Abstract
The importance of CD4+ Th1 cells during the effector phase of the antitumor response has been overshadowed by emphasis on CD8+ cytotoxic T lymphocytes (CTLs). To determine their respective functions, we purified antigen-primed T cells from tumor-draining lymph nodes and separately activated CD4+ and CD8+ subsets in vitro. Adoptive transfer of CD4+ T effector cells (TEs) combined with CD8+ TEs provided synergistic therapy for mice bearing subcutaneous, intracranial, or advanced pulmonary metastases. CD4+ TEs augmented IFN-γ production by CD8+ TEs when cells were stimulated by tumor digest–containing antigen-presenting cells (APCs). CD4+ TEs infiltrated and proliferated extensively in pulmonary tumors, while also stimulating tumor antigen–specific CD8+ T cells. By contrast, CD8+ TEs showed minimal intratumoral proliferation in the absence of CD4+ cells or when systemically transferred CD4+ cells were prevented from infiltrating pulmonary tumors by pretreatment with pertussis toxin. Irradiation of CD4+ T cells immediately prior to adoptive transfer abrogated their intratumoral proliferation and direct antitumor efficacy but did not block their capacity to stimulate intratumoral CD8+ TE proliferation or tumor regression. These results highlight the importance of cross-presentation of tumor antigens during the effector phase of immunotherapy and suggest that approaches to stimulate CD4+ TE function and boost APC cross-presentation within tumors will augment cancer immunotherapy.
Introduction
T-cell–mediated adoptive immunotherapy of cancer has several advantageous features, yet the optimal method to activate T cells and ideal composition of effector cells have not been defined for clinical applications.1–6 Tumor-specific T cells are highly enriched in certain anatomic sites: for example, within tumors or in tumor-primed lymph nodes.7–9 Moreover, antigen-primed T cells display differential expression of cell adhesion molecules and chemokine receptors providing a method to purify them through fluorescence-activated cell sorting (FACS) or magnetic-bead sorting while also removing regulatory T cells.10–14 Because TCR specificity is preserved in progeny cells, it is feasible to escalate the therapeutic effect through in vitro numeric amplification of tumor-reactive T cells. Of importance, once tumor-reactive T cells have been sufficiently enriched, antigen-independent mitogenic stimuli can be used with less risk of amplifying irrelevant or autoreactive T cells.15 The in vitro sequestration of T cells also permits cytotoxic tumor therapy or immunomodulation to be administered to the host without detrimental effects on effector cells
The majority of adoptive immunotherapy studies have used systemic administration of IL-2 to enhance survival of effector CD8+ T cells. However, our previous studies demonstrated that systemic IL-2 inhibits infiltration of transferred T cells into intracranial tumors or advanced pulmonary metastases.16,17 Recently, additional concerns about the optimal therapeutic use of IL-2 have been raised, based on stimulatory effects of IL-2 for regulatory T cells.18–20 There is also considerable interest in using IL-15 as a mitogen during T cell adoptive transfer, but it has not been clinically evaluated.21 Moreover, transcellular presentation by antigen-presenting cells (APCs) may be its dominant mode of presentation in vivo.22 A logical approach is to direct the beneficial mitogenic effects of IL-2 or IL-15 specifically unto tumor-reactive CD8+ T cells by restricting its production to sites of antigen expression rather than to administer it systemically.23 CD4+ T cells transiently produce IL-2 upon antigen stimulation. In addition, monocytes produce active surface IL-15 after stimulation with IFN-γ.24 Therefore, one strategy is to administer tumor-reactive CD4+ T cells along with effector CD8+ T cells with the expectation that they will encounter tumor antigens in proximity to each other.
Most solid tumors arise from tissues that do not express class II MHC molecules and therefore are not able to directly stimulate CD4+ TEs. In previous studies, we demonstrated that tumor antigen–primed CD4+ cells are able to completely eliminate MHC class II–negative tumors.25 Moreover, in studies using bone marrow chimeras that restricted tumor antigen presentation to bone marrow–derived APCs and prevented direct recognition of tumor cells, adoptively transferred CD8+ and CD4+ TEs were nevertheless able to completely eliminate established tumors.26 Several studies have indicated that disruption of stroma or antiangiogenic responses might account for some of the indirect mechanisms of tumor destruction.27–30 Thus, there seem to be important antitumor mechanisms that supplement direct cytotoxic T lymphocyte (CTL)–mediated tumor destruction.
In this study, we investigated whether tumor-reactive CD4+ and CD8+ TEs work in parallel, or whether CD4+ TEs augment the antitumor efficacy of CD8+ TEs. By focusing on the effector phase of the immune response, we address different CD4-helper mechanisms from those that are involved in the priming phase. In 3 stringent models of established tumors, we observed synergistic antitumor responses when equal numbers of CD4+ and CD8+ TEs were administered. Our results indicate that interactions between CD4+ TEs and tumor-associated macrophages lower the threshold for CD8+ responsiveness. A second mechanism is enablement of intratumoral CD8+ T-cell proliferation. These results highlight the importance of identifying MHC class II tumor antigens and optimizing strategies to augment effector CD4+ T-cell numbers and function for tumor immunotherapy.
Materials and methods
Animals and cell lines
Female C57BL/6N mice were purchased from the Biological Testing Branch, Frederick Cancer Research and Development Center, NCI (Frederick, MD), and Thy1.1 congenic B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in an SPF environment, and used at 8 to 12 weeks of age. Experimental protocols were approved by the Cleveland Clinic IACUC. The 3-methylcholanthrene–induced fibrosarcomas MCA205 and MCA207 originally derived in B6 mice have been maintained in vivo by serial subcutaneous transplantation and were used between passages 4 to 8. Single-cell suspensions were prepared from solid tumors by digestion with a mixture of 0.1% collagenase type IV, 0.01% DNase I, and 2.5 U/mL hyaluronidase type V (Sigma-Aldrich, St Louis, MO) in HBSS for 2 to 3 hours at room temperature. Then tumor cells were filtered through a 100-μm nylon mesh, washed, and suspended in HBSS for intravenous, subcutaneous, or intracranial inoculation.
Isolation and activation of tumor-draining lymph node (TDLN) T-cell subsets
B6 mice were inoculated subcutaneously with 1.5 × 106 MCA205 tumor cells in the lower flank region bilaterally; 12 days later, TDLNs were removed and mechanically teased apart with 20-G needles, and a single-cell suspension was prepared. CD62Llow cells were isolated by depletion of CD62Lhigh cells using magnetic-activated cell sorting (MACS) beads (Miltenyi Biotech, Auburn, CA) as previously described.31 CD4+ cells were subsequently purified by depletion of CD8+ cells followed by positive selection with CD4 MACS beads.32 Alternatively, CD8+ cells were purified by depletion of CD4+ cells followed by positive selection of CD8+ cells. CD62Llow or purified CD4+ or CD8+ T cells were suspended in complete medium (CM) at 2 × 106/mL and activated with plate-bound anti-CD3 mAb (145-2C11; ATCC, Rockville, MD) for 48 hours in 24-well plates at 37°C in 5% CO2. Activated cells were washed, counted, and suspended at 0.5 × 105/mL in CM with IL-2 (4 U/mL; Chiron, Emeryville, CA), with or without rmIL-7 (10 ng/mL) or rhIL-23 (2 ng/mL) (each from R&D Systems, Minneapolis, MN) and then diluted to 105/mL on days 5 and 9. For long-term expansion, cultures were stimulated with anti-CD3 mAb for 14 hours on days 21 to 23 and every 21 days thereafter.
FACS analysis and intracellular IFN-γ staining
FITC, PE, or Cychrome-conjugated antibodies (CD4, CD8, CD11b, I-Ab, and isotype-matched rat Ig) were purchased (BD Biosciences PharMingen, San Diego, CA). Cell surface phenotypes were measured by direct immunofluorescence staining with conjugated mAbs, and stained cells analyzed using the CellQuest Software (BD Biosciences Immunocytometry, San Jose, CA). T cells were assayed for production of intracellular IFN-γ by stimulating T cells with a single cell suspension of either MCA205 or MCA207 tumor digest at a 1:1 ratio, cultured MCA205, or anti-CD3 mAb. Brefeldin A (10 μg/mL) was added at hour 5 and cells were harvested at hour 20. The cells were then washed and pretreated with FcR block, followed by staining for 30 minutes with a mixture of FITC-conjugated anti-CD8 and Cychrome-conjugated anti-CD4. Washed cells were fixed with 2% paraformaldehyde for 20 minutes, permeabilized with 0.3% saponin, and incubated for 40 minutes with PE-conjugated INF-γ at 4°C. Unbound mAbs were removed by 2 washes with 0.3% saponin in PBS.
Adoptive immunotherapy
Therapeutic efficacy of activated T cells was assessed in 3 tumor models in C57Bl/6N mice. Pulmonary metastases were established by intravenous inoculation of 3 × 105 MCA205 tumor cells, subcutaneous tumors by inoculation of 1.5 × 106 cells, and intracranial tumors by transcranial inoculation of 1 × 105 cells. Three days later for subcutaneous or intracranial tumors or 10 days later for pulmonary metastases, mice received sublethal total body irradiation (TBI, 5 Gy) from a 137Cs Irradiator (J. C. Shephard & Associates, Glendale, CA) several hours prior to intravenous transfer of the indicated number of in vitro–activated T cells. In some experiments, CD4+ T cells were treated with pertussis toxin (100 ng/mL; Sigma Chemical, St Louis, MO) for 16 hours, then washed and resuspended prior to adoptive transfer as previously described.33 Mice bearing pulmonary tumors were killed on days 18 or 21 after tumor inoculation, and the lungs were insufflated with India ink and the number of tumor nodules on the surface was enumerated. Subcutaneous tumors were measured in 2 perpendicular dimensions 2 to 3 times per week with digital calipers, and recorded as tumor area (mm2). Mice bearing intracranial tumors were followed for survival or were killed when neurologic symptoms were apparent.
In vivo trafficking
T cells derived from MCA205 TDLN CD62Llow T cells from Thy1.1 congenic mice were activated for 29 days then labeled with CFSE and injected intravenously into B6 hosts bearing established 10-day pulmonary metastases. At the designated time points after adoptive transfer, mice were killed and lungs and spleen were harvested. Single cell lymphocyte suspensions were prepared from lungs by enzymatic digestion followed by Percoll density gradient centrifugation as described.34 Cells were counted and stained with anti-CD8 or anti-CD4 and analyzed by FACS. The number of Thy1.1 T cells in the lungs was calculated by multiplying the total lymphoid cell count by their percentage on FACS analysis.
IFN-γ ELISA assay
Serum was collected from mice 24 hours after adoptive transfer of T cells and assayed using an IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA).
Statistical analysis
The significance of differences between groups was analyzed by the Wilcoxon rank-sum test or by the Student t test. A 2-tailed P value of less than .05 was considered significant.
Results
CD4+ TEs plus CD8+ TEs synergistically mediate tumor regression
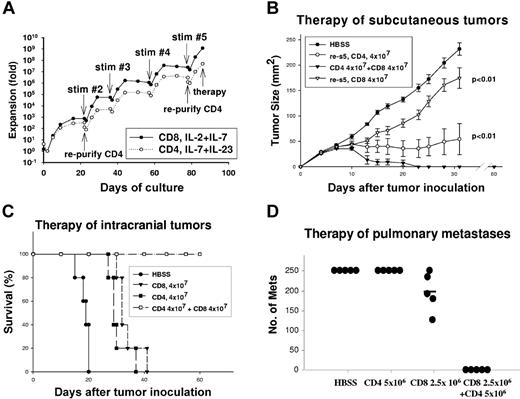
We have previously observed that T-cell adoptive immunotherapy of established MCH class I antigen–positive, class II antigen–negative tumors is inhibited if either the CD4+ or CD8+ T-cell subset is depleted in hosts immediately after adoptive transfer. There are certain anatomic sites such as intracranial or subcutaneous tumors that are particularly dependent on CD4+ TE administration to achieve complete tumor regression.16,35 In these models, systemic high-dose IL-2 cannot replace CD4 helper functions, in fact IL-2 inhibits trafficking of T cells to intracranial or subcutaneous locations and abrogates efficacy.17 This provided a rationale to explore the mechanisms through which CD4+ cells augment CD8+ cells during the effector phase of the antitumor response. Tumor-primed T cells were harvested from LNs draining progressively growing subcutaneous tumors 12 days after inoculation. LN cells were depleted of naive CD62Lhigh cells—yielding between 7% to 15% of the initial cell number—which were 3% CD8+ and 16% CD4+ and 80% CD4CD8−. The CD4+ subset was additionally purified by depletion of CD8+ cells followed by positive selection using CD4 magnetic beads.
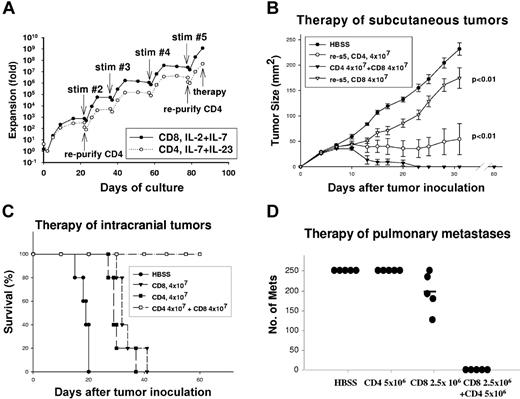
Total CD62Llow cells were activated with anti-CD3 mAb for 2 days, then cultured in the presence of IL-2 and IL-7 for an additional 20 days leading to an initial proliferative burst followed by a plateau. By contrast, purified CD4+ T-cell cultures were supplemented with IL-7/IL-23, which supports the proliferation of effector memory CD4+ T cells.36 The T cells were subsequently stimulated with immobilized anti-CD3 mAb for 14 hours every 3 weeks, which resulted on day 85 in CD8 cell expansion of 109-fold, and CD4 cell expansion of 107-fold (Figure 1A). IL-2/IL-7 cytokine support led to predominance of CD8+ T cells, and FACS analysis on day 85 of culture demonstrated more than 95% CD8+, with 0.5% CD4+ cells. The dominance of CD8+ cells in what were initially mixed cultures is presumably due to the more rapid intrinsic proliferative rate of murine CD8+ T cells compared with CD4+ cells and is consistent with our previous observations.32,37 CD4+ T-cell cultures were supplemented with IL-7 and IL-23 with exclusion of exogenous IL-2. Although CD8+ cells were depleted prior to the initiation of cultures, it was necessary to deplete residual CD8+ T cells from the CD4+ T-cell cultures on days 21 and 79 with the final phenotype on day 85, 89% CD4+, 0.4% CD8+, and 10% double negative.
Hyperexpanded CD4+ and CD8+ T cells synergistically mediate tumor regression. (A) CD62Llow TDLN cells, or the purified CD4+ subset thereof, were activated as described in “Materials and methods” with anti-CD3 restimulations every 21 days as indicated. The total proliferation is plotted on a semi-log10 scale. (B) Mice bearing 3-day subcutaneous tumors (n = 5/group) were treated with TBI, and then received intravenous transfer of HBSS, 4 × 107 CD4+ T cells, 4 × 107 CD8+ T cells, or the combination of 4 × 107 CD4+ and CD8+ T cells activated for 85 days. Mice treated with the combination of CD4+ and CD8+ T cells had complete regression and superior therapeutic response than CD4+ or CD8+ T cells alone; error bars indicate SEM (P < .01 for each). (C) Mice bearing 3-day intracranial tumors (n = 5/group) were treated with TBI, and then received HBSS, CD4+, CD8+ T-cell subsets alone, or the combination of CD4+ and CD8+ T cells activated for 85 days. Survival for the CD4+ and CD8+ T-cell subsets was significantly better than HBSS control (P < .01 for each), but survival for the CD4+ combined with CD8+ T-cell group was significantly better than either the CD4+ or CD8+ T-cell subset (P < .01). (D) Mice bearing 10-day pulmonary metastases were treated with TBI then received the indicated number of CD4+ or CD8+ T cells alone or the combination of CD4+ and CD8+ T cells activated for 135 days. The number of metastases was counted on day 21. The combination of CD4+ and CD8+ T cells was superior to either subset alone (P < .01).
Hyperexpanded CD4+ and CD8+ T cells synergistically mediate tumor regression. (A) CD62Llow TDLN cells, or the purified CD4+ subset thereof, were activated as described in “Materials and methods” with anti-CD3 restimulations every 21 days as indicated. The total proliferation is plotted on a semi-log10 scale. (B) Mice bearing 3-day subcutaneous tumors (n = 5/group) were treated with TBI, and then received intravenous transfer of HBSS, 4 × 107 CD4+ T cells, 4 × 107 CD8+ T cells, or the combination of 4 × 107 CD4+ and CD8+ T cells activated for 85 days. Mice treated with the combination of CD4+ and CD8+ T cells had complete regression and superior therapeutic response than CD4+ or CD8+ T cells alone; error bars indicate SEM (P < .01 for each). (C) Mice bearing 3-day intracranial tumors (n = 5/group) were treated with TBI, and then received HBSS, CD4+, CD8+ T-cell subsets alone, or the combination of CD4+ and CD8+ T cells activated for 85 days. Survival for the CD4+ and CD8+ T-cell subsets was significantly better than HBSS control (P < .01 for each), but survival for the CD4+ combined with CD8+ T-cell group was significantly better than either the CD4+ or CD8+ T-cell subset (P < .01). (D) Mice bearing 10-day pulmonary metastases were treated with TBI then received the indicated number of CD4+ or CD8+ T cells alone or the combination of CD4+ and CD8+ T cells activated for 135 days. The number of metastases was counted on day 21. The combination of CD4+ and CD8+ T cells was superior to either subset alone (P < .01).
Despite extensive proliferation of TEs, they maintained therapeutic efficacy against established MCA205 tumors in multiple anatomic sites. Mice bearing 3-day subcutaneous tumors received conditioning with TBI (5 Gy) followed by adoptive transfer of 4 × 107 CD4+ or CD8+ T-cell subsets or the combination of the 2. There was retardation of tumor growth by CD8+ T cells at this subtherapeutic dose (P < .01 versus controls; Figure 1B). CD4+ TEs had an even greater therapeutic effect manifested by prevention of tumor growth for up to 30 days (P < .01 versus controls). However, CD4+ TEs alone did not induce complete tumor regression. By contrast, the combination of CD4+ and CD8+ TEs induced rapid complete tumor regression with no evidence of tumor recurrence on day 60, at which time cured mice successfully rejected MCA205 tumor challenge. Similarly, for intracranial tumors (Figure 1C), host TBI followed by adoptive transfer of 4 × 107 CD4+ or CD8+ T cells led to prolongation of survival compared with controls (P < .01). However, the combination of CD4+ and CD8+ T cells was curative in all mice (P < .01) versus single TE subsets. T cells were subsequently tested for efficacy against 10-day pulmonary metastases after additional expansion for 135 days at which time the CD8+ TEs had proliferated more than 1012-fold and the CD4+ TEs more than 109-fold. As demonstrated (Figure 1D), CD8+ TEs alone had minimal efficacy at a dose of 2.5 × 106 and CD4+ TEs had no detectable efficacy at 5 × 106 cells. The combination of 2.5 × 106 CD8+ and 5 × 106 CD4+ TEs completely eliminated tumors (P < .01 vs CD8+ or CD4+ alone). An independent experiment of similar design, using T cells propagated for 78 days, showed that the combination of 1.25 × 106 each CD4+ and CD8+ T cells resulted in fewer lung metastases (49 ± 13) compared with 1.25 × 106 CD8+ T cells (148 ± 26) or 5 × 106 CD4+ T cells alone (> 250). The combination of CD4+ and CD8+ TEs was superior to CD4 cells alone (P < .001) or CD8 cells alone (P = .049).
To examine whether the synergistic effects observed were due merely to loss of certain functions during the course of prolonged in vitro culture, purified CD4 and CD8 cells were activated in culture for 9 days. The combination of 2.5 × 106 each of CD4+ and CD8+ T cells had superior efficacy to 5 × 106 CD4+ T cells (P = .014) or 5 × 106 CD8+ T cells (P < .01). This experiment indicates that twice the number of either the CD4+ or CD8+ subset alone did not have the same efficacy as the mixture and argues for a qualitative synergistic effect rather than a quantitative effect of the CD4+ and CD8+ TE subsets.
CD4+ T cell–APC interaction augments CD8+ TE function
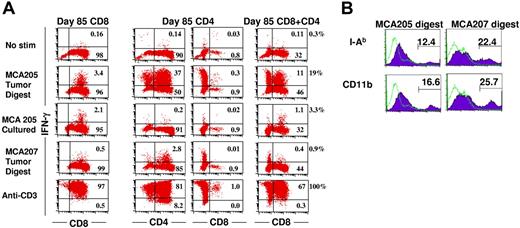
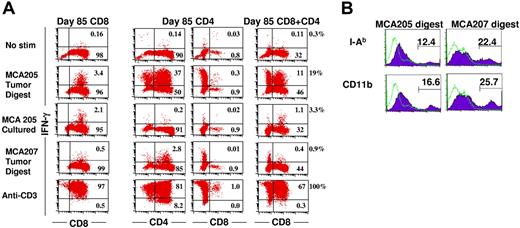
We previously observed that early in culture (day 9), a high percentage of the CD4+ (30%) and CD8+ (30%) TEs produces IFN-γ when stimulated with MCA205 tumor digest and also exhibits antigenic specificity as manifested by minimal response to MCA207 tumor digest.34 However, after prolonged culture activation (85 days) and 109-fold expansion, the percentage of CD8+ T cells producing IFN-γ upon stimulation with tumor digest diminished to 3.5% (Figure 2A first column). This indicates that either the frequency of tumor-reactive cells has declined due to preferential expansion of irrelevant T cells or that the CD8+ T cells require a higher threshold for effective stimulation following prolonged culture. In vitro–cultured MCA205 cells stimulate the CD8+ TEs nearly as effectively (2.2%) as tumor digest. By contrast, the CD4+ T cells display high reactivity (37%) to tumor digest (Figure 2A second column). As demonstrated in Figure 2B, MCA205 tumor digest contains 12% I-Ab and 17% CD11b+ APCs. Of interest, in the CD4 cultures, there is a subset of CD4/CD8 double-negative cells (Figure 2A third column). These cells are also NK1.1 negative, but do produce IFN-γ in response to tumor digest. The CD4+ TEs are completely unresponsive to in vitro–cultured MCA205 cells, which express MCH class I but not MCH class II molecules. The tumor-associated APCs present predominantly unique tumor antigens to CD4+ T cells because there is minimal response of MCA205-reactive CD4+ cells to MCA207 tumor digest, which contains 22% I-Ab, and 26% CD11b+ APCs (Figure 2B). Of interest, when CD4+ cells are mixed 1:1 with CD8+ T cells in the presence of MCA205 tumor digest, they dramatically increase the percentage of CD8+ cells that produce IFN-γ from 3.5% to 19% (Figure 2 fourth column). These data indicate that the loss of reactivity exhibited by hyperexpanded CD8+ T cells on day 85 of culture is, in part, due to decreased sensitivity to tumor antigens, which is reversed in the presence of CD4-APC interactions.
CD4+ T cells enhance the production of IFN-γ by CD8+ T cells in response to subcutaneous tumor digest. (A) CD4+ or CD8+ T-cell subsets derived from TDLN CD62Llow cells activated for 85 days were mixed with enzymatically digested single cell suspensions derived from subcutaneous MCA205 or MCA207 tumors, a single cell suspension of in vitro–cultured MCA205 or immobilized anti-CD3 mAb. Brefeldin A was added at hour 5 and cells were harvested at hour 20, permeabilized and stained for IFN-γ and CD4 and CD8. First column shows CD8+ TEs alone; second column, CD4+ TE cultures stained for CD4+ cells; third column, CD4+ TE cultures stained for CD8+ cells; and fourth column, CD8+ TEs and CD4+ TEs mixed at a 1:1 ratio and stained for CD8+ cells. The percentage of total cells is indicated in each compartment, whereas the percentage of IFN-γ–producing cells among the CD8+ T-cell subset is indicated next to the fourth column. (B) Subcutaneous MCA205 or MCA207 tumors were enzymatically digested to a single cell suspension, treated with FcR blocking antibody and stained with anti–I-Ab or anti-CD11b mAb then analyzed by FACS.
CD4+ T cells enhance the production of IFN-γ by CD8+ T cells in response to subcutaneous tumor digest. (A) CD4+ or CD8+ T-cell subsets derived from TDLN CD62Llow cells activated for 85 days were mixed with enzymatically digested single cell suspensions derived from subcutaneous MCA205 or MCA207 tumors, a single cell suspension of in vitro–cultured MCA205 or immobilized anti-CD3 mAb. Brefeldin A was added at hour 5 and cells were harvested at hour 20, permeabilized and stained for IFN-γ and CD4 and CD8. First column shows CD8+ TEs alone; second column, CD4+ TE cultures stained for CD4+ cells; third column, CD4+ TE cultures stained for CD8+ cells; and fourth column, CD8+ TEs and CD4+ TEs mixed at a 1:1 ratio and stained for CD8+ cells. The percentage of total cells is indicated in each compartment, whereas the percentage of IFN-γ–producing cells among the CD8+ T-cell subset is indicated next to the fourth column. (B) Subcutaneous MCA205 or MCA207 tumors were enzymatically digested to a single cell suspension, treated with FcR blocking antibody and stained with anti–I-Ab or anti-CD11b mAb then analyzed by FACS.
Intratumoral proliferation of effector CD8+ TEs is augmented by CD4+ TEs
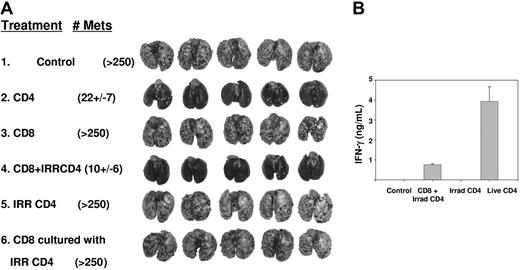
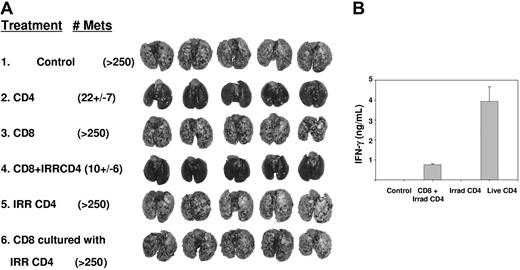
To define whether CD4+ T cells directly augment CD8+ T-cell proliferation and function within 10-day pulmonary metastases, we transferred CD4+ TEs, which had been irradiated immediately prior to adoptive transfer, in combination with live CD8+ TEs. Although 107 nonirradiated CD4+ cells provided nearly complete tumor regression, efficacy was abrogated following irradiation (Figure 3A group 2 vs 5). When mice were treated with a subtherapeutic dose of 2.5 × 106 CD8+ TEs in combination with 107 irradiated CD4+ TEs, there was nearly complete regression of established tumors (group 3 vs 4). This indicates that the capacity of irradiated CD4+ T cells to help CD8+ TE function was retained even though they lost their stand-alone antitumor competence. CD4+ help was required in vivo, during the period of CD8+ T-cell effector function, because addition of irradiated CD4+ T cells during in vitro culture of CD8+ cells did not augment their therapeutic activity (group 6). The relative efficacy of nonirradiated versus irradiated CD4+ TEs was tested in a separate experiment of similar design. This demonstrated superior efficacy for 2.5 × 106 nonirradiated CD4+ TEs versus 5 × 106 irradiated CD4+ TEs when administered with 5 × 106 live CD8+ TEs (P = .008).
Irradiated CD4+ T cells can provide help for effector CD8+ T cells. (A) Mice bearing 10-day pulmonary metastases were conditioned with TBI then received live CD4+ T cells (107), CD 8+ T cells (2.5 × 106), irradiated CD4+ T cells (107), the combination of CD8+ (2.5 × 106) and irradiated CD4+ T cells (107), or CD8+ T cells (2.5 × 106) that were culture activated in vitro in the presence of irradiated CD4+ T cells. Mice treated with live CD4+ T cells or the combination of irradiated CD4+ combined with CD8+ T cells each had superior response (P < .01) compared with untreated, CD8+ T cells alone, irradiated CD4+ T cells, or CD8+ cocultured with irradiated CD4+ T cells. (B) Serum was collected from 2 mice from each of the indicated groups 24 hours after T-cell transfer and ELISA for IFN-γ was performed.
Irradiated CD4+ T cells can provide help for effector CD8+ T cells. (A) Mice bearing 10-day pulmonary metastases were conditioned with TBI then received live CD4+ T cells (107), CD 8+ T cells (2.5 × 106), irradiated CD4+ T cells (107), the combination of CD8+ (2.5 × 106) and irradiated CD4+ T cells (107), or CD8+ T cells (2.5 × 106) that were culture activated in vitro in the presence of irradiated CD4+ T cells. Mice treated with live CD4+ T cells or the combination of irradiated CD4+ combined with CD8+ T cells each had superior response (P < .01) compared with untreated, CD8+ T cells alone, irradiated CD4+ T cells, or CD8+ cocultured with irradiated CD4+ T cells. (B) Serum was collected from 2 mice from each of the indicated groups 24 hours after T-cell transfer and ELISA for IFN-γ was performed.
As a surrogate marker for T-cell function in vivo, serum was collected from additional sentinel mice 24 hours after T-cell transfer and analyzed for IFN-γ. There were high levels of IFN-γ (> 4000 pg/mL) in recipients of live CD4+ T cells but undetectable levels in recipients of irradiated CD4+ T cells, and intermediate levels for recipients of irradiated CD4+ and live CD8+ T cells (Figure 3B). In other experiments, adoptive transfer of up to 4 × 107 CD8+ T cells in the absence of CD4+ T cells did not lead to detectable amounts of IFN-γ in serum at 24 hours. Production of IFN-γ by CD4+ T cells was transient and serum levels decreased to 200 pg/mL by 48 hours and were undetectable thereafter.
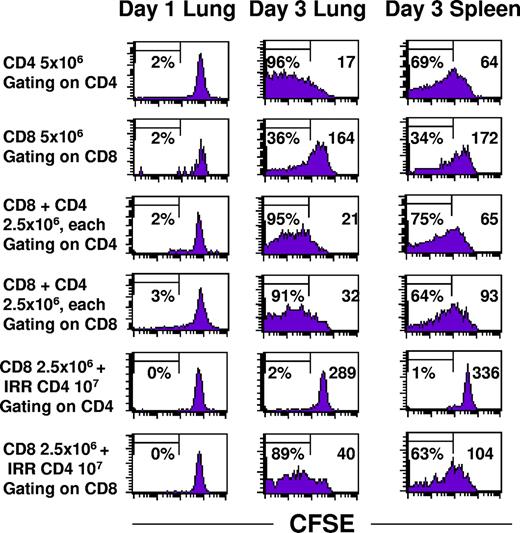
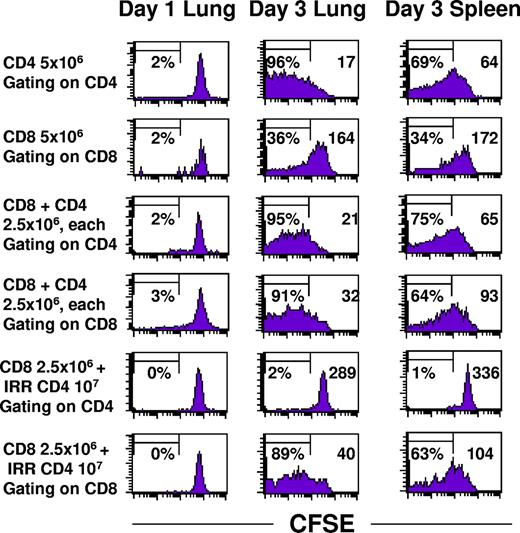
CD4+ TEs had a profound effect on the proliferation of CD8+ TEs within 10-day pulmonary metastases. CD62Llow tumor-draining lymph node cells were prepared from congenic Thy1.1 cells so that adoptively transferred cells could be identified. The CD4+ and CD8+ subsets were each labeled with CFSE prior to adoptive transfer to monitor proliferation of transferred cells in the lungs versus the spleen. Figure 4 demonstrates that intratumoral CD4+ T cells proliferate extensively whether they were transferred alone (upper row) or with CD8+ T cells (third row). Up to 95% of the CD4+ cells have greater than log10-fold CFSE dilution compared to day 1. There were fewer CD4+ cells that had proliferated extensively in the spleen compared to the lung, and the mean fluorescence intensity (indicated by the inset numeral in the upper right-hand portion of each graph) was approximately 3-fold greater. The basal rate of CD8+ TE proliferation (Figure 4 second row) was dramatically increased when live CD4+ TEs (fourth row) or irradiated CD4+ TEs (sixth row) were also administered. The mean fluorescence intensity for CD8+ T cells alone was 164 compared with 32 when live CD4+ cells were cotransferred or 40 with irradiated CD4+ T cells.
CD4+ T cells, whether viable or irradiated, augment proliferation of CD8+ T cells. Congenic Thy1.1 CD4+ and CD8+ T cells derived from TDLN were labeled with CFSE immediately prior to intravenous adoptive transfer into hosts with 10-day pulmonary metastases. Lungs and spleens were harvested on days 1 and 3 and single cell suspensions were analyzed for CFSE staining intensity within the gated Thy1.1 subset. The percentage of cells demonstrating at least 10-fold reduction in CFSE intensity is indicated on the left side and the mean fluorescence intensity is indicated on the right side of each graph.
CD4+ T cells, whether viable or irradiated, augment proliferation of CD8+ T cells. Congenic Thy1.1 CD4+ and CD8+ T cells derived from TDLN were labeled with CFSE immediately prior to intravenous adoptive transfer into hosts with 10-day pulmonary metastases. Lungs and spleens were harvested on days 1 and 3 and single cell suspensions were analyzed for CFSE staining intensity within the gated Thy1.1 subset. The percentage of cells demonstrating at least 10-fold reduction in CFSE intensity is indicated on the left side and the mean fluorescence intensity is indicated on the right side of each graph.
The total number of Thy1.1 CD4+ cells increased dramatically in the tumors but not in the spleen, whereas the number of CD8+ T cells alone did not increase except in the presence of live or irradiated CD4+ T cells (Table 1). Therapeutic efficacy was simultaneously monitored in 2 sentinel mice from each group showing complete tumor regression in recipients of CD8+ T cells in combination with either live or irradiated CD4+ T cells but minimal tumor regression with 5 × 106 CD8+ T cells. Complete therapeutic efficacy was observed with a much higher dose of 4 × 107 CD8+ T cells. Therefore, one mechanism by which CD4+ T cells augment the antitumor efficacy of CD8+ T cells is to induce their proliferation.
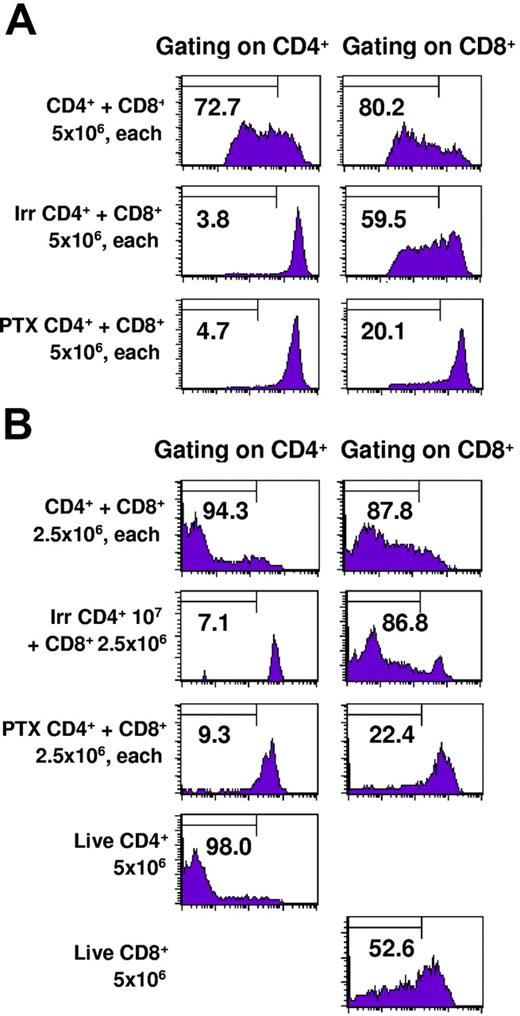
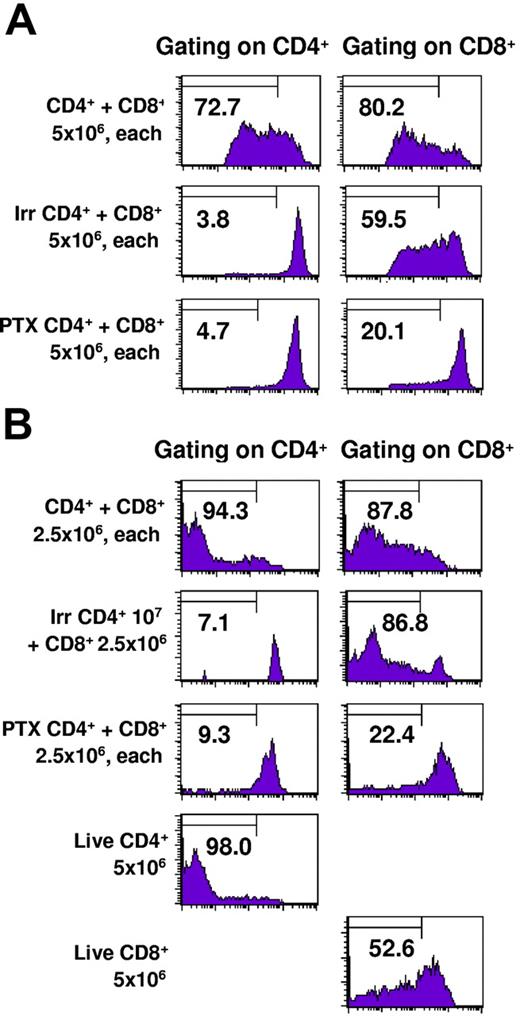
To determine whether intratumoral CD8+ TE proliferation was dependent on the coinfiltration of CD4+ cells, we selectively blocked the capacity of only the CD4+ TE subset to infiltrate tumors by treatment with pertussis toxin (PTX) in vitro immediately prior to adoptive transfer. PTX inhibits G protein–coupled receptors, thereby blocking signaling through lymphocyte chemokine receptors. Our previous studies demonstrated that PTX treatment of effector T cells prevents them from infiltrating into pulmonary metastases and consequently abrogates therapeutic efficacy even though photomicrographs demonstrated that there were numerous T cells in the pulmonary vasculature.33 Of importance, PTX treatment of effector cells is not cytotoxic and does not inhibit their antitumor function when they are coinoculated with tumor cells subcutaneously in a Winn assay. Figure 5A demonstrates that when PTX-treated CD4+ cells are adoptively transferred along with intact CD8+ effector cells there is minimal proliferation of CD8+ cells recovered from the lungs 3 days later. As previously observed, irradiated or untreated CD4+ effector cells derived from the same CD4+ culture were able to induce proliferation of the CD8+ cells. Sentinel mice from this experiment demonstrated complete regression in recipients of intact CD4+ and CD8+ T cells but more than 250 metastases in recipients of intact CD8+ plus PTX–treated CD4+ cells. In Figure 5B, a second experiment of similar design harvested 5 days after adoptive transfer of T cells demonstrates greater CD8+ T-cell proliferation in the presence of intact or irradiated CD4+ T cells. Thus, the presence of intratumoral CD4+ TEs augments the proliferation and therapeutic efficacy of CD8+ TEs.
Pertussis toxin–treated CD4+ T cells fail to traffic into tumors or induce proliferation of CD8+ T cells. TDLN CD4+ T cells were untreated, irradiated, or treated with pertussis toxin and labeled with CFSE. They were mixed with TDLN CD8+ T cells labeled with CFSE and adoptively transferred to irradiated hosts bearing 10-day pulmonary metastases. Lungs were harvested 3 days later and cells were stained for CD4 or CD8 expression and analyzed by FACS. The percentage of cells demonstrating at least 4-fold reduction in CFSE intensity is indicated in each graph.
Pertussis toxin–treated CD4+ T cells fail to traffic into tumors or induce proliferation of CD8+ T cells. TDLN CD4+ T cells were untreated, irradiated, or treated with pertussis toxin and labeled with CFSE. They were mixed with TDLN CD8+ T cells labeled with CFSE and adoptively transferred to irradiated hosts bearing 10-day pulmonary metastases. Lungs were harvested 3 days later and cells were stained for CD4 or CD8 expression and analyzed by FACS. The percentage of cells demonstrating at least 4-fold reduction in CFSE intensity is indicated in each graph.
Discussion
The use of animal models of cancer immunotherapy is somewhat artificial because the characteristics of transplantable murine tumors are likely different from spontaneous human cancer. Moreover, processes such as development of regulatory T cells and immunoediting of tumor cells might be different in human hosts with spontaneous tumors compared with transplantable tumor models.38 However, murine tumor models have elucidated general features of the antitumor response and therefore serve as useful guideposts. In this study, we used adoptive transfer of CD4+ and CD8+ TEs to focus on effector mechanisms within established tumors. Because the hosts received lymphodepletive TBI prior to TE adoptive transfer, the effects of immune regulatory cells may have been less prominent in the short time period examined. Moreover, the MCA205 tumor has been maintained by serial in vivo passage in immunocompetent hosts, and the immune response is directed against naturally occurring weak tumor antigens. For these reasons, there may be several features of CD4+ TE augmentation of the CD8+ TE response that have relevance beyond this model system. Our data indicate that it is preferable to use CD4+ TEs in combination with CD8+ TEs because they promote intratumoral CD8+ cell proliferation as well as mediating distinct mechanisms of tumor regression.
T-cell immunotherapy is dose dependent and one of the potential advantages of adoptive immunotherapy is that it is possible to enrich tumor-reactive subsets of immune cells and numerically amplify them under optimized conditions in vitro. We achieved a high precursor frequency of tumor-reactive T cells in these experiments by harvesting tumor-draining LNs at the peak of their response to local antigen priming. Further enrichment was achieved by in vitro depletion of naive LN T cells, which are CD62Lhigh. The initial population of antigen-primed CD8+ T cells was quite small (eg, only 3% of the subset of CD62Llow cells, which were 8% of the total TDLN cells). Despite the low initial number of CD8+ T cells, repetitive anti-CD3 mAb stimulation and use of low concentrations of IL-2 (4 U/mL) and IL-7 (10 ng/mL) supported rapid expansion to more than 109-fold in 85 days. We do not know whether the activation schedule or cytokine support used here is optimal, but it allowed us to address the function of T cells after extensive antigen-independent activation.
It was more difficult to achieve selective hyperexpansion of murine CD4+ T cells than CD8+ T cells. To do so, we needed to deplete CD8+ T cells several times during prolonged culture and delete exogenous IL-2. Our previous studies indicated that cyclic in vitro stimulation with anti-CD3 mAb preserves a diverse polyclonal population of T cells that maintain antigen-specific effector function and establish memory.32 Although there is diminished per cell therapeutic efficacy after 107-fold proliferation compared with limited 10-fold expansion achieved in many of our previous studies, the aggregate antitumor effect is much greater simply due to a greater number of cells. These experiments illustrate the remarkable capacity of T cells to preserve their function after controlled hyperexpansion in vitro and suggest that separate culture of CD4+ T cells and CD8+ T cells in conditions optimal for proliferation of each subset might facilitate generating sufficient numbers of T cells for therapeutic purposes.
These experiments highlight the importance of CD4+ T cells in augmenting the function of CD8+ T cells at the effector stage of the antitumor response. Combination therapy with CD4+ and CD8+ TEs provided synergistic efficacy against 3 challenging tumor models: 10-day pulmonary metastases, subcutaneous tumors, and intracranial tumors. This study documents the robust intratumoral proliferation of CD4+ T cells within pulmonary metastases and mechanistically extends previous studies using unseparated TDLN cells activated for only 5 days.39 The greater proliferation of CD4+ T cells within the tumor relative to the spleen indicates the key role of antigen presentation by intratumoral MHC class II+ APCs in addition to the homeostatic proliferation provided by host lymphodepletion. It is important to note several key differences in our experimental model compared with the adoptive transfer of pmel-1 CD8+ CTLs used by Restifo's group40 that result in some apparently contradictory conclusions. For example, whereas we isolated antigen-primed CD62Llow cells that maintained this phenotype, Klebanoff et al40 demonstrated a superior therapeutic efficacy for adoptive transfer of activated central memory T cells, which were CD62Lhigh compared with effector memory cells with a CD62Llow phenotype. However, one important aspect of the pmel-1 model is that the CTLs are not sufficiently stimulated in vivo by endogenous levels of gp100 on B16F10 tumors. Effective therapy required in vivo restimulation using viral vectors encoding an altered peptide with higher affinity for murine MHC class I, as well as provision of exogenous IL-2. Presumably, the viral vectors were preferentially acquired and presented to CD62Lhigh central memory T cells by APCs in lymphatic tissue rather than to effector memory cells in tumor tissue. By contrast, in our tumor model, the interactions between CD4+ TEs and intratumoral APCs that have naturally acquired endogenous tumor antigens are sufficient to augment the function of CD8+ TEs. This apparent discrepancy may simply reflect differences in the location of effective antigen presentation and cytokines to CTLs induced by features of the tumor models used.
The observation that intratumoral CD4+ T cells augment the function and proliferation of CD8+ T cells within the tumor also underscores the advantages when T-cell help is specific for the tumor antigens targeted. This has implications for active immunotherapy approaches. It was demonstrated by Kennedy and Celis that the presence of CD4 Th cells at the site of antigen recognition by activated CTLs was able to significantly decrease activation-induced cell death (AICD).41 In our model, AICD of CD8+ T cells may be prominent as noted by the marginal increase in total number of intratumoral CD8+ cells despite vigorous proliferation as documented by CFSE dye dilution. Of interest, in the Kennedy and Celis study, CD4 antigen specificity was not required during ex vivo restimulation, because PADRE-specific or OVA-specific CD4 cells provided equivalent protection of OVA-specific CD8.41 Indeed, several groups have evaluated the addition of foreign helper epitopes such as PADRE and KLH to vaccine formulations to further stimulate the tumor-specific CTL response. This may be useful during antigen priming or restimulation of central memory cells if both antigens can be directed to lymphatic tissue. However, the data presented here indicate that MCH class II antigens that are specific for the tumor, resulting in the generation of CD4+ TEs that home to tumor, will, more likely, have greater efficacy in eliciting an antitumor response from CTLs than an unrelated foreign antigen.
The dramatic increase in proliferation of CD8+ TEs in the presence of intratumoral CD4+ TEs demonstrates another important function. It was noteworthy that even irradiated CD4+ T cells could stimulate proliferation of CD8+ TEs and enhance their antitumor function. The observation that live CD4+ cells had a superior per-cell therapeutic effect compared with irradiated CD4+ TEs when combined with CD8+ cells indicates that CD4 persistence or proliferation is highly important but not absolutely required. CD4+ T cells have the capacity to produce IL-2 after stimulation, and CD25 expression on the hyperexpanded CD8+ T cells indicates their capacity to respond to IL-2 mitogenic stimulation in vivo. It is interesting that Antony et al42 observed greater augmentation of B16F10 tumor therapy when adoptively transferred CD8+ pmel-1 cells and recombinant fowlpox boost were supplemented by cotransfer of CD4 Th cells compared with exogenous IL-2 administration. This indicates that there are other stimulatory factors besides IL-2 that are produced during productive CD4-APC interactions.
In addition, the acquisition of Ly-6C and other memory cell markers by culture-activated CD8 cells (data not shown) suggests that they would also be responsive to IL-15 stimulation. Monocytes are major producers of IL-15 and biologically active surface IL-15 is up-regulated by IFN-γ.24,43 Moreover, IL-15 has been demonstrated to enhance the activity of adoptively transferred CD8+ T cells and enhance antigen-presenting function by tumor-associated dendritic cells (DCs).21,44 This provides a model whereby cell-to-cell interactions between CD4+ T cells and tumor-associated macrophages stimulate production of IL-2 and IFN-γ by CD4+ T cells and IL-15 by macrophages. In addition, the increased systemic availability of IL-7 due to TBI-induced lymphodepletion prior to adoptive transfer could provide survival signals to effector CD8+ T cells. As a result, direct recognition of tumor cells by CD8+ T cells would take place in a cytokine-enriched local environment that would augment their function.
Although enhancement of CD8+ T-cell efficacy is an important function of CD4+ T cells, there is considerable evidence for selection of antigen-loss variants and down-regulation of MHC class I molecules on tumor cells as mechanisms of immune escape from CTLs.38,45,46 This increases the importance of cross-presentation of specific tumor antigens to CD4+ TEs. In this regard, several studies have demonstrated that cross-presentation is sufficient to initiate complete tumor regression in the absence of direct tumor recognition.25–27,47 Presumably, CD4+ T-cell efficacy is mediated through activation of tumor-associated macrophages as well as cytokine effects on endothelium and stromal cells.48,49 Recent clinical studies have shown some therapeutic efficacy of adoptive T-cell therapy in human solid tumors.50 While clinical responses have been observed with the infusion of these predominantly CD8+ polyclonal tumor-specific T-cell lines, only a fraction of the patients has maintained those responses. The augmentation or addition of tumor-specific CD4+ T cells to CD8+ T cells would potentially result in both an enhanced and persistent antitumor response.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grant RO1CA91981 (G.E.P.).
We thank Dr Peter Cohen for comments on the paper, and Richard Caspell for technical support.
National Institutes of Health
Authorship
Contribution: L.-X.W. designed and performed research, and collected and analyzed data; S.S. designed research and analyzed data; M.L.D. analyzed data; and G.E.P. designed the research and wrote the paper.
Conflict-of-interest disclosure: M.L.D. is a consultant to Dendreon. All other authors declare no competing financial interests.
Correspondence: Gregory E. Plautz, Center for Surgery Research, NE6, 9500 Euclid Ave, Cleveland Ohio 44195; e-mail: plautzg@ccf.org.