Abstract
Monosomy 7 (−7) and deletion 7q \del(7q)] are rare in childhood acute myeloid leukemia (AML). We retrospectively collected data on 258 children with AML or refractory anemia with excess blasts in transformation (RAEB-T) and −7 or del(7q) with or without other cytogenetic aberrations \± other]. Karyotypes included −7 (n = 90), −7 other (n = 82), del(7q) (n = 21), and del(7q) other (n = 65). Complete remission (CR) was achieved in fewer patients with −7 ± other compared with del(7q) ± other (61% versus 89%, P < .001). Overall, the 5-year survival rate was 39% (SE, 3%). Survival was superior in del(7q) ± other compared with −7 ± other (51% versus 30%, P < .01). Cytogenetic aberrations considered favorable in AML \t(8;21)(q22;q22), inv(16)(p13q22), t(15;17)(q22;q21), t(9;11)(p22;q23)] (n = 24) were strongly associated with del(7q) and a higher 5-year survival rate compared with del(7q) without favorable cytogenetics (75% versus 46%, P = .03). Patients with −7 and inv(3),−5/del(5q), or + 21 had a 5-year survival rate of 5%. Stem cell transplantation analyzed as a time-dependent variable had no impact on overall survival. However, patients not achieving CR had a 31% survival rate after stem cell transplantation. Childhood AML with chromosome 7 aberrations represents a heterogeneous group of disorders with additional cytogenetic aberrations having a major prognostic impact which should be reflected in future risk-group stratification.
Introduction
Monosomy 7 (−7) is the most common acquired abnormality in hematopoietic cells of children with myelodysplastic syndrome (MDS) being found in about 40% of patients.1–3 In contrast, −7 or deletion of the long arm of chromosome 7 \del(7q)] is found in only 4% to 5% of pediatric patients with acute myeloid leukemia (AML).4–9 On the basis of the frequency of chromosome 7 aberrations and on the incidence of MDS and AML,10–12 the annual incidence of −7/del(7q) is estimated to be 0.3 to 0.7 cases of MDS and 0.3 cases of AML per million children younger than 15 years of age
Refractory cytopenia with −7 is associated with a high risk of disease progression and inferior survival.13 Most studies have found −7 to be without prognostic significance in advanced MDS (bone marrow blasts ≥ 5%) and in juvenile myelomonocytic leukemia (JMML),14–16 including children receiving hematopoietic stem cell transplantation (HSCT).17 The British group, however, found a poor outcome in children with MDS and −7.12
Patients with AML and −7 or del(7q) have been reported to have an adverse prognosis; thus, they are treated on high-risk AML protocols and offered HSCT in first remission.18 AML studies have traditionally combined patients with −7 and those with del(7q) because of their low numbers. Two studies that included mainly adult patients suggested that del(7q) is associated with a better outcome compared with that of patients with −7.19,20 A study from the European Working Group on Childhood MDS (EWOG-MDS) suggested that patients with AML and −7 as the sole cytogenetic abnormality experience a poor survival, whereas the probability of survival of patients with del(7q) was comparable to that of other patients with AML.14 Even the largest pediatric AML series has too few patients with chromosome 7 abnormalities to analyze the cytogenetic subgroups −7 and del(7q) separately. We present data from an international collaborative study on 258 children with myeloid leukemia and chromosome 7 abnormalities collected from several major AML study groups as well as from EWOG-MDS.
Patients, materials, and methods
Patients and treatment
Pediatric AML study groups and EWOG-MDS reviewed their records to identify patients younger than 19 years of age whose disease was diagnosed between January 1, 1985, and December 31, 2002. Only children with peripheral blood (PB) or bone marrow (BM) blasts of at least 20% and −7 or del(7q) were included. Patients were eligible if BM or PB karyotype showed at least 3 cells with loss of a whole chromosome 7 or at least 2 cells with deletion of the long arm of chromosome 7. Both karyotypes with or without additional cytogenetic abnormalities were included. Patients with ring chromosome 7, add(7q), or der(7q), as well as those studied only by fluorescence in situ hybridization (FISH) were not eligible. Children with Down syndrome, congenital BM failure disorders (eg, Fanconi anemia, severe congenital neutropenia, and Shwachman syndrome), prior acquired aplastic anemia, previous chemotherapy or radiotherapy, or a preceding documented MDS phase (BM blasts < 20%) for more than 3 months were excluded.
A predefined set of data were collected for each case and sent to the EWOG-MDS Coordinating Study Center in Freiburg, Germany. Data were checked for consistency and completeness. The patients were classified according to the pediatric WHO classification system.21 The reported karyotypes were reviewed and classified according to the International System for Human Cytogenetic Nomenclature.22 Data on patient outcome were locked on August 1, 2004.
Data on 258 eligible patients were submitted from the following study groups (number of patients in parenthesis): Belarus (5); BFM: Austria (6), Germany (19); CCG (47); CPH (2); DCOG (5); EORTC (18); EWOG-MDS: Austria (2), Germany (15), Italy (12), Netherlands (3), Nordic countries (6); GATLA (7); Greece (5); Hong Kong (3); Israel (5); Japan (13); LAME (8); MRC (24); NOPHO (8); Poland (5); POG (25); Spain (1); and St Jude Children's Research Hospital (14).
Patients were treated according to the regional clinical AML trials. The treatment protocols were approved according to local guidelines including informed consent per the Declaration of Helsinki. Patients received anthracycline- and cytarabine-based induction courses and consolidation that included high-dose cytarabine. A few patients received autologous HSCT; these patients were included in the “chemotherapy only” group.23,24 Although HSCT was offered to most patients with a suitable family donor, the number of patients with an HLA-matched family donor who did not undergo HSCT is not known. Some patients were offered unrelated donor HSCT in first complete remission (CR) at the discretion of the treating physician.
Categories of chromosome 7 abnormalities
Patients were assigned to 1 of 4 cytogenetic subgroups according to their karyotype: monosomy 7 alone (−7), monosomy 7 with other cytogenetic aberrations (−7 other), deletion 7q alone \del(7q)], and deletion 7q with other cytogenetic aberrations \del(7q) other]. Favorable cytogenetic aberrations were defined as t(8;21)(q22;q22), t(15;17)(q22;q21), inv(16)(p13q22), and t(9;11)(p22;q23).20,25,26 Patients with additional nonrandom cytogenetic abnormalities were coded according to the additional abnormalities. Only one code per patient was entered. If more than one nonrandom abnormality was observed, then the first abnormality from the following list was coded: inv(16), t(9;11), t(8;21), t(15;17), inv(3)/t(3;3), der(11q), −5/del(5q), del(12p), trisomy 8(+ 8), trisomy 21 (+ 21).
Statistical analyses
The main end point of the outcome analysis was overall survival (OS) measured from the date of diagnosis to the date of death or last follow-up. Event-free survival (EFS) was calculated from the date of diagnosis to first event (relapse or death) or last follow-up whichever occurred first. For OS and EFS analyses patients who did not experience an event were censored at the time of last follow-up. Patients who did not achieve CR were considered as treatment failure at day 0. Six patients received no AML therapy; 1 had no follow-up and was censored at day 0, 2 died before therapy could be initiated (day 1 and day 2 from diagnosis), and 3 were given HSCT without receiving any chemotherapy before conditioning. Actuarial survival curves were constructed using the Kaplan-Meier method to estimate probabilities of OS and EFS. All results are expressed as 5-year probability with the 95% confidence interval (95% CI). The 2-sided log-rank test was used to determine the equality of the survivorship functions in different subgroups.27
Univariate analyses of EFS and OS were performed considering potential risk factors and patient-related variables such as age at diagnosis, sex, and year of diagnosis. For multivariate analysis the Cox proportional hazard regression model was used, including all variables with a P value less than .1 in the univariate analysis.28 HSCT was included as a time-dependent covariate in the model. Continuous variables were divided into quartile categories. Adjacent categories with comparable relative event rates were grouped if needed.
A chi-square test was used to compare differences in percentages for groups. Because normal distribution cannot be assumed, median values and ranges were reported, and nonparametric statistics were used to test differences in continuous variables (Kruskal-Wallis rank test with adjacent post hoc Mann-Whitney U test).29,30 All P values were obtained by performing 2-sided tests and values less than .05 were considered statistically significant. Statistical analyses were performed using SPSS for Windows 11.0 (SPSS Science, Chicago, IL).
Results
Clinical characteristics
Monosomy 7 alone was observed in 90 (35%) patients, −7 other in 82 (32%), del(7q) in 21 (8%), and del(7q) other in 65 (25%) (Table 1). There was a trend toward more males in the group with −7 (63%), and del(7q) other (60%) compared with an even sex distribution in −7 other and del(7q) (P = .15). The median age at diagnosis was 7.7 years (range, birth-18.1 years) without any difference between the cytogenetic subgroups.
Refractory anemia with excess blasts in transformation (RAEB-T) was found in 24 patients; all of them had either −7 or −7 other (Table 1). The 2 patients with AML M3 both had del(7q) with t(15;17)(q22;q21). Hepatomegaly was noted more often in patients with −7 ± other than in those with del(7q) ± other (P < .05; Table 1), whereas the presence of splenomegaly did not correlate with karyotype.
The median white blood cell (WBC) count at diagnosis was 12.8 × 109/L (range, 1-261 × 109/L). The percentage of blasts in PB and BM at diagnosis was lower in −7 and −7 other than in del(7q) and del(7q) other (P < .01 for both PB and BM; Table 1). The lower blast counts in−7 ± other were still significant after exclusion of RAEB-T.
The median time of follow-up for all patients alive was 53 months (range, 0-196 months).
Cytogenetic characteristics
Thirty-nine (48%) of the patients with −7 other had 2 or more additional aberrations. Unidentified marker chromosomes were noted in 25 (30%) of those with −7 other. Nonrandom aberrations in addition to −7 or del(7q) were found in 51 (62%) of the patients with −7 other and in 46 (71%) of those with del(7q) ± other (Table 2). Two additional nonrandom aberrations were seen in 12 of the 97 (12%) patients with nonrandom aberrations; + 8 was noted in 7 of those cases. The most frequent combination was + 8 and + 21, which was seen in 5 patients. Unrelated clones were observed in only 1 patient. Favorable aberrations \t(8;21), inv(16), t(15;17), and t(9;11)] were observed in 21 patients with del(7q). Only 3 patients with −7 had favorable aberrations, and all of those had t(8;21) (Table 2). In contrast inv(3)/t(3;3), t(9;22), i(17q), and + 21 were mainly associated with −7 (Table 2). Ten patients had abnormalities involving the long arm of chromosome 11 \der(11q)]; the breakpoint included 11q23 in 6 patients but was not specified in the remaining 4 patients. MLL involvement was not routinely tested.
Chromosome abnormalities and survival
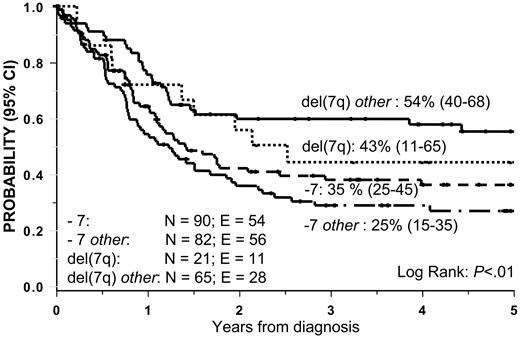
The 5-year OS and EFS rate of patients with del(7q) ± other was superior compared with that of patients with −7 ± other: OS, 51% versus 30%, P < .01 (Figure 1) and EFS, 45% versus 23%, P < .01 (Figure 2).
Overall survival in 258 pediatric patients according to cytogenetic subgroup.
Event-free-survival in 252 patients according to cytogenetic subgroup. The 6 patients not receiving AML chemotherapy were excluded from this analysis.
Event-free-survival in 252 patients according to cytogenetic subgroup. The 6 patients not receiving AML chemotherapy were excluded from this analysis.
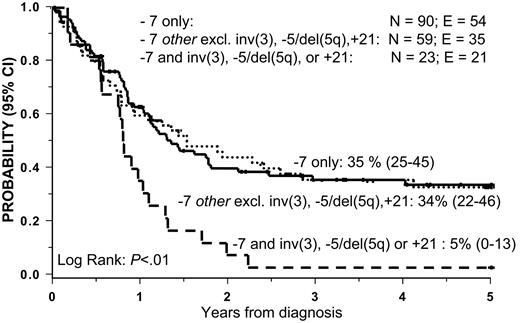
The analysis of survival among patients with −7 other identified 3 cytogenetic subgroups with a particularly poor prognosis: inv(3) (n = 7), −5/del(5q) (n = 8), and + 21 (n = 8). The 5-year OS of these groups was only 5% (Figure 3). The combination of −7, + 8, and + 21 was observed in 5 patients (not included in the + 21 subgroup), 3 of whom are long-term survivors. Three patients had −7 and t(8;21): 2 received HSCT and 1 received chemotherapy only; one died following transplantation and the remaining 2 patients are long-term survivors. The number of patients with t(9;22) and i(17q) was too few for meaningful survival analyses.
Overall survival (OS) according to −7 subgroups. Patients with −7 and inv(3), del(5q)/−5, or +21 had an OS of 5%. OS in the remaining group of patients with −7 other (excluding inv(3), del(5q)/−5, +21) was 34% and in the patients with −7 only OS was 34%.
Overall survival (OS) according to −7 subgroups. Patients with −7 and inv(3), del(5q)/−5, or +21 had an OS of 5%. OS in the remaining group of patients with −7 other (excluding inv(3), del(5q)/−5, +21) was 34% and in the patients with −7 only OS was 34%.
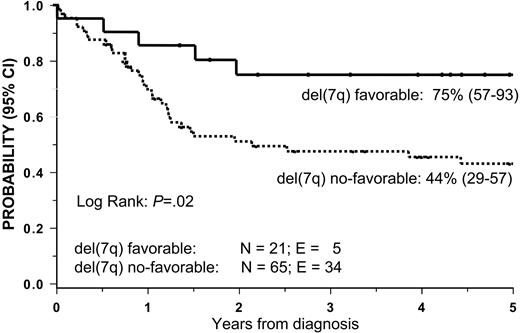
Patients with del(7q) and favorable cytogenetics (n = 21) had a 5-year OS rate of 75% compared with 44% in other patients with del(7q) (P = .02; Figure 4). OS was similar in patients with del(7q) only or with del(7q) other when those with favorable cytogenetics were excluded. Patients with a complex karyotype (3 or more aberrations) did not as a group show impaired survival (data not shown).
Overall survival (OS) according to del(7q) subgroups. Patients with del(7q) and favorable cytogenetics (t(8;21), inv(16), t(15;17), and t(9;11)) had an OS of 75%. Those with del(7q) without favorable cytogenetics had an OS of 44%.
Overall survival (OS) according to del(7q) subgroups. Patients with del(7q) and favorable cytogenetics (t(8;21), inv(16), t(15;17), and t(9;11)) had an OS of 75%. Those with del(7q) without favorable cytogenetics had an OS of 44%.
The combined data from Figures 3 and 4 demonstrate significant differences in the outcome of the various cytogenetic subgroups. Patients with del(7q) with favorable aberration showed the greatest OS (75%), followed by patients with del(7q) without favorable aberrations (44%), −7 excluding inv(3), del(5q), and + 21 (35%), and, finally, those with −7 and inv(3), del(5q), or + 21 showed the poorest survival (5%). However, we found no significant difference in survival between cytogenetic groups with del(7q) without favorable aberrations and those with −7 excluding inv(3), del(5q), and + 21.
The differences in EFS and OS among the cytogenetic subgroups was not only due to different CR rates after AML therapy; the CR rate was 100% in patients with del(7q) favorable and 86% in del(7q) no favorable (not significant, P = .10). The CR rate was 62% in −7 excluding inv(3), −5/5q−, + 21, and 55% in −7 with inv(3), −5/del(5q), or + 21 (not significant). Analyzing only patients who achieved CR the OS was 79% for patients with del(7q) favorable versus 52% in del(7q) no favorable (P = .05).
Treatment and survival
AML induction chemotherapy with cytarabine and anthracycline was given to 252 (98%) patients. CR was achieved in 61% of the patients with −7 ± other and in 89% of those with del(7q) ± other (P < .001). HSCT was performed in 97 (38%) patients who were in first CR (n = 77), whose induction chemotherapy failed (n = 17), or who had not received any chemotherapy (n = 3). HSCT analyzed as a time-dependent covariate had no significant impact on survival among patients who had entered CR.
Seventeen (23%) of the 75 patients who did not enter CR underwent HSCT, which resulted in a 3-year OS of 31%. Six patients are alive with a median follow-up of 36 months from time of HSCT. All 58 patients whose induction therapy failed and who did not receive HSCT died within 17 months (median survival, 5 months).
Multivariate analysis
On the basis of our initial analyses, patients were regrouped into 4 cytogenetic subgroups: del(7q) favorable \inv(16), t(9;11), t(8;21), or t(15;17)], del(7q) no favorable, −7 excluding inv(3), −5/5q−, + 21, and −7 with inv(3), −5/del(5q), or + 21. The univariate analysis identified karyotype and age at diagnoses as factors that significantly influenced the probability of survival (Table 3). The multivariate Cox analysis included the following variables: sex, age at diagnosis, karyotype, WBC count, year of diagnosis, and HSCT as a time-dependent covariate. The strongest prognostic factor was the cytogenetic aberration. Patients with del(7q) favorable showed a better outcome than patients with del(7q) no favorable and −7 ± other (P = .001; Table 4). Other variables associated with superior survival were age younger than 6 years at diagnosis (P = .01) and WBC count less than 20 × 109/L at diagnosis (P = .02). A tendency for a better outcome was observed in patients whose disease was diagnosed during the most recent years of the study (P = .06; Table 4).
Discussion
The present study confirms our previous observation that among children with AML and −7/del(7q) one third has −7 alone, one third has −7 with other aberrations, and one third has del(7q), mostly associated with other cytogenetic abnormalities.14 Males dominated with an overall male-to-female ratio of 1.3. The male predominance was found only in the −7 and del(7q) other subgroups (not significant). Male sex has been associated with −7 as the sole cytogenetic abnormality,14 in contrast to a predominance of girls among those with del(7q).14 The median age at diagnosis of 7.7 years is comparable with that seen in large childhood AML series. Patients with −7/del(7q) lack the incidence peak at 0 to 3 years of age seen in several other cytogenetic subsets of AML.31
The median percentage of blast cells in both PB and BM at diagnosis was lower in −7 ± other than in del(7q) ± other, also after the exclusion of RAEB-T. Although the WHO classification recommends that AML be redefined by a BM blast count of 20% or more,32 there are no pediatric data to support this reclassification, and maintenance of the RAEB-T category is currently recommended.21 The patients with RAEB-T in the present study all showed −7 ± other abnormalities. Multivariate analysis did not show any difference in survival between the RAEB-T and the AML groups.
Complete loss of chromosome 7 as the sole abnormality is the prevailing finding in refractory cytopenia, RAEB, and JMML, whereas patients with RAEB-T often have more complex abnormalities but very infrequently show del(7q).14 Monosomy 7 as the sole abnormality represents 75% of the chromosome 7 abnormalities in MDS but only 35% of the AML cases.14 In contrast, del(7q) represents only 11% of chromosome 7 abnormalities in MDS, as compared with 33% in AML.14 Additional cytogenetic abnormalities are of major prognostic importance, and both −7 and del(7q) may be considered secondary abnormalities with a prognosis depending on the primary cytogenetic change.33 AML with −7 ± other has some similarities with MDS, including low blast count in PB and BM, and poor response to induction chemotherapy. This finding suggests that AML with −7 shares some pathogenic features with MDS rather than with true de novo AML.
Advances in the treatment of childhood AML during the past 2 decades have significantly increased the expectancy of survival.34 Cytogenetic characteristics are now a major factor used to predict outcome. Patients with −7 and those with del(7q) have often been considered together as one cytogenetic subgroup with a poor outcome. However, the present study demonstrates a great degree of heterogeneity among patients with chromosome 7 abnormalities. We found an OS disadvantage in patients with −7 compared with those who had del(7q), but within both groups, additional aberrations had a substantial impact on survival. The CR rate was significantly higher in patients with del(7q) compared with patients with −7 also after exclusion of the favorable and unfavorable cytogenetic aberrations. However, patients with del(7q) without favorable cytogenetics and patients with −7 without inv(3), 5q−, or + 21 who achieved CR had similar survival.
Unlike in adults35 a complex karyotype (3 or more aberrations) in pediatric patients had no prognostic significance per se; however, the outcome was strongly associated with the type of additional aberrations.
Outcome was particularly poor in patients with −7 and inv(3)(q21q26). Monosomy 7 is found in approximately 30% of adults with t(3;3) or inv(3)(q21q26). The increased expression of the EVI-1 gene located at chromosome 3q26 is associated with poor survival.36–38 Of interest, we found that an additional chromosome 21 gain was associated with poor survival, although the few patients who had both + 8 and + 21 did not appear to have a poor outcome. A poor outcome has also been noted in adults with AML with −7 and + 21.39 We observed a considerable number of patients with −7 and a marker chromosome among the additional aberrations. Previous investigations have shown that marker chromosomes may contain chromosome 7 material,14,40,41 indicating that these patients may have only a partial loss of chromosome 7. The karyotypes including marker chromosomes were not systematically analyzed in the present study.
Patients with favorable cytogenetics and del(7q) had a superior outcome. The presence of favorable cytogenetics appears to be the main determinant of outcome irrespective of additional aberrations.20 As previously reported,42 favorable cytogenetic aberrations are rarely associated with −7 \only 3 (4%) of 82 patients with −7 other in the present study also had t(8;21)]. The number of patients is too low to make any conclusions about the outcome for the unusual association of −7 with aberrations considered favorable in AML. The prognosis of patients with del(7q) favorable is dictated by the favorable aberrations, and del(7q) may be considered as a secondary abnormality, the same may be true for −7 with favorable cytogenetics, although the data for this group are very limited. The WHO classification considers each of the favorable cytogenetic aberrations as separate diagnostic groups without specifying whether patients with additional cytogenetic aberrations should be included.32 The present study together with data from the UK group20 suggest that AML with inv(16), t(8;21), and t(15;17) are diagnostic entities with favorable outcomes regardless of the presence of additional aberrations. The outcome for patients with −7 was mainly determined by the additional cytogenetic aberrations. Of interest, monosomy 7 also loses its predictive value in childhood acute lymphoblastic leukemia (ALL) when patients with Philadelphia chromosome–positive disease are excluded from the analyses.43
About 50% of children with MDS treated with intensive chemotherapy enter CR, but only 15% to 30% remain in CR.15,44–47 The present study showed a similar CR rate of 60% in −7 ± other. Among those who entered CR the OS rate was 43%. Those who received HSCT showed no significant survival advantage, when HSCT was analyzed as a time-dependent covariate. Patients who did not enter CR but had residual disease when they received an HSCT had a long-term probability of OS of 31%. Although not the result of a direct comparison, all 58 patients who did not enter CR and who did not receive HSCT died. The data suggest that HSCT is a reasonable treatment option in nonresponding patients; however, we found no evidence for recommending HSCT in patients without preceding MDS who achieved CR on chemotherapy.
Compared with patients with del(7q) ± other, those affected by myeloid malignancies with −7 ± other had a lower percentage of PB and BM blast cells at diagnosis and only very infrequently had favorable cytogenetic aberrations. Patients with −7 ± other experienced a lower likelihood of CR (61% versus 89%) and a lower OS (30% versus 51%) compared with those who had del(7q). Patients with −7 and 3q or 5q aberrations or + 21 had a very poor outcome. However, patients with del(7q) associated with inv(16), t(9;11), t(8;21), or t(15;17) had an OS of 75%. We conclude that patients with −7 and those with del(7q) should not be considered as one cytogenetic subgroup. Additional cytogenetic aberrations greatly affect the prognosis of patients in each subgroup.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Angela McArthur for editorial assistance.
This work was supported by the International Pediatric AML Group of the I-BFM-SG and the European Working Group on Childhood myelodysplastic syndrome (EWOG-MDS).
Authorship
Contribution: H.H. conveyed and planned the study, analyzed the data, and wrote the paper; H.H., E.F., and S.C.R. reviewed the karyotypes; P.N. performed the statistical analyses; and C.M.N. provided funding. All authors participated in data collection as well as critical review and final approval of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of childhood leukemia groups and centers that submitted patient data to the study appears as Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Henrik Hasle, Department of Pediatrics, Aarhus University Hospital Skejby, Denmark; e-mail: hasle@dadlnet.dk.