Abstract
We explored the physiologic role of endothelial cell apoptosis during development by generating mouse embryos lacking the inhibitor of apoptosis protein (IAP) survivin in endothelium. This was accomplished by intercrossing survivinlox/lox mice with mice expressing cre recombinase under the control of the endothelial cell specific tie1 promoter (tie1-cre mice). Lack of endothelial cell survivin resulted in embryonic lethality. Mutant embryos had prominent and diffuse hemorrhages from embryonic day 9.5 (E9.5) and died before E13.5. Heart development was strikingly abnormal. Survivin-null endocardial lineage cells could not support normal epithelial-mesenchymal transformation (EMT), resulting in hypoplastic endocardial cushions and in utero heart failure. In addition, 30% of mutant embryos had neural tube closure defects (NTDs) that were not caused by bleeding or growth retardation, but were likely due to alterations in the release of soluble factors from endothelial cells that otherwise support neural stem cell proliferation and neurulation. Thus, regulation of endothelial cell survival, and maintenance of vascular integrity by survivin are crucial for normal embryonic angiogenesis, cardiogenesis, and neurogenesis.
Introduction
Embryonic development depends on the establishment of a complex network of blood vessels to meet the functional demands of each organ system.1,2 During vasculo/angiogenesis, factors that regulate endothelial survival are balanced to provide resistance to exogenous stresses, to facilitate vascular regression during vessel remodeling, and to promote endothelial proliferation and migration during sprouting. Prominent among these factors is vascular endothelial cell growth factor (VEGF). VEGF stimulates angiogenesis by promoting endothelial growth, migration, and survival via interactions with VEGF receptors, PI3 kinase (PI3K), beta-catenin, and VE-cadherin, which in turn leads to activation of Akt and up-regulation of antiapoptotic proteins such as nitric oxide, Bcl-2, Bcl-XL, XIAP, and survivin.3 Withdrawal of VEGF results in vessel regression, and inactivation of a single VEGF allele causes profound defects with endothelial apoptosis.4
Other factors that modulate endothelial survival include angiopoietin-1 and -2, which promote survival and apoptosis, respectively.5,6 Inhibitors of angiogenesis, such as endostatin,7 interleukin-12,8 and cyclo-oxygenase-2 inhibitors9 induce apoptosis via suppression of Bcl-2 and Bcl-XL. Remarkably, mice in which Bcl-2, Bcl-XL, or XIAP have been inactivated10–12 do not exhibit obvious angiogenic defects, and thus the in vivo significance of these pathways in angiogenesis is raised. Indeed, the physiologic relevance of endothelial apoptosis is poorly documented due to lack of genetic models in which regulators of apoptosis have specifically been inactivated in the endothelium. Delineating the role of endothelial apoptosis, however, may lead to elucidation of clinically relevant endothelial-derived signal pathways. Furthermore, studies indicating that organogenesis is regulated by endothelial factors13–15 additionally underscores the importance of better characterizing functional properties of the endothelium during and after fetal development.
Survivin is an inhibitor of apoptosis protein (IAP) and a regulator of mitosis that is highly expressed in proliferating cells, but which is at low levels in quiescent cells.16,17 Its importance in endothelial biology is supported by several lines of evidence. Survivin is up-regulated in cultured endothelial cells by VEGF, placental growth factor (PlGF), angiopoietin-1, and basic fibroblast growth factor (bFGF).5,18–20 Vascular tube formation in vitro is dependent on the induction of survivin,19,21 and in vivo survivin regulates vein graft hyperplasia.22 Resistance to vascular injury and chemotherapy may be mediated by increased endothelial survivin,23,24 and new vessel formation is accompanied by increased endothelial survivin.19,25 Finally, endothelial survivin is a target to suppress tumor angiogenesis26,27 (reviewed in Altieri28 ).
In spite of these advances, elucidation of the in vivo role of survivin in angiogenesis has been confounded by the fact that inactivation of survivin in mice causes embryonic lethality before embryonic day 4.5 (E4.5).29,30 To delineate the function of endothelial survivin, we used the cre-lox system in mice to inactivate the survivin gene in endothelial cells. The results highlight the importance of survivin in maintaining vascular integrity during development, and provide novel insights into the close relationship that exists between angiogenesis, cardiogenesis, and neurogenesis.
Materials and methods
Generation of mice lacking endothelial cell survivin
Mice in which the survivin gene is flanked by loxP sites (survivinlox/lox mice) were previously generated.31 Mice expressing cre recombinase under the control of the tie2 promoter (tie2-cre mice) were provided by Prof M. Yanagisawa (Howard Hughes Medical Institute, Dallas, TX), and cre excision was confirmed to be endothelial specific using a lacZ reporter mouse line.32 Mice expressing cre under the control of the endothelial-specific tie1 promoter33 (tie1-cre mice) were provided by Dr E. Gustafsson (Lund University, Sweden). Using ROSA26R reporter mice, functional cre expression, driven by the tie1 promoter, is fully restricted to the embryonic vasculature from E8 to E14.5. No nonendothelial cre is detectable between E8 and E14.5. To inactivate endothelial survivin, tie1-cre or tie2-cre mice were first crossed with survivinlox/lox mice. A breeding strategy was established in which 25% of offspring were expected to carry the tie1-cre or tie2-cre transgene with both survivin gene alleles floxed. Experiments were performed with approval from the University of Leuven ethics committee.
Mouse and embryo genotyping
Genotyping to determine whether the floxed survivin gene was cre excised was performed by polymerase chain reaction (PCR) on tail DNA as reported.31,32 The tie1-cre and tie2-cre transgenes were detected by PCR using primers cre403 (antisense 5′-GATGCCGGTGAACGTGCAAAACAGGCTC-3′) and cre850 (sense 5′-CGCCGTAAATCAATCGATGAGTTGCTTC-3′), which generates a 450-bp amplicon.
Isolation of embryos and immunohistologic staining
Embryos were removed from pregnant females at specified times and examined. Live embryos were defined as having discernible embryonic structures with recognizable organ features, a regularly beating heart, and microscopically visible circulating blood. Embryos were fixed in 2% paraformaldehyde or Z-fix (Anatech, Hayward, CA), dehydrated, and embedded in paraffin for histologic analyses. Rabbit anti–rat thrombomodulin antibodies were a gift from Dr R. W. Jackman (Boston University, MA), and anti-Prox1 antibodies were prepared in-house. Rabbit anti-cre antibodies were provided by Prof G. Schütz (Heidelberg, Germany).
Detection of apoptosis and hypoxia
Apoptosis was detected by TUNEL staining using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA). Hypoxic regions in the embryos were detected with the hypoxyprobeTM-1 kit (Chemicon). Pimonidazole (60 mg/kg of body weight) was injected into the tail vein of pregnant females 2 hours prior to killing, after which the embryos were dissected and paraffin sections were prepared for immunostaining.
Microscopy
For confocal microscopy, 100-μm thick cryosections of fixed embryos were prepared. Colocalization of endothelial and smooth-muscle cells was achieved by staining sections with rat anti–murine PECAM (CD31) mAb (1:10 dilution; BD Biosciences, Palo Alto, CA) and rabbit anti-NG2 antibodies (1:200 dilution; Chemicon). Different fluorescence-tagged secondary antibodies were used for specific detection. Z-stacks of confocal images were collected at 5-μm intervals and presented as maximum intensity projections. To colocalize survivin with thrombomodulin (TM), rabbit anti–murine survivin antibodies34 (R51, 1:200 dilution; gift from Dr G. Shaw, University of Florida, Gainesville) and goat anti–murine TM antibodies (1:200, gift from Dr N. Esmon, University of Oklahoma, Norman) were used. For transmission electron microscopy (TEM), embryos were fixed, embedded, and stained for visualization using an electron microscope (model JEM-1200EX; Jeol, Peabody, MA) as described.35
Histologic sections were visualized with a Zeiss Axioplan 2 imaging microscope (Carl Zeiss, Thornwood, NJ). The microscope was fitted with Zeiss Plan-Neofluor objective lenses: 2.5×/0.07 NA, 5×/0.15 NA, 10×/0.30 NA, and 40×/0.75 NA. Confocal images were obtained with the Zeiss CLS M510 confocal laser scanning microscope installed on a Zeiss AxioVert 100M microscope. To visualize whole embryos, the Zeiss Fluorosteromicroscope V. 12, with 0.8× and 1.5× lenses, was used. All images were captured using a Zeiss Axiocam MRC5 camera and digitized using Zeiss AxioVision 4.6 computer imaging software.
Whole-mount staining for CD31
Whole-mount immunostaining of embryos with rat anti–mouse CD31 antibody was performed as reported,36 with visualization accomplished with BCIP/NBT Color Development Substrate (Promega, Madison, WI).
Endothelial cell cultures
Primary neonatal endothelial cells from the hearts of mice were generated as described37 and cultured at 37°C, 5% CO2 in DMEM containing 1% l-glutamine, 1% Na-pyruvate, 0.04% heparin, 20% fetal calf serum (FCS), and 3 mg/100 mL Endothelial Cell Mitogen (Biomedical Technologies, Stoughton, MA). Endothelial cells used at passages 3 to 7, were more than 95% pure based on expression of TM and CD31. Polyoma middle T antigen–transformed murine endothelial cells (fEND5s) were previously characterized,38 and cultured in DMEM containing 10% FCS.
RNA isolation and real-time PCR
TaqMan quantitative real-time PCR (RT-PCR) was performed as reported.25 Total RNA was isolated in TRIzol reagent (Life Technologies, Merelbeke, Belgium) according to the manufacturer's instructions. Standard curves for RT-PCR were generated from plasmids containing cDNA encoding murine survivin or VEGF. Gene expression levels, in triplicate for each sample, were calculated as the number of mRNA copies relative to the number of mRNA copies of the hprt gene (hypoxantine-guanine-phosphoribosyltransferase—using primers HPRTF (sense 5′ ttatcagactgaagagctactgtaatgatc) and HPRTR (antisense 5′ ttaccagtgtcaattatatcttcaacaatc) and probe (5′-(JOE)-tgagagatcatctccaccaataacttttatgtccc-(TAMRA)-3′) in the same sample.
Isolation and characterization of neurospheres
Neurospheres of neural stem cells (NSCs) were isolated from the subventricular zone of brains of wild-type mice, cultured in Neurobasal medium (Invitrogen, Merelbeek, Belgium) containing 1 × B27 supplement (Gibco, Gent, Belgium), 20 ng/mL EGF, 20 ng/mL bFGF (R&D Systems, Abingdon, United Kingdom), 2 μg/mL heparin, and 2 mM glutamine, and used at passages 4 to 8.39 When in culture as free-floating neurospheres, NSCs were characterized by their ability to self-renew without differentiating when more than 95% of the cells expressed nestin and RC2 without detectable expression of GFAP, β-tubulin-III, or NeuN. When plated in adhesive wells, multipotent NSCs gave rise to mature NeuN+, β-tubulin-III+ neurons, and GFAP+ astroglia cells.
Coculture of NSCs with endothelial cells
Endothelial cells from survivin+/+ and survivin+/− mice30 were seeded in collagen-coated 6-well transwells (0.4-μm pore size; Costar, Corning, NY), 100 000 cells per well, and cultured in Neurobasal medium supplemented with 20 ng/mL bFGF, 1 × B27 supplement, and 2 mM glutamine. After 4 hours, neurospheres from 6-week old wild-type mice were seeded in the lower chambers of the transwell dishes coated with poly-l-ornithine and laminin (Sigma, St Louis, MO), as reported.40 After 7 days, prior to NSC differentiation,40 the transwells with endothelial cells were removed and adherent neural cells were fixed in 4% paraformaldehyde and analyzed by immunofluorescence microscopy for GFAP, β-tubulin-III, RC2, or nestin. Cells were counted in 14 microscopic fields per well, and the average was calculated from a minimum of 3 wells each. Alternatively, neural cells were suspended and fixed, and GFAP, RC2, and nestin positivity was quantified by fluorescence-activated cell sorting (FACS).
Coculture of embryos with conditioned media
Murine microvascular endothelial cells (fEND5s) or murine embryonic fibroblasts were cultured until preconfluent. After 48 hours, conditioned media were removed and frozen for later use in cocultures. Wild-type murine E8.5 embryos were cultured for 24 hours as described41 but in conditioned media, after which embryos were scored for neural tube closure by a trained blinded observer. A scoring system, based on the stages of closure as described by MacDonald et al,42 was established: a score of 0 indicates no fusion of the neural folds; a score of 1, contact of neural folds at the cervical region (Closure 1); a score of 2, cervical region closed and extending bidirectionally, and the anterior neural folds in contact at the region of the posterior prosencephalon (Closure 2); a score of 3, fusion present at the most anterior part of the prosenchephalon, extending caudally toward Closure 2; a score of 4, fusion of the most anterior neural folds complete, and the rhombencephalon region still open; and a score of 5, complete fusion of the rhombencephalon region (Closure 4) (Figure S6, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Statistical methods
Statistical analyses were conducted with the computer program InStat 3.0 (GraphPad Software, San Diego, CA). Means are provided with SD unless otherwise noted. P values were determined using the unpaired t test, and groupwise comparisons were determined by Wilcox-ranked sum testing.
Results
Disruption of the survivin gene in endothelial cells and embryonic lethality
Since endothelial survivin expression during prenatal development has not been well documented, we first immunostained histologic sections of wild-type E9.5 to E12.5 murine embryos with antisurvivin antibodies. These confirmed that survivin is widely expressed in many tissues, and that it also colocalizes with the endothelial-specific receptor thrombomodulin throughout the vasculature (Figure S1). To study the physiologic role of endothelial survivin, we inactivated the survivin gene in endothelial cells by cross-breeding mice in which both alleles of the survivin gene are flanked by loxP sites (survivinlox/lox)31 with transgenic mice expressing cre recombinase under the control of the endothelial specific tie1 promoter (tie1-cre mice33 ). tie1-cre mice have similarly been used to examine the function of endothelial PDGF-B.43 Using ROSA26R mice, tie1-cre expression was confirmed to be entirely restricted to the endothelium from E8 to E14.5.33 Minimal ectopic expression of cre was only evident in adult mice. We confirmed that cre is restricted to the endothelium during early embryonic development by immunostaining histologic sections of E10.5 tie1-cre/survivinlox/wt embryos with anti-cre antibodies (Figure S2). Cre staining was absent in all nonendothelial cells, including the neuroepithelium, while it was readily detectable throughout the vasculature.
The offspring from interbreeding tie1-cre mice with survivinlox/lox mice were genotyped by PCR (Figure S3). Tie1-cre/survivinlox/wt mice appeared healthy under nonstress conditions. Cross-breeding tie1-cre/survivinlox/wt and survivinlox/lox mice resulted in 112 healthy offspring; 28% tie1-cre/survivinlox/wt, 38% survivinlox/wt, and 34% survivinlox/lox. No viable tie1-cre/survivinlox/lox mice were detected, indicating that disruption of the survivin gene in endothelial cells results in embryonic lethality.
Bleeding in tie1-cre/survivinlox/lox embryos
From E9.5 to E12.5, we found live embryos of all expected genotypes in a normal Mendelian distribution (Table 1). At E13.5, tie1-cre/survivinlox/lox embryos were underrepresented, and when present, were resorbing or had unrecognizable organ features.
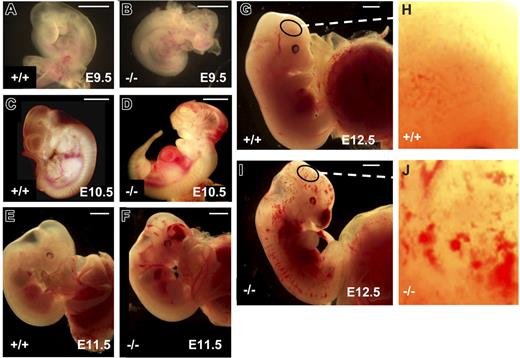
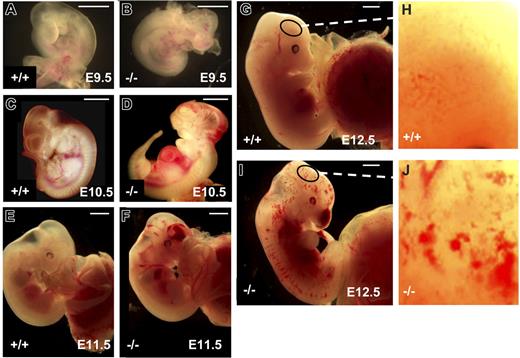
E9.5 tie1-cre/survivinlox/lox embryos were normal in size, without evidence of anemia, but 25% to 30% had prominent, dilated vessels and numerous hemorrhages on the head, neck, back, and around the heart (Figure 1A-B). At E10.5, tie1-cre/survivinlox/lox embryos were normal in size, but 50% to 70% (n > 15) exhibited severe bleeding (Figure 1C-D). The incidence and severity of the hemorrhages increased during development, and at E12.5, more than 95% of the tie1-cre/survivinlox/lox embryos suffered serious bleeding, and a few of these were smaller than the normal embryos, suggesting growth retardation.
Hemorrhages in embryos lacking endothelial survivin. Survivinlox/lox (A,C,E,G) and tie1-cre/survivinlox/lox (B,D,F,I) embryos were examined at E9.5 to E12.5. Embryos lacking endothelial cell survivin (B,D,F,I) exhibit evidence of vasodilatation and diffuse bleeding in the head, trunk, and intersomitic regions, and around the heart. Scale bars equal 1.0 mm. (H,J) High-power view (× 10) of the surface of the head of the embryos in panels G and I, respectively, reveals the extensive microvascular bleeding in the tie1-cre/survivinlox/lox embryos.
Hemorrhages in embryos lacking endothelial survivin. Survivinlox/lox (A,C,E,G) and tie1-cre/survivinlox/lox (B,D,F,I) embryos were examined at E9.5 to E12.5. Embryos lacking endothelial cell survivin (B,D,F,I) exhibit evidence of vasodilatation and diffuse bleeding in the head, trunk, and intersomitic regions, and around the heart. Scale bars equal 1.0 mm. (H,J) High-power view (× 10) of the surface of the head of the embryos in panels G and I, respectively, reveals the extensive microvascular bleeding in the tie1-cre/survivinlox/lox embryos.
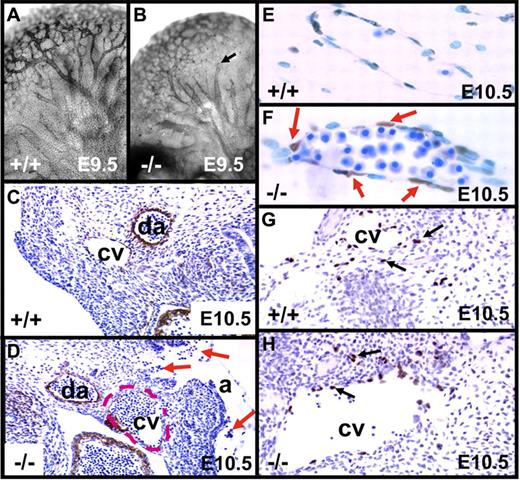
Whole-mount immunostaining of E9.5 embryos with the endothelial marker CD31 revealed normal vessel branching, with formation of complex vascular plexuses in tie1-cre/survivinlox/lox and survivinlox/lox embryos. However, CD31 staining in tie1-cre/survivinlox/lox embryos (n = 5 of 5), best appreciated in the brain, was notably weaker (Figure 2A-B), and the larger vessels were more dilated, often ending abruptly with a cut-off appearance. H&E-stained sections at E9.5 revealed hemorrhages only in the tie1-cre/survivinlox/lox embryos (n = 6 of 8), with nucleated erythrocytes in the amniotic space and extravascular tissue (not shown). The hearts of E9.5 tie1-cre/survivinlox/lox embryos appeared normal in size and shape, with blood in the pericardial space of more than 70% of the mutant embryos (see the next section). The anterior cardinal veins at E10.5 were dilated and often filled with blood, and the surrounding endothelial lining cells were discontinuous, resulting in extravascular blood accumulation (Figure 2C-D). In most embryos (n = 7 of 8), these vessels also exhibited poor staining with CD31 and anti-TM antibodies. Endothelial cell apoptosis in E10.5 tie1-cre/survivinlox/lox embryos was confirmed by TUNEL staining (Figure 2E-F), consistent with the diminished or absent expression of endothelial survivin in the mutant embryos (Figure S2). Smooth-muscle cell actin staining of vessels was not affected by loss of endothelial survivin (not shown). Lymphangiogenesis, first visualized at E8.5 to E9.0 with Prox1+ cells migrating from the cardinal vein, was intact in both genotype E10.5 embryos (Figure 2G-H).
Phenotypic changes in embryos lacking endothelial survivin. (A-B) E9.5 embryos were whole-mount stained for CD31 expression, revealing vascular patterning in head and brain. Arborization of vessels was largely intact in both genotypes, but in the tie1-cre/survivinlox/lox embryos (−/−), vessels were more dilated, often with a “cut-off” appearance (arrow). (C-H) Transverse histologic sections of E10.5 embryos. (C-D) Compared with survivinlox/lox embryos (+/+), those lacking endothelial survivin (−/−) exhibit notably less intense antithrombomodulin antibody (endothelial-specific) staining of the cardinal vein (cv), which had a discontinuous endothelial border, and was dilated (border shown with red dashed line), congested with blood, and “leaking” nucleated red blood cells into the amniotic space (arrows). da indicates dorsal aorta. (E-F) Higher-power view of transverse section of E10.5 embryos reveal that the cardinal vein endothelium of tie1-cre/survivinlox/lox embryos is strongly TUNEL positive. (G-H) Prox-1 staining reveals the presence of lymphatic endothelial cells (arrows) emanating from cardinal veins (cv) in both genotypes. Magnifications were the same for paired panels A-B, C-D, E-F, and G-H.
Phenotypic changes in embryos lacking endothelial survivin. (A-B) E9.5 embryos were whole-mount stained for CD31 expression, revealing vascular patterning in head and brain. Arborization of vessels was largely intact in both genotypes, but in the tie1-cre/survivinlox/lox embryos (−/−), vessels were more dilated, often with a “cut-off” appearance (arrow). (C-H) Transverse histologic sections of E10.5 embryos. (C-D) Compared with survivinlox/lox embryos (+/+), those lacking endothelial survivin (−/−) exhibit notably less intense antithrombomodulin antibody (endothelial-specific) staining of the cardinal vein (cv), which had a discontinuous endothelial border, and was dilated (border shown with red dashed line), congested with blood, and “leaking” nucleated red blood cells into the amniotic space (arrows). da indicates dorsal aorta. (E-F) Higher-power view of transverse section of E10.5 embryos reveal that the cardinal vein endothelium of tie1-cre/survivinlox/lox embryos is strongly TUNEL positive. (G-H) Prox-1 staining reveals the presence of lymphatic endothelial cells (arrows) emanating from cardinal veins (cv) in both genotypes. Magnifications were the same for paired panels A-B, C-D, E-F, and G-H.
Embryos lacking endothelial cell survivin have heart defects
Given the observed peripheral hemorrhage, a hallmark of embryonic heart failure,44 and the requirement of the endothelial lineage during endocardial cushion cell formation,45,46 we further assessed the hearts of E9.5 to E13 tie1-cre/survivinlox/lox, tie1-cre/survivinlox/wt, and survivinlox/lox embryos (n = 36 total; Figure 3). Histologic analysis revealed that all the hemorrhaging tie1-cre/survivinlox/lox embryonic hearts were strikingly abnormal (n = 12 of 12). In normal hearts, the endocardial cushions are mesenchymal swellings that form within the wall of the embryonic heart as the primordia of the future valves and septa.44,47 Initially, the atrioventricular (AV) endocardial mesenchymal cushions are formed from overlying endothelial cells undergoing epithelial-mesenchymal transformation (EMT) around E9 to E9.5 and colonizing the primitive acellular cushions. Subsequently, the outflow tract (OFT) mesenchymal cushions are formed from cells derived from both endocardial cells via EMT and cardiac neural crest cells that populate the OFT around E10 to E10.5.48
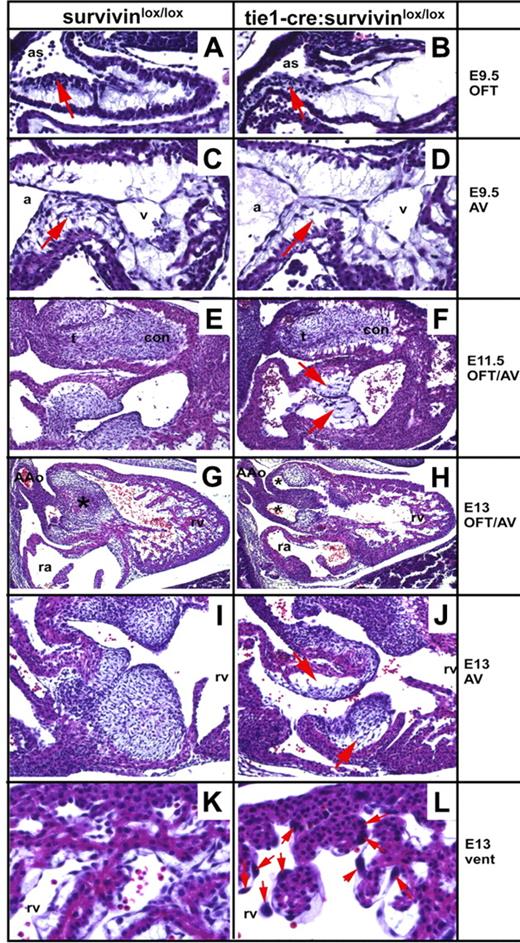
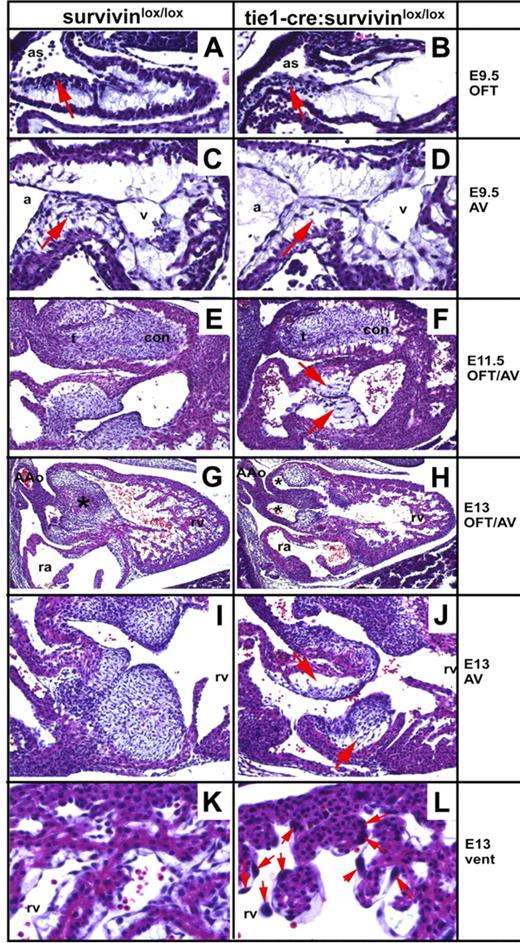
Hypoplastic endocardial cushions in embryos lacking endothelial survivin. Transverse histologic sections of survivinlox/lox (A,C,E,G,I,K) and tie1-cre/survivinlox/lox (B,D,F,H,J,L) embryos were examined at E9.5 to E13.0. Both wild-type and mutant E9.5 embryos exhibit the start of cardiac neural crest colonization of the OFT (arrows in A-B). Embryos lacking endothelial cell survivin exhibit reduced E9.5 AV EMT (arrow in panel D) that subsequently results in sparsely seeded, undersized AV cushions that are detached from the adjacent myocardium (F,J). Note the few enlarged endocardial cushion mesenchymal cells in hypoplastic E11.5 mutant AV cushions (arrows in panel F). Endocardial-EMT in the OFT is also affected and results in hypoplastic conal cushions (F,H). The E13 tie1-cre/survivinlox/lox OFT mesenchymal septum is undersized and misaligned, and exhibits double outflow right ventricle (DORV), as both the future pulmonary trunk and aorta exit the right ventricle (indicated by 2 stars in panel H), instead of the aorta exiting the left ventricle and the pulmonary trunk exiting the right ventricle (indicated by 1 star in panel G). The survivin-deficient endothelium overlying the E13 cardiomyocytes and endocardial cushions is abnormal, as there are fewer endothelial nuclei and many of the remaining nuclei are grossly enlarged (arrows in panel L). a indicates common atria; AAo, aortic arch; as, aortic sac; con, conal OFT cushions; t, truncal OFT cushions; ra, right atria, rv, right ventricle; and v, common ventricle.
Hypoplastic endocardial cushions in embryos lacking endothelial survivin. Transverse histologic sections of survivinlox/lox (A,C,E,G,I,K) and tie1-cre/survivinlox/lox (B,D,F,H,J,L) embryos were examined at E9.5 to E13.0. Both wild-type and mutant E9.5 embryos exhibit the start of cardiac neural crest colonization of the OFT (arrows in A-B). Embryos lacking endothelial cell survivin exhibit reduced E9.5 AV EMT (arrow in panel D) that subsequently results in sparsely seeded, undersized AV cushions that are detached from the adjacent myocardium (F,J). Note the few enlarged endocardial cushion mesenchymal cells in hypoplastic E11.5 mutant AV cushions (arrows in panel F). Endocardial-EMT in the OFT is also affected and results in hypoplastic conal cushions (F,H). The E13 tie1-cre/survivinlox/lox OFT mesenchymal septum is undersized and misaligned, and exhibits double outflow right ventricle (DORV), as both the future pulmonary trunk and aorta exit the right ventricle (indicated by 2 stars in panel H), instead of the aorta exiting the left ventricle and the pulmonary trunk exiting the right ventricle (indicated by 1 star in panel G). The survivin-deficient endothelium overlying the E13 cardiomyocytes and endocardial cushions is abnormal, as there are fewer endothelial nuclei and many of the remaining nuclei are grossly enlarged (arrows in panel L). a indicates common atria; AAo, aortic arch; as, aortic sac; con, conal OFT cushions; t, truncal OFT cushions; ra, right atria, rv, right ventricle; and v, common ventricle.
The E9.5 tie1-cre/survivinlox/lox hearts underwent correct looping, the myocardium was unaffected, and normal trabeculation occurred. Moreover, the cardiac neural crest was beginning to colonize the E9.5 tie1-cre/survivinlox/lox OFT (Figure 3B; arrow). However, initial AV EMT of the endocardial endothelium was reduced in mutant hearts (Figure 3D; arrow), and a few oversized mesenchymal endocardial cushion cells were present in the mutant AV cushions. At E11.5, both the tie1-cre/survivinlox/lox OFT and AV endocardial cushions were visibly reduced in size, detached from the adjacent myocardium, and more sparsely seeded (Figure 3F). The hypoplastic AV cushions were most affected and populated by fewer mesenchymal endocardial cushion cells, and these were clearly oversized when compared with the controls (Figure 3F; arrows). The hypoplastic tie1-cre/survivinlox/lox OFT cushions were sparsely populated within the more proximal conal region, but appeared relatively unaffected in the distal truncal cushion region. Variable hypoplastic AV cushions were also seen in the hearts of some tie1-cre/survivinlox/wt E11.5 and E13 embryos (n = 3 of 8; not shown).
At E13, the endothelium overlying the cardiomyocytes and endocardial cushions was abnormal, with fewer endothelial nuclei, many of which were grossly enlarged (Figure 3L; arrows). The cardiomyocyte morphology and arrangement in the mutant embryos appeared intact, although ventricular trabeculation was reduced (Figure 3G-H). The extracellular matrix (ECM) between the overlying endothelium and trabeculae was almost absent in the tie1-cre/survivinlox/lox embryos, and was otherwise detached from the adjacent cardiomyocytes in other places (Figure 3J). The E13 tie1-cre/survivinlox/lox embryo hearts had an OFT misalignment problem, as both the future pulmonary trunk and aorta exited the right ventricle (double-outflow right ventricle; Figure 3H, indicated by 2 asterisks), instead of the normal situation where the aorta and pulmonary trunk exit the left and right ventricle, respectively (Figure 3G; indicated by an asterisk). The right AV bulbar cushion tissue (Figure 3H) was reduced in the hearts of the tie1-cre/survivinlox/lox embryos. When comparing hearts from survivinlox/lox (Figure 3G,I) and tie1-cre/survivinlox/lox (Figure 3H,J) embryos, both the OFT and AV endocardial cushions in the mutant embryos were reduced in size—particularly the AV cushions (indicated by arrows in Figure 3J). The right AV cushion of the future tricuspid valve was intact, but there was a reduction of seeded cushions, particularly adjacent to the myocardial inducing layer (Figure 3J). Moreover, the AV cushions in the tie1-cre/survivinlox/lox hearts failed to fuse, in contrast to the controls (Figure 3I). Similar to the trabeculae, there were visibly fewer endothelial nuclei overlying both the OFT and AV cushions (Figure 3L), with several regions of gaps and some large endothelial nuclei.
Placenta and yolk sac vessels
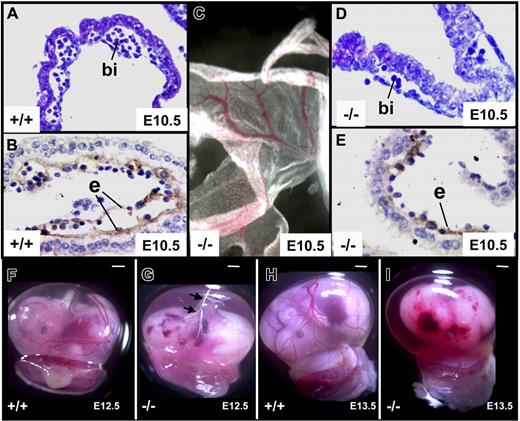
The placentas and yolk sac vasculature of E10.5 tie1-cre/survivinlox/lox embryos appeared grossly normal (Figure 4). No microscopic abnormalities were detected in the placentas of E10.5 mutant embryos (n > 10). In contrast, the luminal endothelial layer of the yolk sac vessels had a friable appearance and was discontinuous and/or flattened, and blood islands were often disrupted (Figure 4). These changes were more apparent at E12.5, when the yolk sac vessels of all embryos (n = 10 of 10) were visible grossly but often empty of blood, coincident with the placenta becoming pale (Figure 4F-I).
Yolk sac vessels, blood islands, and placenta. Sections of yolk sac vessels from survivinlox/lox (+/+) (A-B) and tie1-cre/survivinlox/lox (−/−) (D-E) were stained with H&E (A,D) and antithrombomodulin antibodies (B,E). While the vasculature of the −/− yolk sacs appeared remarkably grossly intact (C), histologic studies (D-E) revealed several areas with discontinuities of the flattened endodermal endothelial layers (e), and blood islands (bi) that were smaller and with fewer hematopoietic precursor cells. (F-I) At E12.5, branching yolk sac vessels were detectable in +/+ and −/− embryos, but they were frequently empty of blood in the −/− embryos (vessels marked with white line and arrows in panel G), and this was most often associated with a pale placenta. By E13.5, all −/− embryos that were detected were nonviable and resorbing (I). Scale bar equals 1 mm (G-I).
Yolk sac vessels, blood islands, and placenta. Sections of yolk sac vessels from survivinlox/lox (+/+) (A-B) and tie1-cre/survivinlox/lox (−/−) (D-E) were stained with H&E (A,D) and antithrombomodulin antibodies (B,E). While the vasculature of the −/− yolk sacs appeared remarkably grossly intact (C), histologic studies (D-E) revealed several areas with discontinuities of the flattened endodermal endothelial layers (e), and blood islands (bi) that were smaller and with fewer hematopoietic precursor cells. (F-I) At E12.5, branching yolk sac vessels were detectable in +/+ and −/− embryos, but they were frequently empty of blood in the −/− embryos (vessels marked with white line and arrows in panel G), and this was most often associated with a pale placenta. By E13.5, all −/− embryos that were detected were nonviable and resorbing (I). Scale bar equals 1 mm (G-I).
Structure of endothelial cells and pericytes
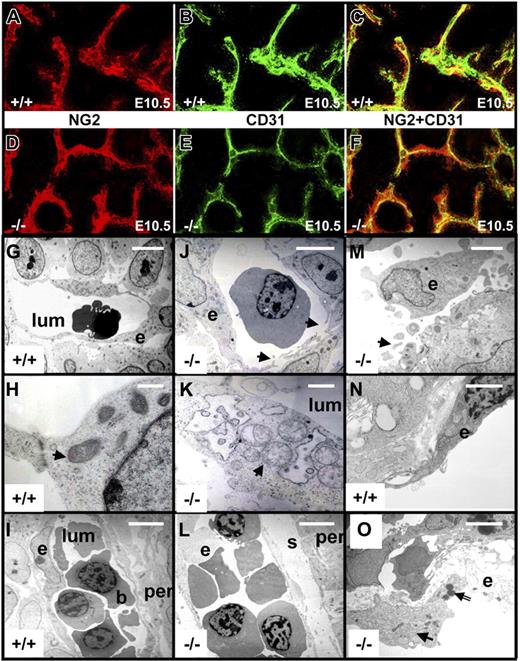
Confocal microscopy was used to visualize the microvasculature of E10.5 embryo sections (Figure 5A-F). NG2 is a proteoglycan predominantly expressed by pericytes and smooth-muscle cells during vascular morphogenesis.49 Anti-NG2 antibody staining of transverse embryo sections through the midbrain region was similar in both genotype embryos, whereas the intensity of CD31 staining was consistently less in tie1-cre/survivinlox/lox embryos (n = 5 of 5). Merging of CD31/NG2 signals indicated less overlap of staining of endothelial cells and pericytes in the tie1-cre/survivinlox/lox embryos, suggesting a pericyte–endothelial cell gap.
Structural studies of brain microvasculature in E10.5 embryos. Transverse sections of nonhemorrhagic brains from survivinlox/lox (+/+) and tie1-cre/survivinlox/lox (−/−) E10.5 embryos were cut at the level of the midbrain and stained with pericyte-specific NG-2 antibodies (A,D) and endothelial-specific CD31 antibodies (B,E), and the confocal images were overlaid (C,F). There was less intense CD31 staining in −/− embryos, with evidence of a gap between the endothelial and pericyte layers (F) compared with the +/+ (C) embryos. Transmission EM was used to examine brain microvessels (G-M) and yolk sac vessels (N-O) of survivinlox/lox (+/+) and tie1-cre/survivinlox/lox embryos. The endothelial cells (e) lining blood-filled (b) vessel lumens (lum) in +/+ embryos were smooth, continuous, and without gaps; contained normal mitochondria (arrow in panel H); and were immediately adjacent to surrounding pericytes (per) (I). In contrast, endothelial cell surfaces of −/− embryos were discontinous (arrows in panel J), with luminal and abluminal vesicles (arrow in panel M) and fragments of endothelial cells extending into the lumen (M), with strikingly abnormal and swollen mitochondria (arrow in panel K), and a wide space(s) between pericytes and endothelial cell layers (L). Compared with yolk sac vessels from +/+ embryos (N), yolk sac vessels from −/− embryos (O) were comprised of endothelial cells with attentuated plasma membranes, dilated endoplasmic reticulum (arrow in panel O), and nuclear chromatin condensation (double-stemmed arrow in panel O). Scale bar indicates 5 μm for panels G, J, I, and L; 2.5 μm for panel M; and 1 μm for panels H, K, N, O.
Structural studies of brain microvasculature in E10.5 embryos. Transverse sections of nonhemorrhagic brains from survivinlox/lox (+/+) and tie1-cre/survivinlox/lox (−/−) E10.5 embryos were cut at the level of the midbrain and stained with pericyte-specific NG-2 antibodies (A,D) and endothelial-specific CD31 antibodies (B,E), and the confocal images were overlaid (C,F). There was less intense CD31 staining in −/− embryos, with evidence of a gap between the endothelial and pericyte layers (F) compared with the +/+ (C) embryos. Transmission EM was used to examine brain microvessels (G-M) and yolk sac vessels (N-O) of survivinlox/lox (+/+) and tie1-cre/survivinlox/lox embryos. The endothelial cells (e) lining blood-filled (b) vessel lumens (lum) in +/+ embryos were smooth, continuous, and without gaps; contained normal mitochondria (arrow in panel H); and were immediately adjacent to surrounding pericytes (per) (I). In contrast, endothelial cell surfaces of −/− embryos were discontinous (arrows in panel J), with luminal and abluminal vesicles (arrow in panel M) and fragments of endothelial cells extending into the lumen (M), with strikingly abnormal and swollen mitochondria (arrow in panel K), and a wide space(s) between pericytes and endothelial cell layers (L). Compared with yolk sac vessels from +/+ embryos (N), yolk sac vessels from −/− embryos (O) were comprised of endothelial cells with attentuated plasma membranes, dilated endoplasmic reticulum (arrow in panel O), and nuclear chromatin condensation (double-stemmed arrow in panel O). Scale bar indicates 5 μm for panels G, J, I, and L; 2.5 μm for panel M; and 1 μm for panels H, K, N, O.
We more precisely defined the vascular defect with TEM (Figure 5G-O). E10.5 tie1-cre/survivinlox/lox and survivinlox/lox embryos without obvious evidence of bleeding were examined (Figure 5). Endothelial cells of microvessels in the midbrain region of tie1-cre/survivinlox/lox embryos were heterogeneous in size and shape. Cellular processes and vesiculation were evident at luminal and abluminal sides, with endothelial discontinuities frequently seen. Whole endothelial cells and/or fragments were often detected in vessel lumens (Figure 5M). Nuclear morphology revealed chromatolysis with finely dispersed chromatin, and less frequently, chromatin condensation, while the nucleoli retained an electro-dense property. The mitochondria of affected endothelial cells (Figure 5K) formed large aggregates, exhibited dilated and disorganized cristae, and were intermingled with swollen tubular endoplasmic reticulum (ER). Pericytes that were adjacent to the endothelial cells were structurally normal, but often not apposed to the endothelium (Figure 5L-M). Brain microvessels were often packed with erythrocytes. Large-vessel endothelia, including those of the aorta, were similarly affected (not shown). Yolk sac vessel endothelial cells from tie1-cre/survivinlox/lox embryos had attenuated plasma membranes with dilated ER, protrusion of vesicles from the irregular cell surface, and nuclear chromatin condensation (Figure 5N-O). Overall, the tie1-cre/survivinlox/lox embryos exhibited diffuse vascular endothelial apoptosis associated with an increased pericyte-endothelial gap.
Embryos lacking endothelial cell survivin have neural tube closure defects
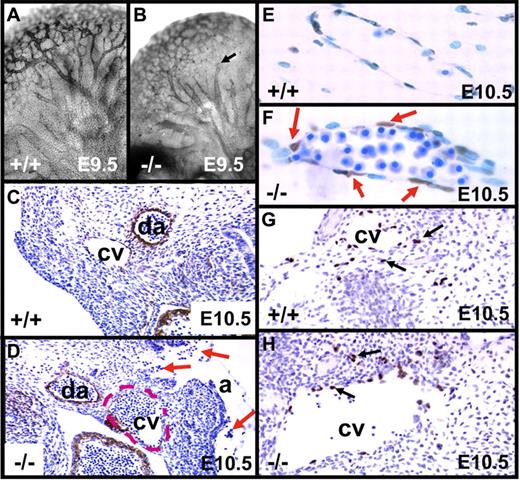
Approximately 30% of the tie1-cre:survivinlox/lox embryos had neural tube defects (NTDs) (11 of 30 at E9.5; 14 of 58 at E10.5; and 11 of 29 at E12.5) that ranged from delayed closure to exencephaly (Figure 6A-C). NTDs were not detected in sibling control survivinlox/lox embryos, and their appearance in the mutant embryos did not correlate with the severity of bleeding in the brain, frequently occurring in the apparent absence of hemorrhage. NTDs are commonly observed in association with embryonic lethal phenotypes in which developmental retardation occurs prior to or during normal neural tube closure. This was not the case for the tie1-cre/survivinlox/lox embryos, as developmental retardation was not observed until E12.5. Moreover, there was no evidence of cre expression in neurons of tie1-cre/survivinlox/wt embryos33 (Figure S2), excluding the possibility that the survivin gene in neurons was excised. Nonetheless, we attempted to confirm our findings using a different endothelial-specific promoter. Tie2-cre/survivinlox/lox embryos were therefore generated. The phenotype of these, however, was more severe, probably due to the earlier expression of tie2 at E7.50 Thus, no live tie2-cre/survivinlox/lox embryos were detected at E11.5, and at E10.5, all exhibited growth retardation and bleeding (not shown); no useful information relevant to the NTD was obtained. Nonetheless, the importance of endothelial survivin in vascular integrity was confirmed.
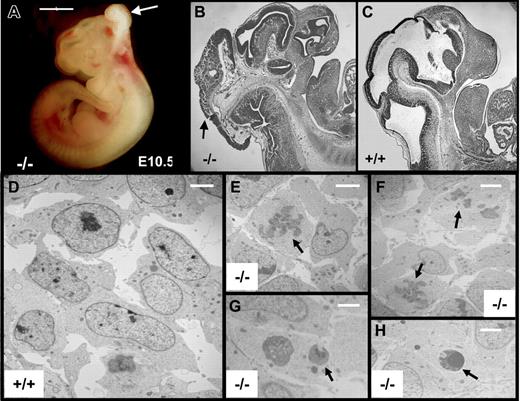
NTDs and TEM studies of brains of E10.5 embryos. (A) Representative E10.5 tie1-cre/survivinlox/lox embryo (−/−) with exencephaly (arrow). Scale bar indicates 1 mm. (B-C) Sagittal sections of the head of E10.5 embryos stained for H&E, showing exencephaly (arrows) in −/− embryo. (D-H) Survivinlox/lox (+/+) and tie1-cre/survivinlox/lox (−/−) embryo sections were examined by EM. Neural cells with mitotic figures (arrows in panels E-F) or apoptotic bodies (arrows in panels G-H) were frequently seen in the hindbrains of −/− embryos but not in those of +/+ (D) embryos. Scale bar indicates 2 μm for panels D-H.
NTDs and TEM studies of brains of E10.5 embryos. (A) Representative E10.5 tie1-cre/survivinlox/lox embryo (−/−) with exencephaly (arrow). Scale bar indicates 1 mm. (B-C) Sagittal sections of the head of E10.5 embryos stained for H&E, showing exencephaly (arrows) in −/− embryo. (D-H) Survivinlox/lox (+/+) and tie1-cre/survivinlox/lox (−/−) embryo sections were examined by EM. Neural cells with mitotic figures (arrows in panels E-F) or apoptotic bodies (arrows in panels G-H) were frequently seen in the hindbrains of −/− embryos but not in those of +/+ (D) embryos. Scale bar indicates 2 μm for panels D-H.
Dysregulated expression of proteins involved in neurulation
NTDs are associated with faulty regulation of apoptosis, altered neuroepithelial cell division and differentiation, mesenchymal cell cytoskeletal defects, changes in telomerase activity, or abnormalities in pyrimidine synthesis (reviewed in Copp et al51 ). Indeed, we documented by TEM multiple neural cells either with mitotic figures, or with apoptotic bodies in the brains of E10.5 tie1-cre/survivinlox/lox embryos (Figure 6D-H)—morphologies visibly more frequent than in controls. VEGF plays a central role in embryonic angiogenesis4,52 and neurogenesis.53,54 We therefore measured VEGF expression in the embryos. VEGF mRNA levels were elevated more than 3-fold in E12.5 tie1-cre/survivinlox/lox embryos (0.021 ± 0.010 versus 0.0061 ± 0.003 vegf mRNA copies/copy hprt; n = 9, P < .05). VEGF protein levels, measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems), were also higher in lysates of tie1-cre/survivinlox/lox embryos (241 ± 5 pg/100 ng protein; n = 5) versus survivinlox/lox embryos.(39 ± 9 pg/100 ng protein; n = 5) Neither hypoxia-inducible factor 1α (HIF1α) nor HIF2α—DNA binding proteins that enhance VEGF gene transcription—were increased in E10.5 tie1-cre/survivinlox/lox embryos by immunostaining or Western immunoblots (not shown), although pimonidazole studies revealed hypoxia in the neuroectoderm of the E10.5 tie1-cre/survivinlox/lox embryos that was not detected in corresponding regions of survivinlox/lox embryos (Figure S4). The data suggest that altered expression of endothelium-derived factors in the tie1-cre/survivinlox/lox embryos may contribute to dysregulated expression of proteins that control neural cell division and apoptosis, resulting in neurulation defects.
Survivin-dependent endothelial cell–mediated NSC proliferation and neural tube closure
During embryonic development, the neuroepithelial wall of the neural tube is composed of NSCs that are important in neural tube closure. Endothelial cells release soluble factors that promote NSC proliferation and inhibit their differentiation.40 We predicted that survivin-dependent alterations in expression of these factors might result in NTDs. To test this hypothesis, we first used transwells and cocultured wild-type NSCs from neurospheres with equal numbers of cultured endothelial cells, the latter of which expressed normal or low levels of survivin. Because survivin−/− endothelial cells could not be generated, we compared survivin+/+ and survivin+/− endothelial cells. By RT-PCR, survivin+/+ and survivin+/− endothelial cells transcribe 1.9 ± 0.2 and 0.6 ± 0.2 survivin mRNA copies per 1000 copies hprt, respectively, and under quiescent conditions, the survivin+/− cells are more permeable to dextran (Figure S5), suggesting a functional defect.
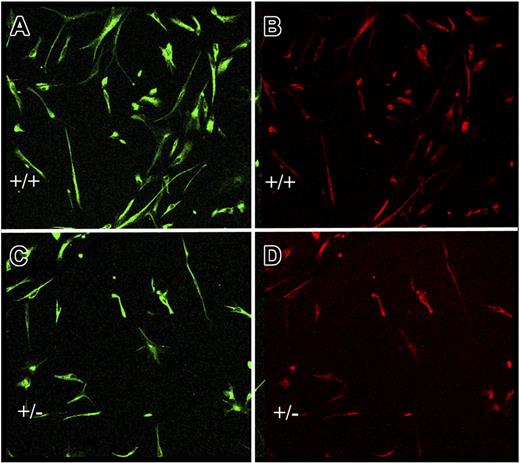
During coculture of the NSCs with either genotype endothelial cells, there were no differences in endothelial cell number, morphology, or growth rate. After coculture with survivin+/− endothelial cells (7 days), the total number of cells, more than 95% of which were primitive NSCs identified by nestin and RC2 positivity, was significantly less (42% ± 8%; n = 8, P < .05) than in the presence of survivin+/+ endothelial cells (Figure 7). We considered that survivin+/− endothelial cells might secrete increased amounts of VEGF, thereby altering NSC proliferation/differentiation. Indeed, the amount of VEGF protein secreted by survivin+/− endothelial cells in 48 hours was 116 ± 5 pg/mL versus 18 ± 12 pg/mL for survivin+/+ endothelial cells (P < .05; n = 2 experiments, each in triplicate). However, addition of exogenous recombinant active VEGF (R&D Systems) up to 100 ng/mL to conditioned media from survivin+/+ endothelial cells had no additional effect on NSC proliferation or differentiation (not shown). In fact, the trend (nonstatistical) was for VEGF to increase the numbers of primitive NSCs. Consistent with the first reports of this method, in which differentiation of the NSCs occurs after 7 days,40 we found that less than 5% of cells were GFAP+ or β-tubulin-III+ (not shown). The results indicate that expression of survivin by endothelial cells impacts on release of soluble factors that regulate NSC proliferation.
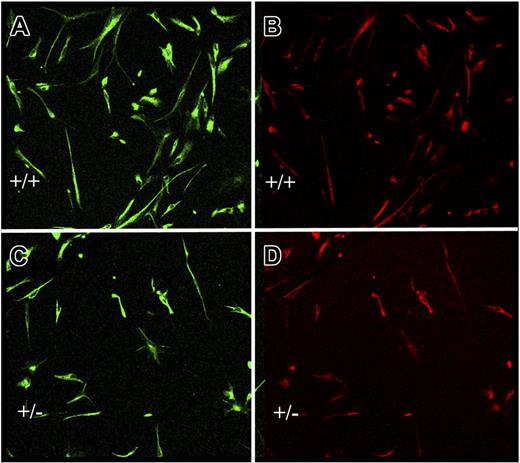
Endothelial cell–neural stem cell cocultures. Neural stem cells from wild-type mice were cocultured in transwells for 7 days with equal numbers of endothelial cells from survivin+/+ (A-B) or survivin+/− (C-D) mice, as described in “Materials and methods.” Adherent neural cells were coimmunostained for nestin (A,C) and RC-2 (B,D). Shown is a representative microscopic field, which indicates that there are approximately 2-fold more undifferentiated neural stem cells after coculture with survivin+/+ endothelial cells.
Endothelial cell–neural stem cell cocultures. Neural stem cells from wild-type mice were cocultured in transwells for 7 days with equal numbers of endothelial cells from survivin+/+ (A-B) or survivin+/− (C-D) mice, as described in “Materials and methods.” Adherent neural cells were coimmunostained for nestin (A,C) and RC-2 (B,D). Shown is a representative microscopic field, which indicates that there are approximately 2-fold more undifferentiated neural stem cells after coculture with survivin+/+ endothelial cells.
We directly assessed whether diminished endothelial survivin could affect neural tube closure by culturing E8.5 wild-type embryos in conditioned media from either survivin+/+ or survivin+/− endothelial cells, and monitoring embryonic neural tube closure over time. Neural tube closure was scored from 0 (open) to 5 (closed) (“Materials and Methods”; Figure S6). After 24 hours, scores for neural tube closure were 2.6 ± 0.4 (n = 7) in embryos cultured in survivin+/+ endothelial-conditioned media, but significantly lower (1.7 ± 0.7; n = 7) in embryos cultured in survivin+/− endothelial-conditioned media. Once again, exogenous VEGF added to the survivin+/+ endothelial-conditioned media had no effect. We also compared conditioned media from wild-type murine endothelial cells (fENDs) with that from wild-type murine embryonic fibroblasts. Over 24 hours, neural tube closure progressed significantly further with endothelial-conditioned media compared with fibroblast-conditioned media (scores: 3.3 ± 0.4 for endothelial versus 1.8 ± 0.5 for fibroblasts; n = 9-11, P < .05).
Discussion
By specifically inactivating the survivin gene in endothelial cells in mice, we show that lack of endothelial expression of survivin during embryonic development results in (1) in utero lethality due to widespread endothelial cell apoptosis, with loss of vascular integrity and embryonic hemorrhage; (2) defects in heart development; and (3) delayed neural tube closure. These findings illustrate the intimate relationship between angiogenesis, cardiogenesis, and neurogenesis, and highlight the important role that a regulator of vascular endothelial cell survival, such as survivin, plays in development and organogenesis.
Survivin, angiogenesis, and vascular integrity
Multiple cellular processes, genes, and gene products are involved in embryonic angiogenesis, coordinated to generate a stable, functional vascular network. Genetic studies in mice have illustrated the critical requirement for VEGF in embryonic vascular development.4 Others have highlighted the importance of pericyte-derived signals in supporting endothelial function, sprouting angiogenesis, and vessel maturation. For example, PDGF-B, PDGF-receptor β, the transcription factor LKLF,55 sphingosine-1 phosphate, Edg-1, and Rac are crucial for vascular smooth muscle cell and pericyte migration, and vascular integrity,56–58 while TGF-β1,59 endoglin,60 activin-like receptor kinase (ALK) 1,61 Smad5,62 ERK,63 quaking,64 and connexin65 collectively promote vessel maturation via mesenchymal differentiation and proliferation of smooth-muscle cells around nascent vascular endothelium. Limited knowledge exists with respect to the specific role of the endothelial cell in signaling to its surroundings or how endothelial apoptosis impacts on developmental angiogenesis. As an isolated event, endothelial apoptosis has been reported with deficiencies of murine angiopoietin-1,66 tie2,67 VE-cadherin,3 B-Raf,68 endothelial integrin-linked kinase (ILK),69 with dysregulation of FGF-R signaling,70 with endothelial-specific inactivation of Notch1,71 and with combined inactivation of sphingosine kinases 1 and 2.15 These were all characterized by embryonic bleeding, similar to the tie1-cre/survivinlox/lox embryos, but with some notable differences.
Given the extent of endothelial damage in the tie1-cre/survivinlox/lox embryos, it was surprising that vascular sprouting and branching was able to proceed until E12.5 to E13. This may be partly explained by incomplete cre excision of the floxed endothelial survivin gene. However, using the same transgenic tie1-cre mice, 50% to 90% excision efficiency was reported for activation of the lacz gene in the ROSA26R mice33 and inactivation of the Pdgfb gene in Pdgf lox−/− mice.43 Due to the limited sensitivity of available antibodies against cre recombinase and survivin, it was not possible to quantify cre excision efficiency. It is also possible that, in response to endothelial injury, recruitment of endothelial and vascular smooth-muscle progenitors, partly in response to a stress-induced increase in VEGF, is enhanced, and/or that smooth-muscle cells transdifferentiate into endothelial cells, providing a source of renewal.72 Regardless, the ability of the mouse embryo to accommodate to this pathologic condition indicates that smooth-muscle cells or pericytes and/or residual basement membrane are able to at least partly provide vascular support in the face of profound endothelial loss, and is the rationale for considering “combinatorial” antiangiogenic therapies in cancer that simultaneously target both the endothelium and the pericyte.73
Endothelial cells provide signals that modulate cardiac development
Much is known about the anatomical and cellular events during cardiogenesis that control endocardial EMT,48 and many genes have been identified which are involved in the formation of the cushions.46,74 However, virtually nothing is known about what happens to the mesenchymal cushion cells after EMT, and how they are maintained remodel to form mature valve leaflets. This transformation is thought to result from a region-specific balance between cellular proliferation, apoptosis, and cushion differentiation.75,76 Evidence supports the concept that apoptosis in the developing heart is a normal occurrence and is required for size reduction, fusion of adjacent cushions, shaping of the endocardial cushions and elimination of cells that fail to differentiate.77 It is likely that there are local triggering mechanisms that determine areas of cell death.77,78 Our studies demonstrate that expression of endothelial survivin in the heart is required for normal endocardial function during EMT, and that survivin-deficient endothelial cells are ineffective during endocardial cushion formation. The hypocellular AV cushion phenotype was highly penetrant and the most severely affected region within the heart. Consistent with a lack of tie1-cre–mediated recombination within the neural crest–derived distal OFT cushions,79 the OFT cushions were less affected than the largely endocardially derived AV cushions. Similarly, as the endothelium is known to influence adjacent myocardial outgrowth and remodeling, but tie1-cre is not expressed in epicardial lineage,33 survivin-deficient endothelial cells secondarily blunted trabeculation but, as expected, failed to affect the compact ventricular myocardial layer. Further studies will be aimed at determining whether loss of survivin affects the cushions and cardiomyocytes via a non–cell autonomous manner (ie, too few endothelial cells due to elevated endocardial apoptosis or diminished proliferation that may yield inadequate EMT signals), or a cell autonomous manner (ie, lack of required EMT signal expression in remaining survivin-deficient endocardial lineage). It will also be important to determine whether the sparse survivin-deficient cushion cells are able to undergo normal remodeling to give rise to mature valve leaflets. These studies will provide much-needed insights into the mechanisms underlying congenital heart defects.
Endothelial cells provide signals that modulate neurogenesis and neurulation
Molecular pathways involving cross-talk with endothelial cells have not only been identified for the heart, but also for the adrenal gland, pancreas, kidney, liver, and bone (reviewed in Cleaver and Melton13 ), and more recently for neurulation.15 In vitro studies indicate that endothelial cells provide trophic support for neurons,80 and can induce differentiation of NSCs to the endothelial lineage.40,81 Conversely, the neural tube directs embryonic perineural vascular plexus formation via the secretion of VEGF.82 There has, however, been only limited in vivo evidence of a role for the endothelium in modulating neurogenesis, dysregulation of which is linked to a defect in neurulation. Recently, Mizugishi et al15 demonstrated that the embryonic angiogenic defect caused by deficiencies of sphingosine kinases 1 and 2 in mice, also results in delayed neural tube closure in up to 20% of E12.5 embryos. Embryos lacking endothelial survivin have a similar phenotype, but with a higher penetrance of the NTD.
NTDs are common and debilitating, and the underlying mechanisms are poorly understood.51 Most research has focused on genes/proteins that regulate neuroepithelial function. However, isolated reports of NTDs in, for example, the loop-tail mouse,83 or mice exposed to excess vitamin A,84 support a vascular pathogenesis. Additional evidence, beyond that of Mizugishi et al,15 is provided in embryos lacking endothelial ILK or Notch1, both of which have widespread endothelial apoptosis and delayed neural tube closure.69,71 Which endothelial factors contribute to the regulation of neurulation remain unknown. VEGF may enhance BDNF, bone morphogenic protein-2 (BMP-2), and bFGF secretion by endothelial cells, which in turn regulate proliferation and/or differentiation of NSCs, a process controlled by endothelial Ids85 and Notch signaling. The tie1-cre/survivinlox/lox embryos with NTDs displayed irregular patterns of expression of several genes that are implicated in normal neural development, including Pax6, nestin, and Id1 (not shown), an effect not due to cre excision of neuronal survivin. The tie1-cre/survivinlox/lox embryos also express higher levels of VEGF, dysregulation of which is known to impact on neurogenesis,53,54 as well as affecting levels of nestin, Pax6, and Id1.86–88 Could the VEGF contribute to NTDs in the tie1-cre/survivinlox/lox embryos? Arguing against this is the fact that modest increases in VEGF in transgenic mice do not cause NTDs.52 On the other hand, embryos lacking the tumor suppressor Lkb1 have enhanced expression of VEGF, and die at E11.0 with NTDs, cephalic mesenchymal cell death, and vascular abnormalities.53 It is thus possible that the high VEGF levels in the tie1-cre/survivinlox/lox embryos contribute to the NTDs, but it is probable that other factors from the survivin-null endothelial cells play a major role. The fact that approximately 70% of the tie1-cre/survivinlox/lox embryos escaped a NTD may be attributed to differences in the extent of endothelial damage, and consequently, they could express crucial “neurulation” factors that allowed these embryos to develop beyond a critical stage for neural tube closure. Delineation of these endothelial neurulation factors and the genes that regulate their expression will be important both diagnostically and therapeutically.
Summary
These studies highlight the critical physiologic role that endothelial cell survivin plays in maintaining prenatal vascular integrity, and furthermore provides a novel paradigm in which the primary defect underlying the development of congenital birth defects may reside at the level of the endothelial cell.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Monica Autiero for critically reviewing the manuscript; An Zwijsen for advice and technical support; Rhonda Rogers for histologic assistance; Stephane Plaisance, Mieke Dewerchin, and Peter Carmeliet for helpful discussions; and all the staff at the Center for Transgene Technology and Gene Therapy (CTG) for their support.
E.M.C. was supported by grants from the Belgian Federation Against Cancer and the Flanders Foundation for Scientific Research (FWO; grant no. G.0382.02). S.J.C. was supported by grants from the National Institutes of Health (HL60714) and the Indiana University Department of Pediatric/Cardiology.
National Institutes of Health
Authorship
Author contributions: F.Z. designed, performed, and analyzed research and wrote the paper; F.L. designed, performed, and analyzed research; A.D.V. and S.P. performed research; L.M. designed research; R.A.A. supervised research; Y.J. performed research; P.M. and P.H. performed and designed research; H.O. designed, performed, and analyzed research; C.R. performed research; D.C. designed research; S.J.C. performed and analyzed research and wrote the paper; and E.M.C. designed and analyzed research and wrote the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Edward M. Conway, Center for Transgene Technology and Gene Therapy, University of Leuven (KULeuven), Flanders Institute for Biotechnology (VIB), Gasthuisberg O&N1, Herestraat 49, 9th floor, 3000 Leuven, Belgium; e-mail: ed.conway@med.kuleuven.be.