Abstract
The signals mediating human plasma cell survival in vivo, particularly within secondary lymphoid tissue, are unclear. Human tonsils grafted into immunodeficient mice were therefore used to delineate the mechanisms promoting the survival of plasma cells. Tonsillar plasma cells were maintained within the grafts and the majority were nonproliferating, indicating a long-lived phenotype. A significant depletion of graft plasma cells was observed after anti-CD20 treatment, consistent with the expression of CD20 by most of the cells. Moreover, anti-CD52 treatment caused the complete loss of all graft lymphocytes, including plasma cells. Unexpectedly, anti-CD3, but not anti-CD154, treatment caused the complete loss of plasma cells, indicating an essential role for T cells, but not CD40-CD154 interactions in plasma cell survival. The in vitro coculture of purified tonsillar plasma cells and T cells revealed a T-cell survival signal requiring cell contact. Furthermore, immunofluorescence studies detected a close association between human plasma cells and T cells in vivo. These data reveal that human tonsil contains long-lived plasma cells, the majority of which express CD20 and can be deleted with anti-CD20 therapy. In addition, an important role for contact-dependent interactions with T cells in human plasma cell survival within secondary lymphoid tissue was identified.
Introduction
During T cell-dependent responses, B cells, on encountering antigen (Ag), traffic to the T-cell zones of secondary lymphoid tissue, where cognate interactions with primed T cells occur. This results in extrafollicular expansion of plasmablasts and the rapid secretion of low-affinity antibody (Ab), as well as development of germinal centers (GCs) and differentiation of long-lived plasma cells (PCs) that produce high-affinity Ab.1–3 Circulating Ag-specific Abs, cumulatively termed serologic memory, are critical in protection against infection, with crucial roles in both adaptive and innate responses.4
Given the relatively short half-life of serum immunoglobulin,5 maintenance of Ag-specific Ab levels requires continuous immunoglobulin secretion by either short-lived PCs that are perpetually replenished or long-lived, Ag-specific PCs. The Ag-independent polyclonal activation and differentiation of memory B cells may also contribute to the maintenance of serologic memory in humans through the gradual replacement of long-lived PCs.6 Following immunization of mice, long-lived, nonproliferating Ag-specific PCs are thought to migrate from their sites of generation within secondary lymphoid tissue to the bone marrow (BM) where they persist, contributing to serum Ag-specific Ab levels.7–9 These cells may persist for the life span of the animal.7–9
Long-lived PCs also reside in the spleen and lymph nodes (LNs) demonstrating that PC persistence is not limited to the BM.8–12 The survival of PCs is thought to be mediated by a combination of soluble and cell contact-dependent signals derived from the local environment.4,13–16 Whether the signals that maintain PC survival differ between BM and other sites where PCs persist is unknown. Inflammatory sites also appear to acquire the capacity to support PC survival because PCs were detected in the kidneys of NZB/W mice,17 humans with systemic lupus erythematosus,18 and synovium of patients with rheumatoid arthritis.19,20
Because autoreactive PCs are a critical component of both systemic and organ-specific autoimmune diseases, the characterization of the signals mediating their survival is of great interest. In vitro culture studies using murine PCs isolated from BM showed that IL-5, IL-6, TNF, CXCL12, as well as CD44 signaling, each individually improved PC survival, whereas the combination of IL-6 and anti-CD44 Abs improved this survival further.15 Coculture of BM-derived PCs with BM stromal cells improved their survival in an IL-6–dependent manner14 and recombinant IL-6 supported the maturation of peripheral blood PCs to a nondividing BM phenotype.21,22 However, despite impaired Ab responses in IL-6−/− mice, IL-6 was found not to be essential for PC survival in vivo.15,23 These studies emphasize the complexity and possible redundancy of PC survival signals in vivo.
CD20 is a B cell-specific surface molecule whose expression is initiated during late pre-B-cell development and only lost during PC maturation.24,25 Anti-CD20 Abs (rituximab) have been used to treat several autoimmune diseases as well as B-cell malignancies.26,27 Rituximab treatment causes the depletion of B-cell populations in the circulation. However, mature PCs are not thought to be affected and serum levels of Abs are usually not diminished by rituximab treatment.26–28 Furthermore, some mouse B cells survive anti-CD20 treatment because of their environment within secondary lymphoid tissue.29 The ability of rituximab to deplete B cells and PCs within human secondary lymphoid tissue is unclear and there is an obvious need to characterize this. Importantly, many tonsillar and other tissue PCs retain expression of CD20, suggesting that they may be targets of this Ab therapy.
Although the BM appears to be a critical site for PC persistence in mice, the situation is less clear in humans and secondary lymphoid tissue may play a more important role in maintaining PC populations.11 A model in which intact lymphoid tissue is maintained is necessary to examine the factors influencing human PC survival effectively. To study human lymphoid cells within their host tissue, xenochimeric mouse models have been developed by the grafting of human tissue into immunocompromised mice.30–32 Here we describe the use of human tonsil grafts to study PC survival within secondary lymphoid tissue. The tonsil grafts supported the persistence of nonproliferating immunoglobulin-secreting cells. Treatment with anti-CD20 Abs caused the partial depletion of these cells, whereas treatment with anti-CD52 Abs resulted in the total loss of lymphocytes from the grafts. Surprisingly, PCs failed to survive within the grafts after T-cell depletion and the survival of purified tonsillar PCs in vitro was improved when cultured in direct contact with tonsillar T cells. Furthermore, PCs in direct contact with T cells were detected within the tonsil grafts as well as in tonsil and LNs, indicating that human PC survival in secondary lymphoid tissue may be mediated through contact-dependent T-cell signals.
Materials and methods
Clinical samples
Tonsil samples were obtained from children younger than 10 years of age undergoing routine tonsillectomy. Spleen and LN samples were obtained from donors following trauma. Peripheral blood was obtained from healthy blood donors. All human studies were approved by the Warren G. Magnuson Clinical Center Institutional Review Board and informed consent was obtained according to the Declaration of Helsinki. Bone marrow was obtained from patients with osteoarthritis at the time of joint surgery. Digestion of tonsils with collagenase was done as previously described.33
Xenochimeric mice
NOD/RAG−/−/perforin−/− mice, aged 8 to 15 weeks, were anesthetized and 3 tonsil pieces, each approximately 2 mm3 in size, were inserted under the skin on the left flank of each mouse. Mice received benzathine penicillin G (6000 U/mouse), gentamicin (40 ng/10 g body weight), and buprenorphine (3.5 ng/10 g body weight) before surgery and were maintained on drinking water containing sulfamethoxazole (0.8 mg/mL), trimethoprim (0.16 mg/mL), oxacillin (3.5 mg/mL), and cefpodoxime proxetil (1 mg/mL).
Treatment of mice
Mice received 3 separate injections, immediately before surgery and then 3 and 6 days after surgery. All treatments were administered by tail vein injection. Mice were treated with either rituximab, an anti-CD20 chimeric monoclonal antibody (mAb; human IgG1, 600 μg total), alemtuzumab, an anti-CD52 humanized mAb (human IgG1, 300 μg total), OKT3, an anti-CD3 mAb (murine IgG2A, 300 μg total), or an anti–human CD154 mAb (murine IgG1, 300 μg, Becton Dickinson [BD] PharMingen, San Diego, CA).
Immunohistochemistry
Tissue sections, cut at a thickness of 6 μm, were fixed in acetone and treated with 20% acetic acid and 0.03% H2O2 in phosphate-buffered saline (PBS). Slides were blocked with 1.5% horse serum. Mouse mAbs directed against human CD3, CD27, Ki-67 (BD PharMingen), CD38 (Caltag Laboratories, Burlingame, CA), and CD138 (Diaclone, Stamford, CT) were detected using the Vectastain ELITE ABC kit and the Vector Blue alkaline phosphatase substrate (Vector Laboratories, Burlingame, CA). Mouse anti–human IgD-HRP (Southern Biotechnology, Birmingham, AL) was detected using Vector NovaRED substrate (Vector Laboratories). Rat anti–mouse pan-endothelial cell marker (BD PharMingen) was detected with the Vectastain ELITE ABC kit for rat IgG and Vector NovaRED substrate (Vector Laboratories). PBS/1% bovine serum albumin (BSA) was used as diluent. Sections were mounted using VectaMount mounting medium (Vector Laboratories). Images were obtained at room temperature with an Olympus DP-12 digital camera (Olympus, Center Valley, PA) mounted on an Olympus BX-50 microscope using either a 4×/0.10 NA or a 20×/0.40 NA objective. Figures were prepared using Adobe Photoshop (Adobe Systems, San Jose, CA).
Immunofluorescence and confocal microscopy
Sections were cut, fixed, and embedded as described under “Immunohistochemistry.” The mAbs used were anti–human CD3–Alexa Fluor (AF) 647, CD11c-AF546, CD27-AF647, CD45-AF546, Ki-67-AF546 (BD PharMingen), CD3-AF405, CD138-FITC (Serotec, Raleigh, NC), and anti–mouse CD11b-AF647 (Caltag Laboratories). To detect anti–human κ light chain and λ light chain, polyclonal rabbit Abs were used (Serotec). The Bu-5 Ab reacts with a component of the rough endoplasmic reticulum (RER) and was a kind gift from Debbie Hardie (MRC Center for Immune Regulation, Birmingham, United Kingdom).34 All mAbs were directly conjugated using Alexa Fluor Monoclonal Antibody Labeling Kits (Invitrogen, Carlsbad, CA) according to manufacturer's instructions, except CD3-AF405 and CD138-FITC, which were purchased commercially. The κ and λ light chains were detected with donkey anti–rabbit Cy5 (Jackson ImmunoResearch, West Grove, PA) and Bu-5 was detected with anti–mouse IgG2a-FITC (Southern Biotechnology). Sections were mounted using Vectashield mounting medium (Vector Laboratories). Cross-talk–free confocal images were obtained at room temperature on a Zeiss LSM 510 equipped with either a 20 × or 40 × 1.4 numerical aperture oil immersion lens and 405, 488, 543, and 633 lasers. Figures were prepared using Adobe Photoshop (Adobe Systems, San Jose, CA). For distance measurements, 100 PCs within multiple tissue sections were analyzed using ImageJ 1.34s software (National Institutes of Health, Bethesda, MD). Distances (μm) were measured from the center of each cell to the nearest cell of the given phenotype.
Flow cytometry and cell sorting
Cells were stained with various mAb combinations for 30 minutes on ice in PBS/1% BSA. Antibodies used were anti–human CD3-allophycocyanin-Cy7 (APC-Cy7), CD20-APC-Cy7, CD20-phycoerthrin (PE)–Cy7, CD27-FITC, CD27-PE, CD38-PE, CD38-PE-Cy7, CD45RO-PE, IgD-FITC (BD PharMingen), CD38-APC (BD Immunocytometry Systems, San Jose, CA), CD19-PE-Cy5.5 (Caltag Laboratories), CD138-PE, CD52-PE (Serotec), and anti–mouse CD11b-PE-Cy5 (Caltag Laboratories). Live cells were identified by exclusion of 4′,6-diamidino-2-phenylindole (Invitrogen). Absolute cell counts were calculated using Sphero AccuCount Blank Particles (Spherotech, Libertyville, IL). Stained cells were washed and data collected immediately using a CyAn flow cytometer (DakoCytomation, Fort Collins, CO). Data were analyzed using Summit software (DakoCytomation). Where indicated, cells were sorted using the DakoCytomation MoFlo flow cytometer.
Enzyme-linked immunosorbent assay to detect immunoglobulin
To detect human Ig (hIg), serially diluted serum was incubated overnight on plates coated with either anti–human IgA, IgG, or IgM (Bethyl Laboratories, Montgomery, TX). Bound Ab was detected using goat anti–human IgA, IgG, or IgM-HRP (Bethyl Laboratories) and TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD). Similarly to detect mouse Ig (mIg), plates were coated with anti–mouse IgA, IgG, or IgM (Bethyl Laboratories). Diluted serum and mouse reference serum (Bethyl Laboratories) were detected using goat anti–mouse IgA, IgG, or IgM-HRP (Bethyl Laboratories). All washes were done using Tris-buffered saline (TBS)/0.05% Tween-20. TBS/1%BSA/0.05% Tween-20 was used as diluent.
Enzyme-linked immunospot assay to detect human immunoglobulin
Multiscreen HTS plates (Millipore, Bedford, MA) were coated with anti–human IgA, IgG, or IgM (Bethyl Laboratories). Plates were blocked with RPMI/10% fetal calf serum (FCS). Cells, serially diluted in RPMI/10% FCS/penicillin G (200 U/mL), were added and plates incubated overnight at 37°C. Bound Ab was detected using biotinylated goat anti–human IgA, IgG, or IgM (Caltag Laboratories) and streptavidin-alkaline phosphatase (Sigma-Aldrich, St Louis, MO). Alkaline phosphatase activity was detected using Vector Blue substrate (Vector Laboratories). Spots were analyzed using a Cellular Technology Ltd Series 3B Analyzer (CTL Analyzer, Cleveland, OH).
In vitro culture of tonsillar PCs
Human tonsillar PCs (CD19+, CD38bright), CD4+ T cells and B cells (CD19+, CD38lo, IgD+) were sorted to more than 90% purity and cultured at 50 000 per cell type per round-bottomed well in 200 μL RPMI/10% FCS/penicillin G (200 U/mL). Transwells (0.2-μm pore size, Nalge Nunc International, Rochester, NY) were used to assess cell contact requirements. Cells were cultured at 37°C for up to 48 hours. For blocking studies, anti–human IL-2 (mouse IgG1), anti–human IL-4 (goat IgG), anti–human IL-6 (goat IgG), anti–human IL-10 (goat IgG), recombinant human IL-21R-Fc chimera, goat IgG, or mouse IgG1 (R&D Systems, Minneapolis, MN) and anti–human CD154 mAb (BD PharMmingen) were used at 10 μg/mL, a concentration shown to block activity of the cytokine or interaction molecule specifically. Cells were collected at 24 and 48 hours and analyzed by flow cytometry as described under “Flow cytometry and cell sorting.”
Results
Survival of human PCs and T cells within tonsil grafts
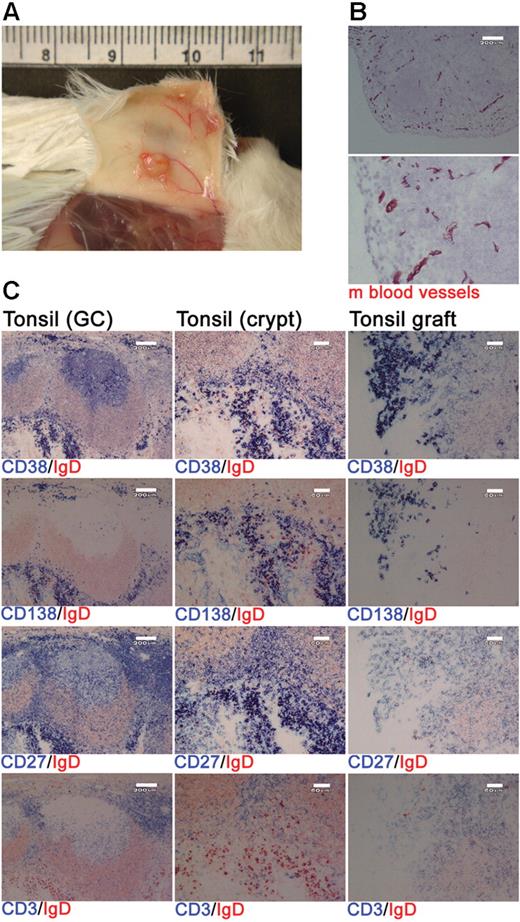
Xenochimeric mice were generated by grafting human tonsil fragments under the skin of NOD/RAG−/−/perforin−/− mice. Postmortem analysis indicated extensive vascularization of the tonsil grafts (Figure 1A) and staining of graft sections for expression of a pan–mouse endothelial cell marker detected numerous mouse blood vessels (Figure 1B). Grafts were removed at 4 weeks after surgery and the cellular composition assessed using immunohistochemistry. The majority of the surviving human cells were either PCs (CD38+, CD138+, CD27+) or T cells (CD3+, CD27+; Figure 1C). Immunofluorescence staining of tonsil sections revealed that cells expressing CD138 also expressed κ or λ light chain, CD27, and an increased amount of RER consistent with a PC phenotype (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Numerous GCs were present in the tonsil before surgery (Figure 1C); however, no such structures were detected in the grafts at 1 or 4 weeks after surgery (Figure 1C and data not shown).
Human PCs and T cells persist within tonsil grafts. Immunohistochemistry was used to assess the cellular composition of tonsil grafts recovered at 4 weeks after surgery. (A) Postmortem examination of a xenochimeric mouse at 2 weeks after surgery showing vascularization of tonsil grafts. (B) Staining of tonsil grafts for expression of mouse (m) blood vessels; lower panel shows enlarged view of upper panel; scale bar represents 200 μm. (C) Serial sections of tonsil (before surgery) and tonsil graft (4 weeks after surgery) were stained for expression of human IgD (in red) and human CD38, CD138, CD27, and CD3 (in blue). Scale bar represents 200 μm (left-hand panels) and 60 μm (central and right-hand panels). Staining is representative of 10 tonsils and tonsil grafts.
Human PCs and T cells persist within tonsil grafts. Immunohistochemistry was used to assess the cellular composition of tonsil grafts recovered at 4 weeks after surgery. (A) Postmortem examination of a xenochimeric mouse at 2 weeks after surgery showing vascularization of tonsil grafts. (B) Staining of tonsil grafts for expression of mouse (m) blood vessels; lower panel shows enlarged view of upper panel; scale bar represents 200 μm. (C) Serial sections of tonsil (before surgery) and tonsil graft (4 weeks after surgery) were stained for expression of human IgD (in red) and human CD38, CD138, CD27, and CD3 (in blue). Scale bar represents 200 μm (left-hand panels) and 60 μm (central and right-hand panels). Staining is representative of 10 tonsils and tonsil grafts.
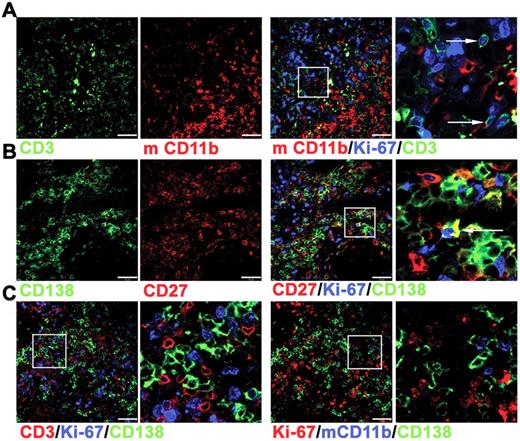
Immunofluorescence studies were used to characterize the surviving cells in tonsil grafts in greater detail. Although nuclear expression of Ki-67 was detected within many CD3+ cells (Figure 2A), only approximately 10% of the CD138+ cells also expressed Ki-67 (Figure 2B), indicating that only a minority of PCs were either in cycle or had recently divided. Notably, Ki-67+ and Ki-67− PCs in direct contact with T cells were detected (Figure 2C), suggesting an interaction between these cells within the graft. Human cells were not detected in either the mouse circulation or within the spleen or liver (data not shown), indicating that human cells did not traffic away from the grafted tissue, as observed in a previous study.31 In contrast to these studies of synovium grafts,31 mouse macrophages expressing CD11b were detected within the tonsil grafts. The CD11b+ cells were distributed unevenly within the grafts, mostly clustered together (Figure 2A) and largely absent in areas rich in PCs and T cells (Figure 2C). Therefore a partial segregation of human and mouse cells appeared to be maintained.
Immunofluorescence analysis of cells within tonsil grafts. Immunofluorescence was used to assess coexpression of multiple markers by the cells within tonsil grafts recovered at 4 weeks after surgery. (A) Three-color staining to detect expression of human CD3 (green), Ki-67 (blue), and mouse (m) CD11b (red). Enlarged view of cells expressing both Ki-67 and CD3 indicated by arrows (far right panel). (B) Three-color staining to detect expression of human CD138 (green), Ki-67 (blue), and human CD27 (red). Enlarged view of cells expressing both Ki-67 and CD138 indicated by arrow (far right panel). (C) Four-color staining (with only 3 of these shown in a given panel) to detect expression of human CD3 (red), human CD138 (green), Ki-67 (blue) in the left 2 panels and human CD138 (green), Ki-67 (red), and mCD11b (blue) in the right 2 panels. Right panel of each pair shows enlarged image. Scale bar represents 50 μm. Staining is representative of 5 tonsil grafts from each of 3 different grafting experiments.
Immunofluorescence analysis of cells within tonsil grafts. Immunofluorescence was used to assess coexpression of multiple markers by the cells within tonsil grafts recovered at 4 weeks after surgery. (A) Three-color staining to detect expression of human CD3 (green), Ki-67 (blue), and mouse (m) CD11b (red). Enlarged view of cells expressing both Ki-67 and CD3 indicated by arrows (far right panel). (B) Three-color staining to detect expression of human CD138 (green), Ki-67 (blue), and human CD27 (red). Enlarged view of cells expressing both Ki-67 and CD138 indicated by arrow (far right panel). (C) Four-color staining (with only 3 of these shown in a given panel) to detect expression of human CD3 (red), human CD138 (green), Ki-67 (blue) in the left 2 panels and human CD138 (green), Ki-67 (red), and mCD11b (blue) in the right 2 panels. Right panel of each pair shows enlarged image. Scale bar represents 50 μm. Staining is representative of 5 tonsil grafts from each of 3 different grafting experiments.
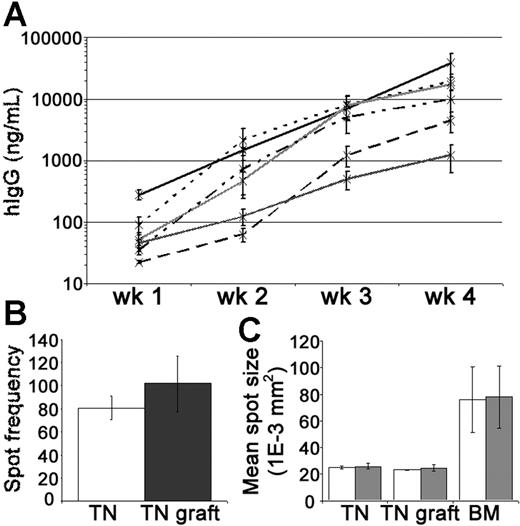
Levels of hIgG in the mouse circulation were measured by enzyme-linked immunosorbent assay (ELISA) to assess PC activity within the tonsil grafts. In groups of xenochimeric mice, the mean levels of hIgG (ng/mL) increased substantially each week (Figure 3A). Given that the half-life of hIgG in the circulation of NOD/RAG−/−/perforin−/− mice is approximately 1 week (data not shown), these data demonstrated the persistence of IgG-secreting human PCs in the grafts. PCs actively secreting hIgG were recovered from the grafts at 4 weeks after surgery (Figure 3B). The rate of immunoglobulin secretion by PCs isolated from the tonsil grafts, as assessed by spot size, remained comparable to that of tonsillar PCs isolated before surgery and was significantly less than that of PCs isolated from BM (Figure 3C). In most experiments, a relatively consistent weekly increase in serum hIgG was noted suggesting that the number of PCs was reasonably constant. However, in some experiments, especially during the first 2 weeks, a marked escalation in the weekly increase in hIgG was detected indicating the generation of new PCs in some grafts.
Tonsil graft PCs secrete high levels of human IgG. Secretion of hIgG by tonsillar PCs was assessed by ELISA and ELISPOT. (A) Weekly mean levels of hIgG (ng/mL) in the circulation of xenochimeric mice over 4-week grafting period in 6 independent experiments; each line represents data from a single experiment; error bars represent SEM. (B) Frequency of spots (hIgG) per 10 000 mononuclear cells recovered from tonsil (TN, □) or 10 000 live mononuclear cells recovered from TN grafts at 4 weeks after surgery (▩). Mean of 3 experiments shown, error bars represent SEM. (C) The mean spot size (1E-3 mm2) of cells secreting hIgG (□) and hIgM (▩) isolated from TN, TN grafts at 4 weeks after surgery, or bone marrow (BM). Mean of 3 experiments shown, error bars represent SEM.
Tonsil graft PCs secrete high levels of human IgG. Secretion of hIgG by tonsillar PCs was assessed by ELISA and ELISPOT. (A) Weekly mean levels of hIgG (ng/mL) in the circulation of xenochimeric mice over 4-week grafting period in 6 independent experiments; each line represents data from a single experiment; error bars represent SEM. (B) Frequency of spots (hIgG) per 10 000 mononuclear cells recovered from tonsil (TN, □) or 10 000 live mononuclear cells recovered from TN grafts at 4 weeks after surgery (▩). Mean of 3 experiments shown, error bars represent SEM. (C) The mean spot size (1E-3 mm2) of cells secreting hIgG (□) and hIgM (▩) isolated from TN, TN grafts at 4 weeks after surgery, or bone marrow (BM). Mean of 3 experiments shown, error bars represent SEM.
Depletion of human PCs following rituximab or alemtuzumab treatment
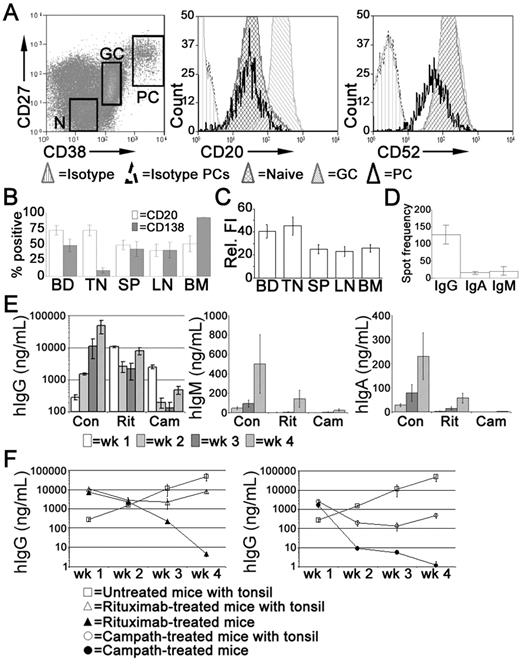
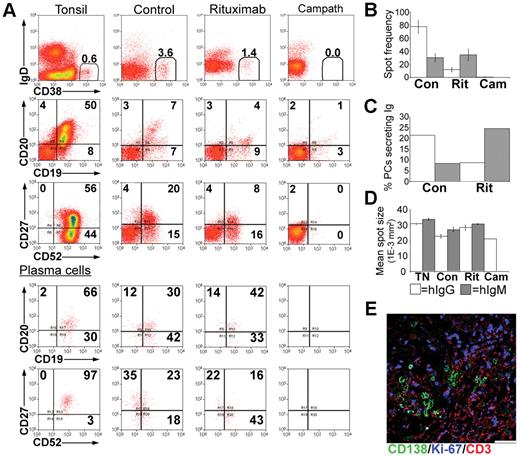
In the tonsil, the majority of PCs express CD20,35 at a density comparable with naive B cells but less than that of GC B cells (Figure 4A). Tonsillar PCs retain expression of the pan-lymphocyte marker CD52 although the density of CD52 expression is reduced compared with other B cells (Figure 4A). In spleen, LNs, and BM, approximately 50% of the PCs expressed CD20 (Figure 4B), at a density significantly less than that of PCs isolated from either blood or tonsil (Figure 4C). Consistent with previous studies,35 the majority of tonsillar PCs lack cell surface expression of CD138 detected by flow cytometry, unlike PCs isolated from BM. Approximately half of the PCs isolated from LNs and spleen expressed CD138 (Figure 4B). The frequency of IgG-secreting cells in the tonsil was approximately 10-fold greater than those secreting IgA or IgM (Figure 4D).
Treatment with rituximab or alemtuzumab reduced serum immunoglobulin levels. Xenochimeric mice were treated with either rituximab or alemtuzumab and levels of hIg in the circulation of the host mice were assessed by ELISA. (A) Expression of CD20 and CD52 by tonsillar PCs (CD19+CD38bright, CD27bright), GC B cells (CD19+CD38+, CD27+), and naive B cells (‘N', CD19+CD38lo, CD27−). Isotype controls for B cells and for PCs are also shown. (B) Percentage of PCs isolated from blood (BD, n = 8), tonsil (TN, n = 5), LN (n = 5), spleen (SP, n = 6), and BM (n = 4) expressing CD20 or CD138. Error bars represent SEM. (C) Relative fluorescence intensity (MFI-positive cells − MFI-negative cells) of CD20 expression of PCs isolated from blood, tonsil, LN, spleen, and BM. Error bars represent SEM. (D) Frequency of tonsillar cells secreting either IgG, IgA, or IgM, assessed by ELISPOT. Mean of 3 experiments shown, error bars represent SEM. (E) Mean levels of hIgG, hIgM, and hIgA (ng/mL) in groups of untreated (Con), rituximab-treated (Rit), or alemtuzumab-treated (Cam) mice. Error bars represent SEM for treatment groups. (F) Mean levels of hIgG (ng/mL) in groups of untreated mice, xenochimeric mice treated with rituximab or alemtuzumab, and NOD/RAG−/−/perforin−/− mice treated with rituximab or alemtuzumab. Error bars represent SEM for treatment groups. Results are representative of 4 experiments.
Treatment with rituximab or alemtuzumab reduced serum immunoglobulin levels. Xenochimeric mice were treated with either rituximab or alemtuzumab and levels of hIg in the circulation of the host mice were assessed by ELISA. (A) Expression of CD20 and CD52 by tonsillar PCs (CD19+CD38bright, CD27bright), GC B cells (CD19+CD38+, CD27+), and naive B cells (‘N', CD19+CD38lo, CD27−). Isotype controls for B cells and for PCs are also shown. (B) Percentage of PCs isolated from blood (BD, n = 8), tonsil (TN, n = 5), LN (n = 5), spleen (SP, n = 6), and BM (n = 4) expressing CD20 or CD138. Error bars represent SEM. (C) Relative fluorescence intensity (MFI-positive cells − MFI-negative cells) of CD20 expression of PCs isolated from blood, tonsil, LN, spleen, and BM. Error bars represent SEM. (D) Frequency of tonsillar cells secreting either IgG, IgA, or IgM, assessed by ELISPOT. Mean of 3 experiments shown, error bars represent SEM. (E) Mean levels of hIgG, hIgM, and hIgA (ng/mL) in groups of untreated (Con), rituximab-treated (Rit), or alemtuzumab-treated (Cam) mice. Error bars represent SEM for treatment groups. (F) Mean levels of hIgG (ng/mL) in groups of untreated mice, xenochimeric mice treated with rituximab or alemtuzumab, and NOD/RAG−/−/perforin−/− mice treated with rituximab or alemtuzumab. Error bars represent SEM for treatment groups. Results are representative of 4 experiments.
Xenochimeric mice were treated with anti-CD20 (rituximab) or anti-CD52 (alemtuzumab) Abs to investigate the ability of these therapies to deplete tonsillar PCs. The mean levels of hIgG, hIgM, and hIgA (ng/mL) in groups of untreated (control), rituximab-treated, and alemtuzumab-treated mice were assessed by ELISA (Figure 4E). However, because both rituximab and alemtuzumab were detected by the anti-hIgG ELISA, levels of hIgG were analyzed in NOD/RAG−/−/perforin−/− mice lacking tonsil grafts but treated with either rituximab or alemtuzumab (Figure 4F). Despite different half-lives, high levels of rituximab and alemtuzumab were maintained in the circulation during the first 2 weeks after treatment, after which the levels had declined sufficiently to allow the identification of newly synthesized hIgG by PCs in the tonsil grafts. The levels of hIgG in rituximab-treated mice at 4 weeks were 5-fold less than control mice. In contrast, levels of hIgG in the circulation of alemtuzumab-treated mice remained over 100-fold lower than in control mice. The levels of hIgM and hIgA in control mice were much lower than levels of hIgG, consistent with the reduced frequency of PCs secreting IgA or IgM in the tonsil. Weekly increases in levels of hIgA and hIgM indicated the persistence of PCs secreting these isotypes in control mice. In both rituximab and alemtuzumab-treated mice, the levels of hIgM and hIgA were dramatically reduced and at the limits of detection until 4 weeks after surgery.
Tonsil grafts were recovered at 4 weeks after surgery and live cells sorted and assessed. PCs recovered from control mice retained expression of CD19, expressed low levels of CD52, and had a range of CD20 expression (Figure 5A). In rituximab-treated mice the CD19+, CD20+ population (B cell) was largely absent, although some CD19+ CD20− cells persisted. The percentage of CD38bright PCs was reduced approximately 3-fold. However, expression of CD19, CD20, and CD52 was comparable to the PCs of control mice (Figure 5A, lower panels). No CD38bright PCs were recovered from the grafts of alemtuzumab-treated mice.
Depletion of immunoglobulin-secreting PCs from tonsil grafts. Tonsil grafts were recovered form xenochimeric mice at 4 weeks after surgery, and cells were assessed by multiparameter flow cytometry, with live cells sorted for analysis of immunoglobulin secretion by ELISPOT. (A) Expression of human IgD, CD19, CD20, CD27, CD38, CD52 in cells from the tonsil before surgery (tonsil), untreated (control), rituximab-treated (rituximab), and alemtuzumab-treated (Campath) mice. The percentage of positive cells in each quadrant and the percentage of PCs (CD38bright) are shown. Lower panels show expression of human CD19, CD20, CD27, CD52 in PCs. (B) ELISPOT analysis of immunoglobulin secretion showing average spot frequency per 10 000 cells in grafts recovered from control (Con), rituximab-treated (Rit), and alemtuzumab-treated (Cam) mice. Bars represent SEM. (C) The percentage of PCs secreting immunoglobulin was calculated by expressing the number of spots as a percentage of the number of PCs in each well. (D) The mean spot size of cells secreting IgG (□) and IgM (▩) isolated from tonsil before surgery (TN), untreated (Con), rituximab-treated (Rit), or alemtuzumab-treated (Cam) mice was calculated. Bars represent SEM. (E) Sections of tonsil grafts from rituximab-treated mice were stained for expression of human CD138 (green), Ki-67 (blue), CD3 (red). Scale bar represents 50 μm. Results are representative of 4 experiments.
Depletion of immunoglobulin-secreting PCs from tonsil grafts. Tonsil grafts were recovered form xenochimeric mice at 4 weeks after surgery, and cells were assessed by multiparameter flow cytometry, with live cells sorted for analysis of immunoglobulin secretion by ELISPOT. (A) Expression of human IgD, CD19, CD20, CD27, CD38, CD52 in cells from the tonsil before surgery (tonsil), untreated (control), rituximab-treated (rituximab), and alemtuzumab-treated (Campath) mice. The percentage of positive cells in each quadrant and the percentage of PCs (CD38bright) are shown. Lower panels show expression of human CD19, CD20, CD27, CD52 in PCs. (B) ELISPOT analysis of immunoglobulin secretion showing average spot frequency per 10 000 cells in grafts recovered from control (Con), rituximab-treated (Rit), and alemtuzumab-treated (Cam) mice. Bars represent SEM. (C) The percentage of PCs secreting immunoglobulin was calculated by expressing the number of spots as a percentage of the number of PCs in each well. (D) The mean spot size of cells secreting IgG (□) and IgM (▩) isolated from tonsil before surgery (TN), untreated (Con), rituximab-treated (Rit), or alemtuzumab-treated (Cam) mice was calculated. Bars represent SEM. (E) Sections of tonsil grafts from rituximab-treated mice were stained for expression of human CD138 (green), Ki-67 (blue), CD3 (red). Scale bar represents 50 μm. Results are representative of 4 experiments.
The enzyme-linked immunospot (ELISPOT) assay confirmed the reduced frequency of IgG-secreting cells 4 weeks after rituximab treatment and their absence after alemtuzumab treatment (Figure 5B). However, the percentage of IgM-secreting cells appeared to increase 4 weeks after rituximab treatment (Figure 5C). Since the ELISA data indicate that all PCs were initially depleted by rituximab treatment, this may reflect the generation of IgM-secreting cells in the graft from a precursor B-cell population that survived rituximab treatment. Importantly, the rate of immunoglobulin secretion of tonsillar graft PCs was unaffected by rituximab treatment (Figure 5D).
To confirm that treatment with rituximab caused the specific depletion of B cells, the distribution of CD3+ cells was assessed by immunofluorescence (Figure 5E). T-cell numbers in these grafts were comparable to those in grafts recovered from control mice (Figure 2A), as was expression of Ki-67 by CD3+ and CD138+ cells (Figure 5E).
PC survival in tonsil grafts is T cell-dependent
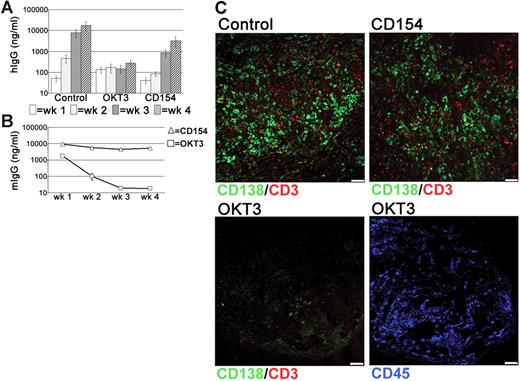
Since CD52 is expressed by all tonsillar lymphocytes, it was possible that the PC depletion following alemtuzumab treatment was related to the loss of other cells that supported PC survival. The persistence of numerous T cells in the grafts, combined with the detection of these cells in direct contact with PCs, suggested that T cells may have a role in PC survival. Therefore, the effects of T-cell depletion were assessed using the anti–human CD3 mAb OKT3. Surprisingly, levels of hIgG in the circulation of OKT3-treated mice remained very low and did not increase over the 4-week grafting period, in contrast to the large weekly increases detected in control mice (Figure 6A). Notably, serum levels of OKT3 declined rapidly in the circulation (Figure 6B). Interactions though CD40-CD154 are essential for the generation of PCs through GC reactions.36–38 Because tonsillar PCs retain expression of CD40,35 xenochimeric mice were also treated with anti-CD154 mAbs to investigate whether signals through CD40-CD154 were involved in either the generation or survival of immunoglobulin-secreting cells within the grafts. In anti-CD154-treated mice the levels of hIgG were reduced, particularly at the 2-week time point. However, by 4 weeks after surgery levels had increased significantly to approximately 5-fold less than control mice (Figure 6A). These data suggest that ongoing T-cell–B-cell interactions through CD40-CD154 generate an immunoglobulin-secreting population within the graft. The variation between experiments in the effect of anti-CD154 treatment (Figure S2) with no effect seen in some experiments and a significant early effect seen in others may be explained by differing frequencies of pre-existing PCs and PC precursors in the different tonsil samples. In all experiments, however, a 3- to 5-fold increase in serum IgG levels was detected between weeks 3 and 4 despite the persistence of large amounts of anti-CD154 mAb (Figure 6B), consistent with the conclusion that CD40-CD154 interactions were not essential for PC maintenance.
T cell-dependent PC survival in tonsil grafts. To assess the role of T cells in PC survival in the grafts, xenochimeric mice were treated with either anti-CD3 (OKT3) or anti-CD154 mAbs. Levels of hIgG and mIgG assessed by ELISA and recovered graft composition assessed by immunofluorescence were analyzed. (A) Mean levels of hIgG (ng/mL) in untreated (Con), OKT3-treated (OKT3), or anti-CD154–treated (CD154) mice. Error bars represent SEM for treatment groups. (B) Mean levels of mIgG (ng/mL) in mice treated with OKT3 or anti-CD154 mAbs. Error bars represent SEM for treatment groups. (C) Tonsil graft sections from control and OKT3-treated and anti-CD154–treated mice stained for expression of human CD138 (green), CD3 (red), and CD45 (blue). Scale bar represents 50 μm. Results are representative of 3 experiments.
T cell-dependent PC survival in tonsil grafts. To assess the role of T cells in PC survival in the grafts, xenochimeric mice were treated with either anti-CD3 (OKT3) or anti-CD154 mAbs. Levels of hIgG and mIgG assessed by ELISA and recovered graft composition assessed by immunofluorescence were analyzed. (A) Mean levels of hIgG (ng/mL) in untreated (Con), OKT3-treated (OKT3), or anti-CD154–treated (CD154) mice. Error bars represent SEM for treatment groups. (B) Mean levels of mIgG (ng/mL) in mice treated with OKT3 or anti-CD154 mAbs. Error bars represent SEM for treatment groups. (C) Tonsil graft sections from control and OKT3-treated and anti-CD154–treated mice stained for expression of human CD138 (green), CD3 (red), and CD45 (blue). Scale bar represents 50 μm. Results are representative of 3 experiments.
Using immunofluorescence, numerous PCs and T cells were detected within the grafts of control and anti-CD154-treated mice (Figure 6C). However, virtually no CD138+ cells were detected in the grafts of OKT3-treated mice and CD3+ cells were absent (Figure 6C) consistent with the loss of PCs following T-cell depletion. Staining with an anti–human CD45 mAb detected numerous lymphocytes within the graft (Figure 6C), demonstrating that not all lymphocytes were depleted following OKT3 treatment.
Improved PC survival in vitro though coculture with T cells
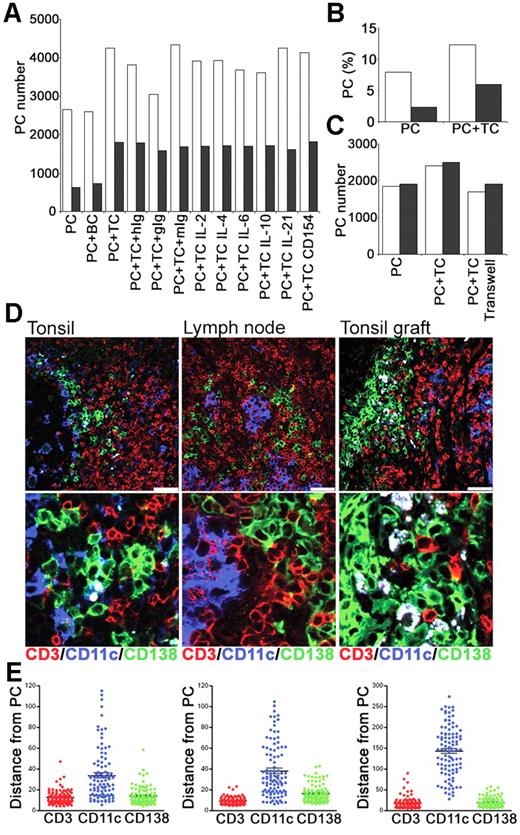
To determine whether interactions with T cells could contribute to PC survival in vitro, CD19+, CD38bright PCs and CD4+ T cells were sorted from tonsil and cultured either alone or together (Figure 7A). Culture of PCs with CD4+ T cells increased the number of PCs recovered at 24 and 48 hours by approximately 50% and 200%, respectively (Figure 7A-B). To control for an effect of increasing cell numbers and thus cell contact, PCs were cultured with B cells (CD19+, CD38low, IgD+) but no improvement in PC survival was detected. When signals through IL-2, IL-4, IL-6, IL-10, or IL-21 were blocked with Abs or specific receptor constructs, the numbers of PCs were comparable to those cultured with T cells alone or with isotype-matched control Abs (Figure 7A). Furthermore, blocking anti-CD154 mAbs (Figure 7A) had no effect on PC numbers, consistent with PC survival being independent of CD40-CD154 signals, and the low level of CD154 expressed constitutively by tonsil CD4+ T cells (data not shown). The coculture of PCs and T cells together in the presence of a transwell or actually separated by a transwell indicated that cell-to-cell contact was required to mediate the T-cell survival signal (Figure 7C).
PCs and T cells interact in vitro and in vivo. Tonsillar PCs and T cells were purified and cultured in vitro. Immunofluorescence was used to assess interactions between PCs, T cells, and CD11c+ DCs in secondary lymphoid tissue. (A) Numbers of PCs recovered at 24 hours (□) and 48 hours (▩) after culture of PCs either alone (PC), with B cells (BC), or with T cells (TC), either alone, with isotype control Abs (hIg, gIg, or mIg) or with anti–IL-2, –IL-4, –IL-6, –IL-10, or –IL-21 or CD154 Abs. Results are representative of 2 independent experiments. (B) Percentage of live PCs recovered at 24 hours (□) and 48 hours (▩). (C) Numbers of PCs recovered at 24 hours after culture in the presence of transwells either alone (PC), with T cells (PC + TC) or with T cells separated by transwell. Results of 2 independent experiments shown. (D) The distribution of CD11c+ DCs relative to PCs and T cells. Expression of human CD138 (green), CD11c (blue), and CD3 (red) in tonsil, lymph node, and tonsil graft at 4 weeks after surgery. Staining is representative of 3 samples of each tissue, scale bar represents 50 μm. (E) The distance (in μm) of 100 (CD138+) PCs to the nearest CD3+, CD11c+ or CD138+ cells was measured in sections of tonsil, LN, and tonsil graft. Mean and SEM are shown. Staining is representative of 4 samples of each tissue type, and scale bar represents 50 μm.
PCs and T cells interact in vitro and in vivo. Tonsillar PCs and T cells were purified and cultured in vitro. Immunofluorescence was used to assess interactions between PCs, T cells, and CD11c+ DCs in secondary lymphoid tissue. (A) Numbers of PCs recovered at 24 hours (□) and 48 hours (▩) after culture of PCs either alone (PC), with B cells (BC), or with T cells (TC), either alone, with isotype control Abs (hIg, gIg, or mIg) or with anti–IL-2, –IL-4, –IL-6, –IL-10, or –IL-21 or CD154 Abs. Results are representative of 2 independent experiments. (B) Percentage of live PCs recovered at 24 hours (□) and 48 hours (▩). (C) Numbers of PCs recovered at 24 hours after culture in the presence of transwells either alone (PC), with T cells (PC + TC) or with T cells separated by transwell. Results of 2 independent experiments shown. (D) The distribution of CD11c+ DCs relative to PCs and T cells. Expression of human CD138 (green), CD11c (blue), and CD3 (red) in tonsil, lymph node, and tonsil graft at 4 weeks after surgery. Staining is representative of 3 samples of each tissue, scale bar represents 50 μm. (E) The distance (in μm) of 100 (CD138+) PCs to the nearest CD3+, CD11c+ or CD138+ cells was measured in sections of tonsil, LN, and tonsil graft. Mean and SEM are shown. Staining is representative of 4 samples of each tissue type, and scale bar represents 50 μm.
PC–T-cell interactions in vivo
To investigate PC–T-cell interactions in vivo, their distribution within secondary lymphoid tissue was assessed using immunofluorescence. Since plasmablast survival and differentiation to PCs in mice may involve association with CD11c+ dendritic cells (DCs),39 it was conceivable that the PC–T-cell interactions were mediated through CD11c+ DCs. Sections of tonsil, LN, and tonsil graft were stained for expression of human CD138, CD11c, and CD3 (Figure 7D). Human PCs (CD138+) were observed in direct contact with T cells (CD3+) in the tonsil, LN, and tonsil graft. In sections of human spleen stained for PCs and T cells, PCs were detected in low frequency scattered within the red pulp, but also in white pulp areas where T cells were clustered (data not shown). Few CD11c+ DCs were detected in tonsil grafts. In contrast, numerous CD11c+ cells were present in the tonsil and LN. Despite this, PC–T-cell interactions were usually distant from CD11c+ DCs. Using ImageJ software, the distance between a PC (CD138+) and the nearest CD3+ cell, CD11c+ cell, and CD138+ cell was measured in sections of tonsil, LN, and tonsil graft (Figure 7E). Measurements were taken from the center of each cell, with a distance between 5 and 10 μm indicating direct contact between cells. A close association between PCs and T cells was evident. Notably a close association between PCs and other PCs was also apparent. In all tissues, the majority of the CD11c+ DCs were distributed more than 20 μm away from PCs, although an occasional DC-CD138+ cell interaction was detected.
Discussion
Here we describe the use of a xenochimeric mouse model to study the survival of human PCs within the secondary lymphoid tissue of their original generation. The tonsil grafts supported the persistence of immunoglobulin-secreting cells for at least 4 weeks. The absence of Ki-67 expression and the kinetics of the hIgG levels in the mouse circulation indicated that most of the PCs were long-lived. Treatment of xenochimeric mice with rituximab caused the specific depletion of B cells and PCs, as assessed by circulating hIg levels, immunofluorescence, flow cytometry, and ELISPOT. This is consistent with the finding that most tonsillar PCs express CD20 at a level comparable with resting naive B cells. No differences in the susceptibility of PCs secreting different isotypes were apparent. Importantly, the level of CD20 expression by tonsil PCs appears sufficient to mediate their depletion through anti-CD20 therapy. The presence of CD20+ PCs in the grafts 4 weeks after rituximab treatment is likely related to the generation of new PCs, largely secreting IgM from surviving B cells through CD40-CD154 interactions with T cells. Since CD19+, CD20− B cells were detected within the grafts of rituximab-treated mice, some cells may have survived rituximab treatment through loss of CD20 expression.40 Notably, the data described here indicate that the tonsil supports long-lived PCs, as described for the spleen and BM.7–9,11 Furthermore, PCs isolated from LNs, spleen, and BM also retained CD20 expression suggesting that rituximab treatment may effectively deplete long-lived PCs in other secondary lymphoid tissue.
In contrast to the partial PC depletion caused by rituximab treatment, treatment with alemtuzumab resulted in the total loss of immunoglobulin-secreting cells from the tonsil grafts. In addition, the alemtuzumab treatment effectively depleted all the other lymphocyte populations including those precursors of immunoglobulin-secreting cells. Because tonsillar PCs retain expression of CD52, this depletion may have been through the direct targeting of these cells. However, given the complete loss of PCs following anti-CD3 treatment, it remains possible that the loss of the PCs was related to the absence of other CD52+ cells required for PC survival.
The surprising effects of anti-CD3 treatment indicated that PC survival within the tonsil grafts required the continued presence of T cells. Although not all lymphocytes were depleted following anti-CD3 treatment, it is conceivable that the PC depletion resulted from nonspecific killing of these cells. However, combined with the detection of PC-T cell interactions and the demonstration in vitro of T cell-dependent PC survival, the data are consistent with a role for T cells in human PC survival.
The mechanism by which T cells might mediate PC survival is unknown. The transwell experiments suggested direct contact between the PCs and T cells was required. Culture with anti–IL-2, –IL-4, or –IL-10 Abs or an IL-21 receptor IgFc construct did not affect tonsillar PC survival in vitro, consistent with roles in B-cell differentiation rather than PC survival.41–44 Although tonsillar PCs express IL-6R,45 anti–IL-6 Abs did not diminish the improved tonsillar PC survival when cocultured with T cells. Therefore, IL-6 does not appear to be involved in T cell-dependent PC survival, although IL-6 derived from other cells may play a role. Studies using BM PCs indicate a critical role for IL-6 produced by stromal cells in PC survival and recombinant IL-6 supported the maturation of peripheral blood PCs.14,20–21 Moreover, human splenic PC immunoglobulin secretion was improved through coculture with splenic stromal cells and dependent on IL-6.46 Interactions with T cells may be sufficient to support PC survival in vivo, explaining the maintenance of immunoglobulin levels in IL-6−/− mice.15,22
The kinetics of the hIgG levels in some xenochimeric mice combined with the reduced levels of hIgG following treatment with anti-CD154 mAbs indicated that immunoglobulin-secreting cells were generated within some tonsil grafts though B-cell–T-cell interactions mediated by CD40-CD154. The detection of CD138+, Ki-67+ cells only within the tonsil grafts and not in freshly isolated tonsil suggests that these were the PCs generated within the grafts. However, this was not observed with all tonsils, possibly because of the variable but low expression of CD154 expressed constitutively by tonsil CD4+ T cells. Because numerous immunoglobulin-secreting cells persisted within the grafts of mice that received anti-CD154 Abs, interactions between CD40 and CD154 were not required for PC survival within the tonsil tissue. Furthermore, anti-CD154 Abs did not block the T cell-dependent increase in PC survival in vitro. Therefore, although PCs retain expression of CD40, interactions with CD154 do not enhance PC survival.
We noted a discrepancy with regard to detection of CD138 by tonsil PCs. As noted previously,35 tonsil PCs did not express CD138 detected by flow cytometry, but CD138 expression by tonsil PCs was easily identified by immunofluorescence microscopy. Costaining for intracellular immunoglobulin light chains and the quantity of RER clearly indicated that the CD138+ cells detected in the tonsil by immunofluorescence microscopy were indeed PCs. Since the same anti-CD138 mAb was used for both procedures, this could not explain the discrepancy. Moreover, tonsil PCs contained abundant mRNA for CD138 (data not shown), indicating that these cells clearly expressed this gene product. The possibilities that low abundance expression or intracellular location might explain the discrepancy were considered. However, a more likely explanation relates to the use of collagenase to obtain single-cell suspensions of tonsil cells. Collagenase digestion of U266 myeloma cells that express large amounts of CD138 led to its rapid and reversible loss (data not shown). Since matrilysin (matrix metalloproteinase 7), a member of the collagenase family, is the principal mediator of the shedding of CD138,47 overlapping substrate specificity between collagenase and matrilysin could explain the loss of CD138 expression by PC during preparation of single-cell suspensions of tonsil cells. Alternatively, mechanical harvesting of tonsil cells without collagenase digestion might yield the CD138− subpopulation exposed to matrilysin-like activity in vivo.
The limited effects of rituximab therapy in reducing serum immunoglobulin levels has been mainly attributed to the loss of CD20 by BM PCs.48,49 Alternatively, effector mechanisms or PC mobilization required for the action of rituximab29 may be deficient in the BM. Anti-CD52 treatment has been extensively used to deplete lymphocytes during organ transplantation.50 Moreover, alemtuzumab effectively depleted PCs from the tonsil grafts of xenochimeric mice and recently was shown to deplete malignant CD52+ PCs transplanted into NOD/SCID mice.51 However, heterogeneity in PC CD52 expression,52 as observed for many lymphocyte Ags,35 indicates that therapies targeting a single molecule may not be uniformly successful. The effects of the anti-CD3 treatment described in this study suggest that targeting T cells might contribute to the depletion of PCs in combination with other agents.
In summary, we describe the use of a xenochimeric mouse model to study human PC survival in secondary lymphoid tissue. Anti-CD20 treatment was able to deplete tonsillar PCs substantially, whereas anti-CD52 treatment caused a total depletion. Anti-CD154 treatment, although affecting the generation of immunoglobulin-secreting cells within the grafts, did not have an impact on PC survival. Importantly this xenochimeric mouse model revealed a novel role for T cells in mediating human PC survival that appeared to be mediated through direct contact between the cells. These data indicate that T-cell signals mediate PC survival within secondary lymphoid tissue and support the use of therapies targeting T cells in addition to B cells in the depletion of human PCs in vivo.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
We thank Dr Gary Sims for technical assistance and scientific discussion. We are very grateful to the NIAMS Animal Facility, in particular Joe Woo, for animal care and surgery assistance. We thank James Simone of the NIAMS flow cytometry facility for sorting cell subsets. All imaging was done at the NIAMS Light Imaging section, with the assistance of Evelyn Ralston and Kristien Zaal.
National Institutes of Health
Authorship
Contribution: D.R.W. designed and performed research, collected and analyzed data, and wrote the paper; C.F. performed research and collected data; R.T.F. performed research and collected data; R.E. designed research; P.E.L. designed research, analyzed data, and wrote the paper; and A.C.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter E. Lipsky, Autoimmunity Branch, National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS), National Institutes of Health, 9000 Rockville Pike, Bethesda, MD, 20892; e-mail: lipskyp@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal