNA expression. (C) Expression of miR-17-92 pri-miRNA transcripts after treatment with imatinib (dark gray bar), lentivirally expressed anti–c-myc shRNA (light gray bar), or simultaneous treatment with imatinib and c-myc RNAi as determined by real-time RT-PCR. Mean and SD of 2 independent experiments.
Function of endogenous miR-17-5p and miR-20a in K562 cells
To analyze miRNA function in vivo, we transfected K562 cells with constructs containing 2 perfectly complementary binding sites for miR-17-5p or miR-20 in the 3′-UTR of the firefly luciferase gene (called “miR-17-5p sensor” and “miR-20a sensor”). Plasmids containing the reverse complement sequence of the respective miR-17-5p or miR-20 binding site served as controls. In this assay, endogenous miRNAs bind to the reporter mRNA and suppress luciferase expression and activity. As shown in Figure 3A, cotransfection of the miR-17-5p and miR-20a antisense oligonucleotides (antagomir-17-5p and antagomiR-20a), but not scrambled oligonucleotides, increases normalized luciferase activity. In addition, luciferase activity is enhanced in K562 cells with RNAi-reduced c-myc expression and can be further increased by antagomir-17-5p or antagomir-20a. As previously observed,15 each antagomir was capable of blocking expression of both reporter genes due to nucleotide similarity between miR-17-5p and miR-20, which differ at only 2 nucleotides.
miR-17-92 function in K562 cells. (A) Inhibition of miR-17-5p and miR-20a function by antagomirs. K562 cells stably transfected with miR-17-5p (i) and miR-20a sensor (ii) luciferase constructs with normal (dcH1-ctrl-shRNA, left columns) or reduced c-MYC expression (dcH1-MYC-shRNA, right columns) were electroporated alone (mock) or with the following antagomirs, as indicated: scrambled, antagomiR-17-5p, or antagomir-20a. The ratio of normalized sensor to control luciferase activity is shown. Mean and SD of 2 independent experiments. (B) Diagram of miR-17-19b, a variant miR-17-92 cluster, transcribed as a polycistronic transcript from an SFFV promoter (long terminal repeat [LTR] of spleen focus–forming virus) inserted in a lentiviral vector, S-17-19b-IEW. The vector encodes EGFP as a marker gene. (C) miR-17-19b expression in 3 independent lentivirally transduced K562 clones (nos. 1-3) in comparison to K562 transduced with empty vector as determined by miR–qRT-PCR. (D) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. K562 cells were lentivirally transduced (transduction efficacy > 95%; data not shown), plated at 104 cells per well 2 days after transduction, and the number of EGFP+ cells was counted. Cell proliferation was standardized to the fastest proliferating cells (clone 1), which was set to 100%. Mean and SD of 2 independent experiments. (E) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls upon reduction of c-MYC expression. The experiments were performed as described in panel D. (F) Imatinib-induced cell death in 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. Ratio of PI-positive cells in the presence of increasing concentrations of imatinib are shown. Mean and SD of 2 independent experiments.
miR-17-92 function in K562 cells. (A) Inhibition of miR-17-5p and miR-20a function by antagomirs. K562 cells stably transfected with miR-17-5p (i) and miR-20a sensor (ii) luciferase constructs with normal (dcH1-ctrl-shRNA, left columns) or reduced c-MYC expression (dcH1-MYC-shRNA, right columns) were electroporated alone (mock) or with the following antagomirs, as indicated: scrambled, antagomiR-17-5p, or antagomir-20a. The ratio of normalized sensor to control luciferase activity is shown. Mean and SD of 2 independent experiments. (B) Diagram of miR-17-19b, a variant miR-17-92 cluster, transcribed as a polycistronic transcript from an SFFV promoter (long terminal repeat [LTR] of spleen focus–forming virus) inserted in a lentiviral vector, S-17-19b-IEW. The vector encodes EGFP as a marker gene. (C) miR-17-19b expression in 3 independent lentivirally transduced K562 clones (nos. 1-3) in comparison to K562 transduced with empty vector as determined by miR–qRT-PCR. (D) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. K562 cells were lentivirally transduced (transduction efficacy > 95%; data not shown), plated at 104 cells per well 2 days after transduction, and the number of EGFP+ cells was counted. Cell proliferation was standardized to the fastest proliferating cells (clone 1), which was set to 100%. Mean and SD of 2 independent experiments. (E) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls upon reduction of c-MYC expression. The experiments were performed as described in panel D. (F) Imatinib-induced cell death in 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. Ratio of PI-positive cells in the presence of increasing concentrations of imatinib are shown. Mean and SD of 2 independent experiments.
Overexpression of miR-17-19b in K562 cells
Since oncogenic ABL variants induce c-MYC and c-MYC activates miR-17-92 expression, we asked whether miRNA overexpression could compensate for silencing of c-MYC. miR-17-19b, a variant of the miR-17-92 polycistron selected for efficient transgenic miRNA expression that lacks the 3′-located miR-92,14 was overexpressed in K562 cells by lentiviral gene transfer (S-17/19b-IEW; Figure 3B). Three individual clones were isolated by limiting dilution, and the relative miRNA expression level compared with control K562 cells is shown in Figure 3C. Expression of miR-17-5p, miR-17-3p, miR-19a, miR-20a, and miR-19b was increased by 2- to 5-fold with clones no. 1 and no. 3, showing higher expression compared with no. 2. Of note, miR-18a expression was not changed compared with control K562 cells for reasons yet to be clarified.
As shown in Figure 3D, overexpression of miR-17-19b enhances cell proliferation compared with K562 cells transduced with empty vector (SEW), with stronger induction of proliferation in clones no. 1 and no. 3 compared with no. 2. As shown above, anti–c-MYC RNAi induces a strong inhibition of cell proliferation in K562 cells (Figure 2Aii). However, miR-17-19b overexpression enhances proliferation of K562 cells with reduced c-MYC expression and partially abrogates the inhibitory effects of anti–c-MYC RNAi. Once again, this effect was more pronounced in clones no. 1 and no. 3 compared with no. 2 (Figure 3E). Finally, since c-MYC is known to be capable of inducing apoptosis, we studied miRNA-dependent cytotoxicity of imatinib in K562 cells. As shown in Figure 3F, propidium iodide (PI) staining demonstrates increased sensitivity to imatinib-induced cell death in K562 cells overexpressing miR-17-19b compared with controls. Again, clones no. 1 and no. 3 were more sensitive to imatinib treatment than clone no. 2.
miRNA expression in CML CD34+ cells from chronic phase and blast crisis
Expression of miRNAs found to be regulated in K562 cells in the initial miCHIP screen was analyzed by miR–qRT-PCR in highly purified CD34+ cells from healthy donors (n = 4) and CML patients in CP (n = 24) and in BC (n = 7). In addition, pri-miR-17-92 and c-MYC expression was analyzed by quantitative RT-PCR. As shown in Table 1, pri-miR-17-92 was consistently overexpressed in 19 of 22 CP CD34+ primary samples analyzed (mean, 12.3-fold) and, at lower levels, in all 7 BC CD34+ primary samples (mean, 3.4-fold) compared with normal CD34+ cells. In parallel, c-myc mRNA was overexpressed in 15 of 19 CML CP CD34+ samples compared with healthy controls (mean 3.1-fold). Interestingly, c-myc expression was more heterogeneous in BC CD34+ cells, with 2 samples showing reduced expression (BC no. 2 and no. 4; mean, 0.7-fold). Mature miRNAs tended to be overexpressed in CP CD34+ cells compared with normal CD34+ cells (mean between 1.9- and 6.0-fold). In particular, miR-17-5p, miR-18a, and miR-106a were up-regulated more than 2-fold in all CML CP samples analyzed. miR-20a, miR-19a, miR-19b, and miR-212 were also predominantly up-regulated in 87.5%, 83%, 75%, and 92%, respectively, of the CD34+ cells from patients in CP. miR-106b expression was mostly unchanged, and miR-142-3p had revealed a more heterogeneous expression pattern. Expression of miR129, the only miRNA up-regulated in K562 cells treated with imatinib or anti–BCR-ABL RNAi, was undetectable by miR–qRT-PCR, presumably due to low expression in primary cells. In contrast to CP, BC CD34+ cells showed unchanged or even down-regulated miRNA expression (mean, 0.3- to 1.6-fold). A striking example of this tendency is patient no. 15 in CP whose cells could be further studied after transformation into BC ( = BC no. 1).
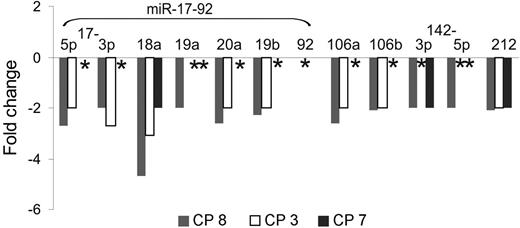
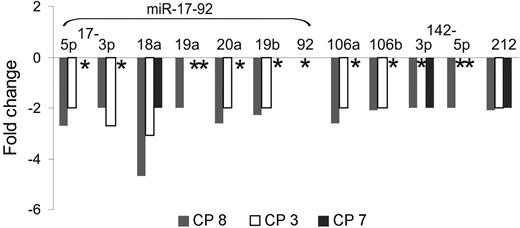
Finally, BCR-ABL–dependent miRNA expression was analyzed in CD34+ cells from 3 CML samples (CP no. 3, CP no. 7, and CP no. 8) upon treatment with imatinib in cytokine-supplemented suspension cultures. As shown in Figure 4, expression of miR-17-92–encoded miRNAs was reduced 2- to 5-fold in CD34+ cells from patients no. 3 and no. 8 but was unchanged in cells from no. 7, where only expression of miR-18a and miR-212 was reduced.
BCR-ABL–dependent miRNA expression in primary CML CD34+ cells. Chronic-phase CML CD34+ cells were cultured for 24 hours with imatinib (1 μM) and cytokines as described previously,12 and miRNA expression was determined by miR–qRT-PCR. ID indicates initial diagnosis in chronic phase. *No change in miRNA expression.
BCR-ABL–dependent miRNA expression in primary CML CD34+ cells. Chronic-phase CML CD34+ cells were cultured for 24 hours with imatinib (1 μM) and cytokines as described previously,12 and miRNA expression was determined by miR–qRT-PCR. ID indicates initial diagnosis in chronic phase. *No change in miRNA expression.
Discussion
Our data demonstrate BCR-ABL–mediated expression of miR-17-92 miRNAs in K562, LAMA-84, and EM-2 CML cell lines dependent upon BCR-ABL tyrosine kinase activity, as demonstrated by the mostly congruent results for imatinib treatment and inhibition of BCR-ABL gene expression by RNAi (Figure 1B-F). Reduced miRNA expression was observed using both miCHIP and miR–qRT-PCR in K562 cells for all miR-17-92 miRNAs, excluding miR-17-3p and miR-92, which seemed not to be regulated in miR–qRT-PCR analyses (with the identity of miR-17-3p as an miRNA still controversial15 ). The reason for this difference is not yet clear but may be due to different sensitivity and/or specificity of both quantification methods. In addition, miR-17-92 expression is regulated by c-MYC in K562 cells in accordance with earlier reports demonstrating binding of c-MYC to mir-17-92 regulatory sequences and its subsequent transcriptional activation.15 Interestingly, we observed differential miRNA suppression by imatinib and anti–c-MYC RNAi as demonstrated for miR-17-3p and miR-92 (independent of BCR-ABL in miR–qRT-PCR analyses) and miR-212 (independent of c-MYC), respectively (Figure 2B), as well as enhanced suppression by combined treatment with imatinib and anti–c-myc shRNAs. In addition, expression of pri-miR-17-92 was also regulated by both BCR-ABL and c-MYC (Figure 2C). However, since miRNA biogenesis is a highly regulated multi-step process,1–4 steady-state miRNA levels can be regulated at different levels, such as pri-miRNA transcription, Drosha-dependent pri-miRNA processing, nuclear export, Dicer-dependent processing into mature miRNAs, incorporation into RISC, and miRNA turnover. This may explain the lack of correlation between pri-miRNA and mature miRNA expression levels, and recent data from Thomson et al4 directly comparing pri-miRNA and mature miRNA expression suggest that Drosha-mediated pri-miRNA processing may be altered in cancer cells. In addition, the rate of processing for individual miRNAs encoded on polycistronic pri-miRNA transcripts may also depend on secondary and tertiary RNA structure. This may well explain the lack of transgenic miR-18a overexpression upon lentiviral gene transfer of a variant miR-17-92, namely the miR-17-19b cluster (Figure 3B-C). Our miRNA expression data support a BCR-ABL–c-MYC–miR-17-92 pathway15,16,22–24 in myeloid cells with the SH2- and C-terminal domain of ABL required for c-MYC induction. Although JAK2 may be involved, the precise molecular mechanisms of BCR-ABL–dependent c-MYC induction still remain unclear.24
In K562 cells, endogenous miR-17-92 miRNAs, namely miR-17-5p and miR-20 which share almost identical miRNA binding sites, are functional as demonstrated by luciferase reporter experiments. In these experiments, reporter gene activity depends on miRNA recognition sites in the 3′-UTR of the reporter and endogenous miRNA expression as described by O'Donnell et al15 (Figure 3A). Using sequence-specific inhibition of miRNAs by antagomirs, we demonstrate miRNA-specific repression of luciferase activity and cooperation of reduced miRNA expression (due to anti–c-MYC RNAi) with antagomirs on induction of luciferase activity. Finally, overexpression of the miR-17-19b cluster in K562 cells induced increased cell proliferation (Figure 3D), antagonized proliferation arrest due to anti–c-MYC RNAi (Figure 3E), and sensitized cells to imatinib-induced cell death compared with controls (Figure 3F). These data are in line with the multiple functions of c-MYC for growth, cell proliferation, and induction of apoptosis (for review see Levens25 and Dang et al26 ). According to the expression data discussed above, the data on miRNA function place the miR-17-92 cluster downstream of c-MYC in K562 cells. Although expression of E2F1, PTEN, and TGFβ have recently been shown to be regulated by miR-17-92–encoded miRNAs,15,27,28 the number and identity of additional validated targets are currently not known.
We also found overexpression of the miR-17-92 cluster in CML CD34+ cells from patients in CP but not in BC compared with normal CD34+ cells (Table 1). In addition, miR-106a was overexpressed in CP but not in BC CD34+ cells, whereas miR-212 was up-regulated in both CP and BC CML CD34+ cells compared with healthy controls. Similarly, c-MYC expression was up-regulated in CP but not in BC CD34+ cells. Although this correlation between enhanced miR-17-92 and c-MYC expression needs further validation in a larger group of samples, it correlates well with the expression data from the K562 cell line. Finally, expression of pri-miR-17-92 transcripts was up-regulated in CP and reduced but still enhanced in BC CD34+ cells. The reason for the differential pri-miRNA and miRNA expression in CP CML and BC is currently not known, but the transition from the myeloproliferative chronic stage to BC may correspond to the general reduction of miRNA expression in human malignancies.10 miRNA overexpression in CP seems to depend upon BCR-ABL tyrosine kinase activity in primary CML cells, like in CML cell lines, as demonstrated by reduced miR-17-92, miR-106a, miR-106b, and miR-212 expression upon imatinib treatment in 2 of 3 primary CP samples analyzed (Figure 4). The reason for the different imatinib response remains unclear, since imatinib inhibited colony formation of CD34+ colony-forming cells in all 3 samples (data not shown).
In summary, these data add miRNAs to the signaling network affected by the BCR-ABL oncoprotein and suggest a BCR-ABL–c-MYC–miR-17-92 pathway in myeloid CML cells. Further studies are required to exactly identify miRNA targets and their functional role in the pathophysiology of CML. Such knowledge may have therapeutic impact in the future, since pathologic miRNA effects may specifically be modulated by anti-miRNA therapeutics such as antagomirs.29
Authorship
Contribution: L.V. and K.B. performed experiments; M.C. and M.U.M. performed miCHIP experiments; B.S. isolated primary cells; A.H. provided primary cells; A.G. prepared the manuscript; M.E. designed research, analyzed data, and wrote manuscript; and M.S. designed and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
M.E. and M.S. contributed equally to this study.
Correspondence: Matthias Eder and Michaela Scherr, Medizinische Hochschule Hannover, Zentrum Innere Medizin Abteilung Hämatologie, Hämostaseologie und Onkologie, Carl-Neuberg Strasse 1, D-30623 Hannover, Germany; e-mail: eder.matthias@mh-hannover.de, m.scherr@t-online.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank G. J. Hannon (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) and J. T. Mendell (Johns Hopkins University School of Medicine, Baltimore, MD) for providing us with the retroviral vector PIG-miR-17-19b and miRNA reporter plasmids, respectively. We acknowledge the contribution of Matthias Hentze and Vladimir Benes (The European Molecular Biology Laboratory [EMBL]) in establishing and maintaining the miCHIP microarray platform, Sabine Schmitt (EMBL) for technical help, and Michael A. Morgan (Medical School Hannover) for critical reading of the manuscript.
This work was supported in part by grants of the Deutsche Forschungsgemeinschaft (SFB 566); H. W. & J. Hector-Stiftung; Wilhelm Sanders-Stiftung (M.S. and M.E.); the Deutsche Krebshilfe; the Competence Network “Acute and chronic leukemias,” sponsored by the German Bundesministerium für Bildung und Forschung (Projektträger Gesundheitsforschung; DLR e.V.-01 GI9980/6) and the European LeukemiaNet within the sixth framework program of the European commission (A.H. and A.G.); and a Cancer Research Net grant (BMBF[NGFN] 201GS0450; M.U.M.).

![Figure 3. miR-17-92 function in K562 cells. (A) Inhibition of miR-17-5p and miR-20a function by antagomirs. K562 cells stably transfected with miR-17-5p (i) and miR-20a sensor (ii) luciferase constructs with normal (dcH1-ctrl-shRNA, left columns) or reduced c-MYC expression (dcH1-MYC-shRNA, right columns) were electroporated alone (mock) or with the following antagomirs, as indicated: scrambled, antagomiR-17-5p, or antagomir-20a. The ratio of normalized sensor to control luciferase activity is shown. Mean and SD of 2 independent experiments. (B) Diagram of miR-17-19b, a variant miR-17-92 cluster, transcribed as a polycistronic transcript from an SFFV promoter (long terminal repeat [LTR] of spleen focus–forming virus) inserted in a lentiviral vector, S-17-19b-IEW. The vector encodes EGFP as a marker gene. (C) miR-17-19b expression in 3 independent lentivirally transduced K562 clones (nos. 1-3) in comparison to K562 transduced with empty vector as determined by miR–qRT-PCR. (D) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. K562 cells were lentivirally transduced (transduction efficacy > 95%; data not shown), plated at 104 cells per well 2 days after transduction, and the number of EGFP+ cells was counted. Cell proliferation was standardized to the fastest proliferating cells (clone 1), which was set to 100%. Mean and SD of 2 independent experiments. (E) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls upon reduction of c-MYC expression. The experiments were performed as described in panel D. (F) Imatinib-induced cell death in 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. Ratio of PI-positive cells in the presence of increasing concentrations of imatinib are shown. Mean and SD of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-045104/4/m_zh80100701090003.jpeg?Expires=1770202044&Signature=ItAReR7k8~Gx1QsadaOrNt-xE60fbfwMq9g7rWN~IuWDHVbKhM50Zd3FkPxHQ2Wd~RCQ684JwNCqLYIZiutRz-HX4lLkHNsHJy5gfNy8~Xe4bmcPrkZWomQHpvFQWvhirm0tqL6D9gjFowiByqNY0XT5WQSu2ujqwIpDp6EFNI-gZWcom1RnNq2rSuL5aReZNQ3wzQn1vrECzPVgUloqqDnF~j~pCtqwzZBJkvHYSbe-yCvVFDb5mSp7JJq05F4J~V1KBuIJSzzFvKImSENT1HGabePvgwZKZrE1fw3yWO5QTTB6HW5PXDlkq4aewkj94wDTCe8riA6POv2C8Vzj7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. miR-17-92 function in K562 cells. (A) Inhibition of miR-17-5p and miR-20a function by antagomirs. K562 cells stably transfected with miR-17-5p (i) and miR-20a sensor (ii) luciferase constructs with normal (dcH1-ctrl-shRNA, left columns) or reduced c-MYC expression (dcH1-MYC-shRNA, right columns) were electroporated alone (mock) or with the following antagomirs, as indicated: scrambled, antagomiR-17-5p, or antagomir-20a. The ratio of normalized sensor to control luciferase activity is shown. Mean and SD of 2 independent experiments. (B) Diagram of miR-17-19b, a variant miR-17-92 cluster, transcribed as a polycistronic transcript from an SFFV promoter (long terminal repeat [LTR] of spleen focus–forming virus) inserted in a lentiviral vector, S-17-19b-IEW. The vector encodes EGFP as a marker gene. (C) miR-17-19b expression in 3 independent lentivirally transduced K562 clones (nos. 1-3) in comparison to K562 transduced with empty vector as determined by miR–qRT-PCR. (D) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. K562 cells were lentivirally transduced (transduction efficacy > 95%; data not shown), plated at 104 cells per well 2 days after transduction, and the number of EGFP+ cells was counted. Cell proliferation was standardized to the fastest proliferating cells (clone 1), which was set to 100%. Mean and SD of 2 independent experiments. (E) Cell proliferation of 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls upon reduction of c-MYC expression. The experiments were performed as described in panel D. (F) Imatinib-induced cell death in 3 miR-17-19b lentivirally transduced K562 cell clones compared with empty vector controls. Ratio of PI-positive cells in the presence of increasing concentrations of imatinib are shown. Mean and SD of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-045104/4/m_zh80100701090003.jpeg?Expires=1770202045&Signature=JzVIoGvYk4e2hwAbRwkc~xYLc~7f6Svo9DZmi4EH~20bml8DSyx9gGFoFPo2tqatgZqZfq2EX2X1S2UG3teVvarxsoojhyYZ5LLdIf2NycJY0ijq9DzqfHGUzQEV8GGt2vfBSndlzmrEr3PCJTskOc3m4owhF7-MyBdw4ed~JuHuwtgRXFq81u0XKl-qzHdxMCyo998TYNeNrx9wAU7~usW~7by2dLJOYjxSU8AMNu5ZX~ZSHDd0aCnBytsLTYn4MBGTDoQTvyRkZRjPmgqXnBnc94LMnZ7BiiQ2U8JMWnzcRnbuemDGl0arCpiw14AIf0OWtaGZelRt~m5u6yVLIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)