Abstract
Interleukin-12 (IL-12), a heterodimeric cytokine (p35/p40) produced mainly from macrophages and dendritic cells, is an important regulator of T-helper 1 cell responses and for host defense. We found that interferon (IFN) consensus sequence binding protein (ICSBP), which is a transcription factor essential for the expression of p40, was expressed in mouse bone marrow–derived mast cells (BMMCs). The transcription levels of p35 and p40 were increased by stimulation of BMMCs with IFN-γ/lipopolysaccharide (LPS). IL-12 was secreted from BMMCs in response to LPS but not by FcϵRI cross-linking. The p40 levels in the peritoneal cavity of mast cell–deficient W/Wv and W/Wv reconstituted with p40−/− BMMCs were significantly lower than those of WBB6F1+/+ and wild-type (WT) BMMC-reconstituted W/Wv in the acute septic peritonitis model. The survival rate of W/Wv reconstituted with p40−/− BMMCs was significantly decreased compared to those of WBB6F1+/+ and WT-BMMC–reconstituted W/Wv, which was due to reduced production of IFN-γ and subsequent impaired activation of neutrophils in the peritoneal cavity. Survival rate of p40−/− mice was also restored by adoptive transfer of WT-BMMCs. These results demonstrate that mast cells play a significant role in the production of IL-12 required for host defense. This is the first report to demonstrate that mast cells are a crucial source of functional IL-12.
Introduction
Interleukin-12 (IL-12) is a cytokine that governs production of interferon-gamma (IFN-γ) in natural killer (NK) cells and CD4+ T cells1,2 and that is involved in the induction and maintenance of T-helper 1 (Th1) cells.3–5 IL-12 also plays important roles in resistance against various infectious agents including viruses, bacteria, and parasites.6,7 The deficiency of IL-12 in knock-out mice causes a severely depressed Th1 response, supporting the role of IL-12 in the Th1 response and resistance to infections.3,8,9 IL-12 (p70) is a heterodimer composed of 2 subunits, p40 and p35, which are encoded by 2 separate genes.10 In many cell types, p35 expression is induced by stimulation with pathogens or their components, such as Gram-negative bacterial lipopolysaccharide (LPS).11 p40 expression is also induced by stimulation of LPS, but is limited to macrophages, dendritic cells, B cells, and neutrophils, among which the first 2 cells are the primary sources of IL-12.1,12–14 The p40 protein also forms a heterodimer with a p35-related protein p19.15 The heterodimer p19/p40, known as IL-23, a member cytokine of the IL-12 family, also regulates Th1-cell responses.16–18 Thus, the p40 protein is considered to be an IL-12–specific subunit required for the functional expression of IL-12.
IFN consensus sequence binding protein (ICSBP), also designated IFN regulatory factor 8 (IRF-8), is a nuclear transcription factor belonging to the IRF family. ICSBP−/− mice cannot show Th1-mediated responses because of a deficiency in IL-12 production.19–21 Several previous studies indicate that ICSBP binds to the Ets site of the p40 promoter to up-regulate promoter activity in response to IFN-γ and LPS stimulation.22–24
We have analyzed the regulation mechanisms of hematopoietic cell development by transcription factors and found (1) cooperation between transcription factors PU.1 and GATA-1, and (2) repression of GATA-1–dependent transactivation by cofactor FOG-1, which causes mast cell–specific gene expression.25–28 In addition, the expression level of PU.1 determines the fate of cell differentiation between mast cells and monocytes.29–31 Although ICSBP is one of the partners of PU.1, the regulatory mechanism for the expression of ICSBP in mast cells and the involvement of ICSBP in the cell fate determination between mast cells and monocytes are unclear.
Mast cells play roles not only as effector cells in allergic disease but also as initiator cells of innate and acquired immune responses against various pathogens.32–35 The innate immune response is initiated by Toll-like receptors (TLRs). Recent reports have demonstrated that mast cells express TLRs and associate with innate immune responses against Gram-negative bacteria via TLR4, a signal transducer of LPS.36,37 In other reports, the mRNAs for the IL-12 components were detected in mouse bone marrow–derived mast cells (BMMCs)38 and in LPS-stimulated human mast cells.39 These observations suggest that mast cells produce IL-12 to contribute to innate immunity. However, IL-12 production in mast cells has not been well analyzed, and the relationship between IL-12 production in mast cells and innate immunity is unknown.
Here, we found that ICSBP is expressed in BMMCs in response to LPS, similar to macrophages and dendritic cells. We also demonstrated that IL-12 is inducibly produced from BMMCs upon LPS stimulation, and that this production increases in the presence of IFN-γ. In addition, we found that IL-12 produced from mast cells was involved in host protection in vivo using a mouse model of acute peritonitis with mast cell–deficient W/Wv mice and W/Wv mice reconstituted with BMMCs from p40-deficient mice.
Materials and methods
Mice
BALB/c, C57BL/6, WBB6F1+/+, and mast cell–deficient WBB6F1-W/Wv mice were purchased from Japan SLC (Hamamatsu, Japan). IL-12/23 p40-deficient (p40−/−) mice (B6.129S1-IL12btm1Jm/J) were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed according to the approved manual of the institutional review board of Juntendo University, Japan.
Preparation of BMMCs
BMMCs were generated from the femoral bone marrow cells of female mice as described previously.40 Cells were incubated in RPMI 1640 (Sigma-Aldrich, St Louis, MO) supplemented with 10% heat-inactivated FCS (Biological Industries, Haemek, Israel), 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μM 2-mercaptoethanol, 10 mM sodium pyruvate, 10 μM MEM nonessential amino acid solution (Invitrogen, Carlsbad, CA), 100 U/mL murine IL-3 (PeproTech EC, London, United Kingdom), and 0.5 U/mL murine stem cell factor (SCF; PeproTech EC) at 37°C in a humidified atmosphere in the presence of 5% CO2. After 4 to 5 weeks of culture, more than 96% of the cells were identifiable as mast cells as determined by toluidine blue staining and fluorescence-activated cell sorting (FACS) analysis of cell surface expression of c-Kit and FcϵRI.
FACS sorting of BMMCs
The 4-week–cultured BMMCs were stained with FITC-conjugated anti–mouse FcϵRIα (MAR-1; eBioscience, San Diego, CA) and PE-conjugated anti–mouse c-Kit (2B8; BD PharMingen, San Diego, CA) after blocking Fc receptors with 2.4G2 (BD PharMingen). Stained cells were sorted using a BD FACSAria Cell Sorter (Becton Dickinson, San Jose, CA) to obtain c-kit+/FcϵRIα+ cells. The purity of sorted cells was confirmed to be 99.8% of c-kit+/FcϵRIα+ cells by FACS analysis.
Peritoneal macrophages and macrophage cell line
Murine peritoneal macrophages (PEC-Mφs) were obtained from peritoneal exudate cells of female BALB/c mice as described previously.31 In brief, mice had been injected intraperitoneally with 2.5 mL thioglycollate broth (Sigma-Aldrich) 4 days previously. Peritoneal exudate cells were washed with ice-cold PBS twice and resuspended in RPMI 1640 containing 10% FCS, and plated on FCS-coated plates. After 1.5-hour incubation at 37°C in a humidified atmosphere in the presence of 5% CO2, nonadherent cells were removed and the remaining adherent cells collected. More than 98% of collected cells were identified to be macrophages by positive staining with anti-F4/80 (BM8; eBioscience) and anti-CD11b (M1/70; BD PharMingen) in FACS analysis. The murine macrophage cell line RAW264.7 was cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μM 2-mercaptoethanol, 10 mM sodium pyruvate, and 10 μM MEM nonessential amino acid solution.
Reverse-transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from either BMMCs or RAW264.7 cells using STAT-60 (Tel-Test, Friendswood, TX) according to the manufacturer's instructions. First-strand cDNA was constructed from 1 μg total RNA with oligo(dT)12-18 as a primer using Superscript II RNase H− reverse transcriptase (Life Technologies, Rockville, MD). PCR was performed using specific primer sets for mouse ICSBP (GenBank accession no. NM_008320: 5′-GCG TGG GAA CCG GCG GCA GGA TG and 5′-CTC AGG CGA GGT GGG GTG CCC GGA C),41 mouse p40 (accession no. M86671: 5′-ATG TGT CCT CAG AAG CTA ACC and 5′-CTA GGA TCG GAC CCT GCA GGG AAC), mouse p35 (accession no. M86672: 5′-ATG TGT CAA TCA CGC TAC CTC C and 5′-TCA GGC GGA GCT CAG ATA GCC),42 mouse p19 (accession no. AF301619: 5′-ATG CTG GAT TGC AGA GCA GTA ATA ATG C and 3′-TTA AGC TGT TGG CAC TAA GGG CTC),15 and species nonspecific GAPDH (5′-AGT ATG ACT CCA CTC ACG GCA A and 5′-TCT CGC TCC TGG AAG ATG GT).43
Quantitative PCR
Expression level of each mRNA was quantitatively analyzed by a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) with cDNA prepared as described above under “RT-PCR analysis” and TaqMan Universal Master Mix (Applied Biosystems). The expression level of ICSBP mRNA detected by TaqMan Gene Expression Assays (Mm00492567_m1) was shown as the ratio to that of GAPDH, which was determined with Rodent GAPDH Control Reagents (Applied Biosystems), by calculation of cycle threshold (Ct) values in amplification plots with a 7500 SDS software (Applied Biosystems).
Western blot analysis of ICSBP
A total of 2.5 × 106 cells of BMMCs or RAW264.7 was lysed by addition of 1 × sampling buffer (62.5 mM Tris-HCl [pH 6.8], 10% glycerol, 2% SDS, 0.1 mg/mL bromphenol blue dye, and 10% 2-mercaptoethanol). The cell lysates were electrophoretically resolved in 10% SDS–polyacrylamide gel and then transferred onto the PVDF membrane (Millipore, Bedford, MA). ICSBP and YY1 were probed by anti-ICSBP goat polyclonal antibody (C-19; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-YY1 mouse monoclonal antibody (H-10; Santa Cruz Biotechnology), respectively. Alexa Fluor 680–conjugated anti–goat IgG or anti–mouse IgG (Molecular Probes, Eugene, OR) was used as the secondary antibody. Infrared fluorescence on the membrane was detected by an infrared imaging system Odyssey (LI-COR Biosciences, Lincoln, NE).
Measurement of cytokine concentration
BMMCs or PEC-MΦs at a concentration of 2 × 105 cells in 150 μL complete medium were incubated in the presence or absence of 100 U/mL murine IFN-γ (PeproTech EC) for 16 hours, and then incubated with the indicated concentration of LPS (Escherichia coli serotype 0111:B4; Sigma-Aldrich) for an additional 24 hours. In the determination of the high-affinity IgE receptor (FcϵRI)–mediated p40 and p70 release, BMMCs were sensitized with 1 μg/mL anti-TNP IgE (BD PharMingen) for 1 hour on ice, washed, resuspended in complete medium at a concentration of 1 × 106 cells/mL, and then incubated in the presence or absence of 50 ng/mL TNP-BSA for 4 hours. In the blocking experiments, BMMCs were preincubated for 48 hours in the medium containing 10 μg/mL anti–mouse IFN-γ rat monoclonal antibody (mAb, R4-6A2; BD PharMingen) or control rat IgG (Sigma-Aldrich) before the cells were stimulated with IFN-γ and LPS. The levels of p40 and p70 in the culture supernatants were determined using a corresponding enzyme-linked immunosorbent assay (ELISA) kit (BD PharMingen) according to the manufacturer's instructions. The degranulation levels of the BMMCs were measured using the β-hexosaminidase release assay as previously described.31
Mast cell reconstitution in W/Wv mice
Mast cell deficiency of 5-week-old W/Wv mice was reconstituted by intraperitoneal injection of 2 × 106 BMMCs from C57BL/6 or p40−/− mice as previously described.33,37,40 The mice were used for experiments at 5 weeks after injection of BMMCs. Reconstitution of mast cells was confirmed by toluidine blue or Alcian blue and safranin staining of the cytospun preparation of peritoneal cells. Blood was collected from the tail into EDTA-coated microcapillary tubes (20-30 μL/sampling) and red cell counts were determined manually. The hematocrit level was determined after centrifugation of blood at 15 000g for 3 minutes.
Cecal ligation and puncture
Cecal ligation and puncture (CLP) was performed as previously described.37,44,45 In brief, mice were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital (Abbott Laboratories, Abbott Park, IL). The cecum was exposed by a 1-cm midline incision on the anterior abdomen and subjected to ligation of the distal half followed by a single puncture with a 0.9-mm needle. The cecum was then squeezed gently, replaced into the abdomen, and closed using a nylon suture for operation. Mice were observed for mortality every day over a period of 14 days.
Estimation of cytokine concentration in peritoneal fluids and serum
Peritoneal exudates were collected from CLP-induced mice at the indicated time points. The concentrations of cytokines in peritoneal fluids were determined by ELISA kits according to the manufacturer's instructions (BD PharMingen).
Bacterial counts in the peritoneal exudates
The number of bacteria in the peritoneal cavity was determined by previously described method.46 In brief, peritoneal fluids collected from mice, which were killed 6 hours after sepsis induction, were placed onto Mueller-Hinton agar dishes (Difco, Hunt Valley, MD). After 18-hour incubation at 37°C, colony-forming units (CFUs) were scored. The results were expressed as log10 of CFUs/mL.
Count of neutrophil migrated to the peritoneal cavity
Peritoneal exudates were collected from CLP-treated mice at 0 or 6 hours after sepsis induction, and total cell numbers were counted. Differential cell counts were conducted on cytospun preparations prepared from each fluid with the May-Grünwald-Giemsa staining (Muto Pure Chemicals, Tokyo, Japan). The results were expressed as the number of neutrophils per cavity.
IL-12 and IL-23 treatment
Statistical analysis
Statistical analysis of most data was performed using Student t test, and data are presented as mean ± SD. Statistical analysis of survival data in CLP experiments was performed using the log-rank test.
Results
ICSBP expression in BMMCs
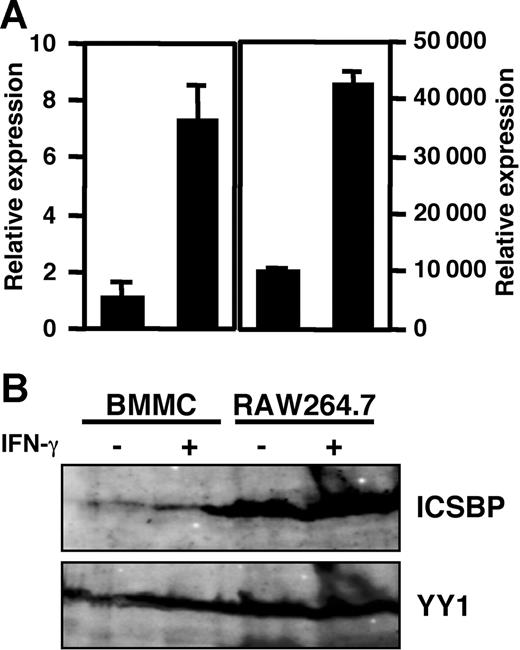
ICSBP, the expression of which is transactivated by IFN-γ stimulation, has been identified as a principal activator for expression of p40 of IL-12 in macrophages.22,23,49 Therefore, we first assessed the expression of the mRNA and protein of ICSBP in BMMCs by RT-PCR and immunoblotting, respectively, using RAW264.7 cells as a positive control.22,50 Quantitative analysis with real-time PCR (Figure 1A) showed that the gene encoding Icsbp was transcribed in BMMCs, and the mRNA level was increased by IFN-γ stimulation. Furthermore, by immunoblotting with specific antibody against ICSBP, the production of ICSBP, which was increased by IFN-γ stimulation, was detected in the lysate of BMMCs (Figure 1B). These results indicate that BMMCs express ICSBP and that the expression level is increased by IFN-γ stimulation.
ICSBP expression in BMMCs. (A) mRNA level of ICSBP determined by real-time PCR. The mRNA level of ICSBP was represented as the ratio to that of BMMCs without IFN-γ stimulation. Data represent mean ± SD of triplicate samples. (B) Western blotting analysis of ICSBP. BMMCs were incubated for 16 hours in the presence or absence of 100 U/mL IFN-γ. RAW264.7 cells, a mouse macrophage cell line, were used as the positive control. Total RNA (1 μg) was used for mRNA analysis and lysates of cells (2.5 × 106) were used for Western blotting. These results are representative of 3 independent experiments that had similar results.
ICSBP expression in BMMCs. (A) mRNA level of ICSBP determined by real-time PCR. The mRNA level of ICSBP was represented as the ratio to that of BMMCs without IFN-γ stimulation. Data represent mean ± SD of triplicate samples. (B) Western blotting analysis of ICSBP. BMMCs were incubated for 16 hours in the presence or absence of 100 U/mL IFN-γ. RAW264.7 cells, a mouse macrophage cell line, were used as the positive control. Total RNA (1 μg) was used for mRNA analysis and lysates of cells (2.5 × 106) were used for Western blotting. These results are representative of 3 independent experiments that had similar results.
Transcription of IL-12–related molecules in BMMCs
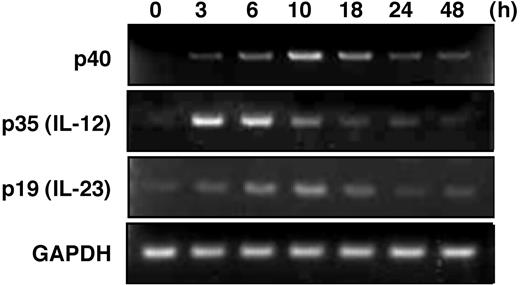
The p40 promoter is known to be transactivated by ICSBP, and the recruitment of ICSBP to a critical Ets site is enhanced by IFN-γ/LPS stimulation.22–24 Therefore, we hypothesized that BMMCs, which express ICSBP, could produce the IL-12 family cytokines, IL-12 and IL-23, in response to IFN-γ/LPS stimulation, as in the cases of macrophages and dendritic cells. To determine the capacity of mast cells to produce IL-12/23, we next assessed the transcriptions of component molecules p40, p35, and p19 in BMMCs by RT-PCR. We analyzed the mRNA levels for the components in IFN-γ–primed BMMCs at appropriate time points after stimulation with LPS (Figure 2). The level of p40 mRNA reached maximum at 10 hours after LPS stimulation. In contrast, a rapid increase in mRNA level peaking at 3 to 6 hours was observed for p35. The level of the p19 mRNA increased with a similar time course to p40, although the response was somewhat dull. These results indicate that the expression of the mRNA of IL-12/23–constituted molecules was induced by stimulation with LPS in BMMCs.
Transcription of IL-12–related molecules in LPS-stimulated BMMCs. RT-PCR analysis of p40, p35, and p19 after LPS stimulation for 0 to 48 hours. BMMCs were stimulated with 1 μg/mL LPS after the cells were preincubated with 100 U/mL IFN-γ for 16 hours. Equality of the RT reaction of isolated RNA was confirmed by amplification of the housekeeping gene GAPDH. These results are representative of 3 independent experiments that had similar results.
Transcription of IL-12–related molecules in LPS-stimulated BMMCs. RT-PCR analysis of p40, p35, and p19 after LPS stimulation for 0 to 48 hours. BMMCs were stimulated with 1 μg/mL LPS after the cells were preincubated with 100 U/mL IFN-γ for 16 hours. Equality of the RT reaction of isolated RNA was confirmed by amplification of the housekeeping gene GAPDH. These results are representative of 3 independent experiments that had similar results.
IL-12 secretion from BMMCs upon LPS stimulation
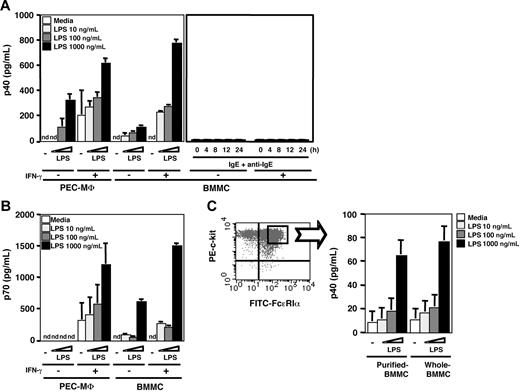
Transcription of both p40 and p35 was observed in LPS-stimulated BMMCs. Then, to examine the IL-12 secretion from mast cells, we measured the levels of p40 and p70 in culture supernatant by ELISA kits (Figure 3). As shown in Figure 3A, BMMCs secreted p40 by LPS stimulation in a dose-dependent manner, whereas unstimulated BMMCs had a p40 secretion level below the detection limit. The secretion levels from BMMCs upon LPS stimulation were greatly enhanced by IFN-γ priming and were comparable to those from PEC-MΦs upon LPS stimulation. Although mast cells express various cytokines such as IL-4, IL-5, IL-6, IL-13, and TNF-α by cross-linking with FcϵRI, a high-affinity IgE receptor, whether IL-12 production in mast cells was activated via FcϵRI was unknown. Therefore, we examined whether BMMCs secreted p40 by FcϵRI cross-linking. By stimulation with IgE antigen, the degranulation of BMMCs was confirmed by the β-hexosaminidase release assay51 (data not shown). However, p40 protein was not detected in the culture media irrespective of the presence or absence of IFN-γ. The p40 protein was also under a detectable level even at later time points (Figure 3A right). We next analyzed the secretion of IL-12 (p70) by LPS stimulation using an ELISA kit established to detect the p70 heterodimer. As shown in Figure 3B, IL-12 was secreted from BMMCs upon LPS stimulation in the same way as p40 secretion from the cells (Figure 3B), and the molarity rate of p40 and p70 was approximately 1:1. Although more than 96% of cells were judged to be have developed into mast cells after 4 weeks of culture of the bone marrow cells (“Materials and methods”), we could not completely exclude the possibility that monocytes and/or monocyte progenitors that had contaminated these bone marrow–derived cells were the source of the IL-12. In an effort to exclude this possibility, we further purified the BMMCs by sorting from 4-week–cultured BMMCs into c-Kit+/FcϵRI+ cells and then analyzing p40 production (Figure 3C). The sorted BMMCs displayed a p40 production profile by IFN-γ/LPS stimulation that was essentially the same as that of whole BMMCs without sorting. These results indicate that BMMCs are capable of secretion of IL-12 in response to LPS, which is similar to macrophages.
IL-12 secretion from BMMCs upon LPS stimulation. ELISA analysis of (A) p40 and (B) p70 in the culture media of cells (2 × 105 cells/150 μL/well) that were incubated with the indicated concentration of LPS for 24 hours after preincubation in the presence or absence of 100 U/mL IFN-γ for 16 hours. To activate BMMCs via FcϵRI, BMMCs were incubated in the presence or absence of 50 ng/mL TNP-BSA for 4 hours (left) or indicated time from 0 to 24 hours (right) after sensitization with 1 μg/mL anti-TNP IgE for 1 hour (A). Murine PEC-MΦs were used as the positive control. (C) Comparison of p40 production level between purified BMMCs by sorting of c-Kithigh/FcϵRIαhigh and nonsorted BMMCs. Both purified and whole BMMCs (1 × 105 cells/150 μL/well) were incubated with the indicated concentration of LPS for 24 hours after preincubation in the presence of 100 U/mL IFN-γ for 16 hours. Data represent mean ± SD of 3 independent experiments. nd indicates not detected (detection limit is 4 pg/mL).
IL-12 secretion from BMMCs upon LPS stimulation. ELISA analysis of (A) p40 and (B) p70 in the culture media of cells (2 × 105 cells/150 μL/well) that were incubated with the indicated concentration of LPS for 24 hours after preincubation in the presence or absence of 100 U/mL IFN-γ for 16 hours. To activate BMMCs via FcϵRI, BMMCs were incubated in the presence or absence of 50 ng/mL TNP-BSA for 4 hours (left) or indicated time from 0 to 24 hours (right) after sensitization with 1 μg/mL anti-TNP IgE for 1 hour (A). Murine PEC-MΦs were used as the positive control. (C) Comparison of p40 production level between purified BMMCs by sorting of c-Kithigh/FcϵRIαhigh and nonsorted BMMCs. Both purified and whole BMMCs (1 × 105 cells/150 μL/well) were incubated with the indicated concentration of LPS for 24 hours after preincubation in the presence of 100 U/mL IFN-γ for 16 hours. Data represent mean ± SD of 3 independent experiments. nd indicates not detected (detection limit is 4 pg/mL).
Effect of IFN-γ released from BMMCs on IL-12 and ICSBP production
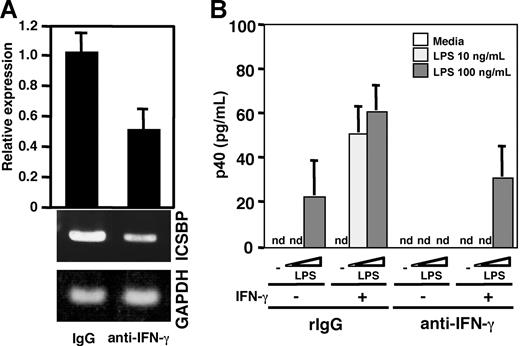
As described in Figure 3, BMMCs secreted p40 and p70 by LPS stimulation even in the absence of IFN-γ priming. We presumed that IFN-γ supplied by the BMMCs themselves could be a major cause of the apparent lack of necessity of IFN-γ priming, as mast cells are known to produce IFN-γ and this IFN-γ production is increased by IL-12 treatment.52 Indeed, an ELISA revealed that IFN-γ was produced in the medium of BMMCs (data not shown). When neutralizing anti–mouse IFN-γ mAb was added into the culture medium, the mRNA level of ICSBP in the BMMCs was markedly decreased to approximately 50%, compared with that of BMMCs treated with control IgG (Figure 4A). Moreover, p40 production in response to LPS stimulation was markedly reduced in BMMCs pretreated with anti–IFN-γ Ab and was partly recovered by addition of IFN-γ into the medium (Figure 4B). These results indicate that IFN-γ contributed to the production of ICSBP and IL-12 in BMMCs.
Contribution of IFN-γ to IL-12 production in BMMCs upon LPS stimulation. (A) Real-time PCR (top) and RT-PCR analysis (bottom) of ICSBP mRNA from BMMCs that were incubated with 100 U/mL IFN-γ for 16 hours after the cells were treated with 10 μg/mL control rat IgG or anti–IFN-γ antibody for 48 hours. These results are representative of 3 independent experiments that had similar results. (B) ELISA analysis of p40 production from control IgG– or anti–IFN-γ antibody–treated BMMCs. The cells were incubated with 10 μg/mL control rat IgG or anti–IFN-γ antibody for 48 hours. Then the cells were incubated with the indicated concentration of LPS for 24 hours after the cells had been preincubated in the presence or absence of 100 U/mL IFN-γ for 16 hours. Data represent mean ± SD of 3 independent experiments. nd indicates not detected.
Contribution of IFN-γ to IL-12 production in BMMCs upon LPS stimulation. (A) Real-time PCR (top) and RT-PCR analysis (bottom) of ICSBP mRNA from BMMCs that were incubated with 100 U/mL IFN-γ for 16 hours after the cells were treated with 10 μg/mL control rat IgG or anti–IFN-γ antibody for 48 hours. These results are representative of 3 independent experiments that had similar results. (B) ELISA analysis of p40 production from control IgG– or anti–IFN-γ antibody–treated BMMCs. The cells were incubated with 10 μg/mL control rat IgG or anti–IFN-γ antibody for 48 hours. Then the cells were incubated with the indicated concentration of LPS for 24 hours after the cells had been preincubated in the presence or absence of 100 U/mL IFN-γ for 16 hours. Data represent mean ± SD of 3 independent experiments. nd indicates not detected.
Mast cells are a source of p40 in a model of acute septic peritonitis
We had confirmed that mast cells produce IL-12 by LPS stimulation in vitro. To evaluate the involvement of mast cells as a source of IL-12 in vivo, we analyzed the cytokine production levels in peritoneal fluids of mice with CLP, a mouse model of acute septic peritonitis. Peritoneal mast cells of mast cell–deficient W/Wv mice were reconstituted with BMMCs either from wild-type (WT) C57BL/6 or p40−/− mice. The reconstitution of peritoneal mast cells derived from W/Wv mice that received BMMCs from WT mice and p40−/− mice was confirmed by positive staining with both Alcian blue and safranin, a feature of connective tissue–type mast cells (data not shown). In addition, we determined hematocrit value and red cell count of each mouse to analyze whether non–mast cell hematopoietic reconstitution initiated by pluripotential c-kit+ progenitors, which may be contaminated in BMMC preparations, occurred in this reconstitution model. W/Wv mice were confirmed to be anemic, with low hematocrit value and red cell count compared with WBB6F1+/+ mice (Table 1). Reconstitutions with WT-BMMCs and with p40−/− BMMCs had no positive effect on hematocrit value and red cell count (Table 1), suggesting that protective effects by non–mast cell hematopoietic reconstitution were not apparently observed in this experiment.
Hematocrit values and red blood cell count of WBB6F1+/+, WBB6F1+/+-W/Wv, W/Wv reconstituted with WT-BMMCs, and W/Wv reconstituted with p40−/− BMMCs
| . | WBB6F1+/+ . | W/Wv . | W/Wv+ WT-BMMCs . | W/Wv+ p40−/− BMMCs . |
|---|---|---|---|---|
| Hct, proportion of 1.0 | 0.493 ± 0.012 | 0.377 ± 0.006† | 0.327 ± 0.084* | 0.360 ± 0.026† |
| Red cell count, × 1012/L | 8.80 ± 1.02 | 2.62 ± 0.31* | 2.61 ± 0.17† | 2.46 ± 0.25* |
| . | WBB6F1+/+ . | W/Wv . | W/Wv+ WT-BMMCs . | W/Wv+ p40−/− BMMCs . |
|---|---|---|---|---|
| Hct, proportion of 1.0 | 0.493 ± 0.012 | 0.377 ± 0.006† | 0.327 ± 0.084* | 0.360 ± 0.026† |
| Red cell count, × 1012/L | 8.80 ± 1.02 | 2.62 ± 0.31* | 2.61 ± 0.17† | 2.46 ± 0.25* |
Mean (± SD) of 3 mice was analyzed each group.
Values or counts in W/Wv mice with and without reconstitution statistically different from those in WBB6F1+/+ mice are indicated with footnote symbols. There are no significant differences among WBB6F1+/+-W/Wv, W/Wv reconstituted with WT-BMMCs, and W/Wv reconstituted with p40−/− BMMCs.
P < .05.
P < .005.
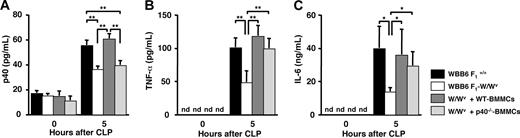
As shown in Figure 5A, a 35% reduction in p40 levels in peritoneal fluids was observed for unreconstituted W/Wv mice compared with congeneric normal WBB6F1+/+ mice, by 5-hour incubation after CLP induction. The reduction in p40 levels of W/Wv mice was not recovered by reconstitution with p40−/− BMMCs, but recovered by reconstitution with WT-BMMCs. In the serum, the p40 levels were reduced by 39% for unreconstituted W/Wv and W/Wv mice reconstituted with p40−/− BMMCs when compared with WBB6F1+/+ and W/Wv mice reconstituted with WT-BMMCs (data not shown). These observations indicate that mast cells are a source of p40 in this model. Previous studies demonstrated that an increase in peritoneal TNF-α level is critical for neutrophil recruitment and bacterial clearance following CLP and that mast cells are an important source of peritoneal TNF-α.33,44,53 IL-6 produced by mast cells is also an important cytokine in local inflammatory reactions by amplifying leukocyte recruitment.37,54 Therefore, we determined the TNF-α and IL-6 levels by CLP induction. By 5 hours of incubation after CLP induction, the levels of TNF-α (Figure 5B) and IL-6 (Figure 5C) in the peritoneal fluids of W/Wv mice reconstituted with p40−/− BMMCs were the same as those of WBB6F1+/+ and W/Wv mice reconstituted with WT-BMMCs, whereas that of unreconstituted W/Wv was markedly lower (50% or less of WBB6F1+/+ and W/Wv mice reconstituted with WT-BMMCs). These observations indicate that the reductions in TNF-α and IL-6 levels in the peritoneal cavity of W/Wv mice by CLP were recovered by reconstitution both with WT-BMMCs and p40−/− BMMCs, suggesting no role of IL-12 in TNF-α and IL-6 production.
Concentration of cytokines in the peritoneal fluids of wild-type, mast cell–deficient, and mast cell–reconstituted mice of acute septic peritonitis. Peritonitis was induced in WBB6 F1+/+ (wild type; closed bar), WBB6 F1-W/Wv (mast-cell deficient; open bar), wild-type BMMC–reconstituted WBB6 F1-W/Wv (hatched bar), and p40−/− BMMC–reconstituted WBB6 F1-W/Wv mice (dotted bar) by CLP. Peritoneal exudates were collected at the indicated time (0 or 5 hours) after CLP induction. Levels of (A) p40, (B) TNF-α, and (C) IL-6 in the peritoneal fluids were determined using ELISA kits. Data represent mean ± SD of 5 mice. *P < .05; **P < .005, significantly different from the mean value of the corresponding control. nd indicates not detected.
Concentration of cytokines in the peritoneal fluids of wild-type, mast cell–deficient, and mast cell–reconstituted mice of acute septic peritonitis. Peritonitis was induced in WBB6 F1+/+ (wild type; closed bar), WBB6 F1-W/Wv (mast-cell deficient; open bar), wild-type BMMC–reconstituted WBB6 F1-W/Wv (hatched bar), and p40−/− BMMC–reconstituted WBB6 F1-W/Wv mice (dotted bar) by CLP. Peritoneal exudates were collected at the indicated time (0 or 5 hours) after CLP induction. Levels of (A) p40, (B) TNF-α, and (C) IL-6 in the peritoneal fluids were determined using ELISA kits. Data represent mean ± SD of 5 mice. *P < .05; **P < .005, significantly different from the mean value of the corresponding control. nd indicates not detected.
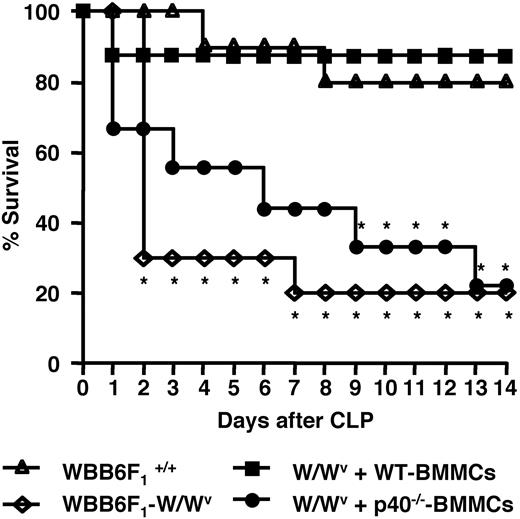
W/Wv mice reconstituted with p40−/− BMMCs exhibit decreased survival compared with WT-BMMC–reconstituted mice
IL-12 plays multiple important roles in bacterial clearance and survival in CLP-mediated polymicrobial infections.55–57 We examined the effects of p40 deficiency in mast cells on survival after CLP. As shown in Figure 6, in CLP-induced acute septic peritonitis, the W/Wv mice reconstituted with p40−/− BMMCs showed a significantly higher mortality rate than the WBB6F1+/+ mice and the W/Wv mice reconstituted with WT-BMMCs, whereas the W/Wv mice without reconstitution with BMMCs showed the highest mortality rate. At 14 days after CLP, 80% of the W/Wv mice reconstituted with p40−/− BMMCs had died, whereas the WBB6F1+/+ mice and the W/Wv mice that carried mast cells expressing IL-12 showed an approximately 80% survival rate. This indicates that IL-12 produced by mast cells contributes to host survival in this model.
Defect in p40 production in mast cells leads to decreased survival following CLP. Survival rates of WBB6 F1+/+ mice (10 mice/group; ▵), W/Wv mice (10 mice/group; ◇), and W/Wv mice reconstituted with wild-type BMMCs (8 mice/group; ■) or p40−/− BMMCs (9 mice/group; ●) following CLP induction are shown. Statistical analysis was performed using the log rank test. *P < .05, significantly different from the WBB6 F1+/+ mice group. These results are representative of 3 independent experiments that had similar results.
Defect in p40 production in mast cells leads to decreased survival following CLP. Survival rates of WBB6 F1+/+ mice (10 mice/group; ▵), W/Wv mice (10 mice/group; ◇), and W/Wv mice reconstituted with wild-type BMMCs (8 mice/group; ■) or p40−/− BMMCs (9 mice/group; ●) following CLP induction are shown. Statistical analysis was performed using the log rank test. *P < .05, significantly different from the WBB6 F1+/+ mice group. These results are representative of 3 independent experiments that had similar results.
Effects of IL-12 produced from mast cells on bacterial clearance–related factors
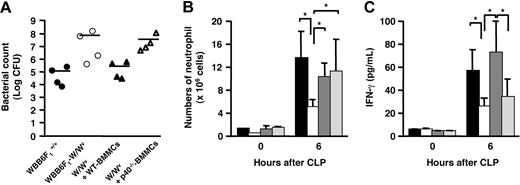
To elucidate the role of IL-12 produced from mast cells in host survival, we analyzed the bacterial counts in the peritoneal cavity of mice after CLP surgery. The CFU in peritoneal exudates of W/Wv mice was significantly higher than that of WBB6F1+/+ mice (Figure 7A). The reduced bacterial clearance of W/Wv mice was normalized by the reconstitution with WT-BMMCs, whereas the reconstitution with p40−/− BMMCs partially restored the reduced bacterial clearance, which was observed in W/Wv (Figure 7A). We then investigated the neutrophil recruitment to the peritoneal cavity, because neutrophils rapidly migrate toward the infection site and play important roles in host defense by releasing a variety of microbicidal mediators. W/Wv mice showed an impaired neutrophil migration compared with WBB6F1+/+ mice (Figure 7B). The neutrophil migration was restored to the level comparable to that of WT mice by WT-BMMC or p40−/− BMMC reconstitution (Figure 7B). This result coincides with the above data that the concentration of TNF-α in the peritoneal cavity (Figure 5C), which is critical for neutrophil migration, was comparable among WBB6F1+/+, WT-BMMC–, and p40−/− BMMC–reconstituted mice. Although the reconstitution with p40−/− BMMCs completely restored the TNF-α level and subsequent neutrophil recruitment, bacterial clearance and host survival were still impaired by the same reconstitution. IL-12 is critical for IFN-γ production, which activates neutrophils to show phagocytic and microbicidal activity and NO synthesis.46 Therefore, we measured IFN-γ concentration in the peritoneal cavity to examine a possibility that the lesser amount of IFN-γ in p40−/− BMMC–reconstituted mice resulted in impaired activation of neutrophil. The concentration of IFN-γ in the peritoneal cavity of W/Wv mice at 6 hours after CLP was significantly lower than that of WBB6F1+/+ (Figure 7C). The reduction of IFN-γ production level in W/Wv mice was completely recovered by the reconstitution with WT-BMMCs. In contrast, the reconstitution with p40−/− BMMCs did not restore the peritoneal IFN-γ level of W/Wv mice (Figure 7C). These results indicate that the absence of IL-12 causes impaired production of IFN-γ at the infection site, leading to reduced activation of neutrophils with normally migrated count, which then results in lower activity of bacterial clearance.
Bacterial counts, neutrophil migration, and IFN-γ concentration in the peritoneal cavity. (A) Bacterial counts in the peritoneal fluids collected from CLP-treated mice at 6 hours after CLP surgery. WBB6 F1+/+ (wild type; ●), WBB6 F1-W/Wv (mast-cell deficient; ○), wild-type BMMC–reconstituted WBB6 F1-W/Wv (▴), and p40−/− BMMC–reconstituted WBB6 F1-W/Wv mice (▵). Results are expressed as log10 of CFU per cavity (n = 4 mice). Horizontal bar represents the mean. (B) Neutrophil migration into the peritoneal cavity of CLP-treated mice at 0 or 6 hours after CLP surgery. WBB6 F1+/+ (■), WBB6 F1-W/Wv (□), wild-type BMMC–reconstituted WBB6 F1-W/Wv (hatched bar), and p40−/− BMMC–reconstituted WBB6 F1-W/Wv mice (dotted bar) in panels B-C. Data represent mean ± SD of 5 mice and *P < .05; significantly different from the mean value of the corresponding control in panels B-C. (C) Concentration of IFN-γ in the peritoneal cavity of CLP-treated mice at 0 or 6 hours after CLP surgery.
Bacterial counts, neutrophil migration, and IFN-γ concentration in the peritoneal cavity. (A) Bacterial counts in the peritoneal fluids collected from CLP-treated mice at 6 hours after CLP surgery. WBB6 F1+/+ (wild type; ●), WBB6 F1-W/Wv (mast-cell deficient; ○), wild-type BMMC–reconstituted WBB6 F1-W/Wv (▴), and p40−/− BMMC–reconstituted WBB6 F1-W/Wv mice (▵). Results are expressed as log10 of CFU per cavity (n = 4 mice). Horizontal bar represents the mean. (B) Neutrophil migration into the peritoneal cavity of CLP-treated mice at 0 or 6 hours after CLP surgery. WBB6 F1+/+ (■), WBB6 F1-W/Wv (□), wild-type BMMC–reconstituted WBB6 F1-W/Wv (hatched bar), and p40−/− BMMC–reconstituted WBB6 F1-W/Wv mice (dotted bar) in panels B-C. Data represent mean ± SD of 5 mice and *P < .05; significantly different from the mean value of the corresponding control in panels B-C. (C) Concentration of IFN-γ in the peritoneal cavity of CLP-treated mice at 0 or 6 hours after CLP surgery.
Effect of adoptive transfer of WT-BMMCs on survival of CLP-treated p40−/− mice
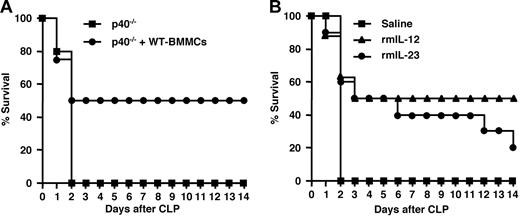
The p40-deficient mice have defects in clearing polymicrobial infections in CLP experiments.46 To evaluate mast cells as a source of IL-12, we compared the survival rates of p40−/− mice with and without adoptive transfer of WT-BMMCs in the CLP experiment. The survival rate of p40−/− mice exposed to CLP was 0% at 2 days after surgery (Figure 8A). In contrast, p40−/− mice transferred with WT-BMMCs by intraperitoneal injection in the same way as reconstitution of W/Wv showed 50% survival rate at 14 days after surgery (Figure 8A). This result suggests that mast cells function as a source of p40, which partially recovers the p40 deficiency of the knock-out mice.
Effects of mast cells, IL-12, and IL-23 on survival after CLP. (A) Effect of transplantation of WT-BMMCs on survival of p40−/− mice exposed to CLP. Survival rates of p40−/− mice (5 mice/group; ■), and p40−/− mice that received a transplant of WT-BMMCs (4 mice/group; ●) after CLP induction are shown. (B) Effect of IL-12 and IL-23 on survival of W/Wv mice exposed to CLP. Survival rates of W/Wv mice with intraperitoneal saline injection (5 mice/group; ■), W/Wv mice with intraperitoneal murine IL-12 injection (8 mice/group; ▴), and W/Wv mice with intraperitoneal murine IL-23 injection (10 mice/group; ●) following CLP induction are shown.
Effects of mast cells, IL-12, and IL-23 on survival after CLP. (A) Effect of transplantation of WT-BMMCs on survival of p40−/− mice exposed to CLP. Survival rates of p40−/− mice (5 mice/group; ■), and p40−/− mice that received a transplant of WT-BMMCs (4 mice/group; ●) after CLP induction are shown. (B) Effect of IL-12 and IL-23 on survival of W/Wv mice exposed to CLP. Survival rates of W/Wv mice with intraperitoneal saline injection (5 mice/group; ■), W/Wv mice with intraperitoneal murine IL-12 injection (8 mice/group; ▴), and W/Wv mice with intraperitoneal murine IL-23 injection (10 mice/group; ●) following CLP induction are shown.
Effects of IL-12 and IL-23 on survival of CLP-treated W/Wv mice
The p40 protein is also a constituent of IL-23 when forming a complex with a p35-related protein, p19. Because involvement of IL-23 in host defense against infectious diseases is also reported,18,58 we examined the effects of recombinant murine IL-12 and IL-23 on survival rates of CLP-treated W/Wv mice. When W/Wv mice were injected intraperitoneally with IL-12 at every 5 days after CLP surgery, 50% of mice were alive after 14 days, whereas all W/Wv mice without cytokine treatment were dead at 2 days after surgery (Figure 8B). When W/Wv mice were injected intraperitoneally with IL-23, the survival rate was the same as that of mice that had been injected with IL-12 cytokine for 5 days; the rate fell to 20% after 14 days (Figure 8B). This result suggests that defect of host survival due to the absence of mast cells is partially rescued by infection of recombinant IL-12 and IL-23.
Discussion
In this study, we demonstrated that mouse BMMCs produce IL-12–related molecules, in response to LPS, and that this production is augmented by preincubation of the cells with IFN-γ. Furthermore, in an acute peritonitis model, we showed that mast cells serve as a source of IL-12 to contribute to bacterial clearance. These observations indicate a novel role for mast cells in innate immune responses to bacterial infections.
In the transcription of p40 induced through TLR4-mediated stimulation by LPS, ICSBP is an essential transcription regulatory factor in macrophages, B lymphocytes, and activated T lymphocytes, and the expression of ICSBP is increased by IFN-γ in these cells.22,23,59 In the present study, we revealed that the ICSBP gene was transcribed in BMMCs and the expression was increased by IFN-γ treatment (Figure 1). We also found that BMMCs inducibly expressed mRNAs for IL-12–component molecules by stimulation with LPS (Figure 2). Indeed, the mRNAs were translated into the proteins of both p40 and IL-12p70 in the culture media of BMMCs (Figure 3). It should be noted that the response to LPS stimulation and the amount of p40 and IL-12 p70 proteins in BMMCs were almost similar to those of macrophages (Figure 3). The molarity rate of the p40 and p70 was approximately 1:1, suggesting that the common p40 molecule was used mainly for formation of IL-12p70 and that a small amount of IL-23 may be produced in BMMCs. The IL-12 production by BMMCs in response to LPS was augmented by preincubation with IFN-γ. This observation is consistent with the enhancement of ICSBP expression by preincubation with IFN-γ (Figure 1), because IL-12 production is enhanced by ICSBP. The BMMCs produced the IL-12 in response to LPS. However, stimulation with cross-linking of FcϵRI did not induce production of IL-12. These data indicate that a transcription signal(s), which is activated through cross-linking of FcϵRI, is not transmitted to transactivate the p40 promoter, even in the presence of ICSBP. Although ICSBP is necessary for the transcription of p40 gene,60 ICSBP is not a sole factor to activate the transcription of this gene. In brief, ICSBP has been shown to form a complex with IRF-123,24 or NFAT22 to transactivate the p40 promoter in response to LPS stimulation in macrophages. NFκB activation, which is mediated by IFN-γ and LPS, involves transactivation of the p40 promoter via an element (−122/−132) close to the ICSBP-binding site (ISRE sequence around −75/−54).61 Activation of NFκB and NFAT is induced by FcϵRI cross-linking in mast cells,62 and induction of IRF-1 expression is usually found with IFN-γ stimulation. Therefore, the reason for no p40 expression by FcϵRI cross-linking is unknown at present. Further detailed analysis revealing the difference between TLR-4– and FcϵRI-mediated activation in mast cells is required to elucidate the transcription factor(s) essential for the activation of the p40 promoter in mast cells.
Mast cells play a prominent role in the early immune response to invading pathogenic bacteria.32–35 The mast cells secrete various mediators including TNF-α, IL-6, and IL-1β when they encounter bacteria; this involves direct interactions with opsonized bacteria and TLR4-mediated interactions with bacterial LPS.37,63 These cytokines produced by mast cells contribute to bacterial clearance by recruiting leukocytes to the inflammatory region. In the early immune response to bacteria, IL-12 also plays a critical role.14 The roles of IL-12 on resistance to infections are to augment the production of IFN-γ and other cytokines from NK and T cells, to enhance the cytolytic activity of cytotoxic T lymphocytes (CTLs) and NK cells, and to stimulate the proliferation of activated NK and T cells.6 Although in a few previous studies transcription of IL-12 had been observed in mast cells in vitro,38,39 the biologic significance of mast cell–derived IL-12 had not been previously elucidated. Therefore, we investigated whether mast cells play a role as a source of IL-12 in vivo and contribute to bacterial clearance using a model of CLP-induced acute septic peritonitis in mast cell–deficient WBB6F1-W/Wv mice and a model of mast cell knock-in mice. In this investigation, the levels of p40, TNF-α, and IL-6 in the peritoneal fluids of W/Wv mice after CLP were significantly lower than those of WBB6F1+/+ mice. The lower cytokine production in the W/Wv mice after CLP was improved to the same level of that in WBB6F1+/+ mice by reconstitution with WT-BMMCs into the peritoneal cavity. In contrast, reconstitution with p40−/− BMMCs into the peritoneal cavity led to the levels of TNF-α and IL-6, but not p40, in W/Wv mice after CLP was improved to the same levels as in WBB6F1+/+ mice (Figure 5). These observations clearly indicate that p40 produced by mast cells is also involved in the survival of mice in the CLP model. During sepsis induced by polymicrobial infections, IL-12 plays important roles in survival and bacterial clearance, as IL-12 administration enhances host resistance and IL-12 neutralization is detrimental in several bacterial infection models.55,56 In CLP-induced acute septic peritonitis, lack of p40 production from mast cells leads to mortality (Figure 6). Bacterial count in the peritoneal cavity at 6 hours after CLP surgery was decreased by reconstitution of WT-BMMCs but not by p40−/− BMMCs (Figure 7A). These observations clearly indicate that IL-12 produced from mast cells plays a crucial role in bacterial clearance for survival. Reduced neutrophil migration in mast cell–deficient mice was restored by reconstitution with p40−/− BMMCs as well as WT-BMMCs (Figure 7B). This result coincides with the completely rescued TNF-α level, which is known to be critical for neutrophil recruitment,33 in p40−/− BMMC–reconstituted mice (Figure 5B). In contrast, IFN-γ level, which is an important factor for neutrophil activation,46 is not recovered by reconstitution of p40−/− BMMCs (Figure 7C). Therefore, we conclude that IL-12 produced from mast cells affects IFN-γ production level at infection site, which is essential for neutrophil activation, and induces subsequent bacterial clearance and survival of host.
Reduced survival rate of p40 knock-out mice is partially rescued by adoptive transfer of WT-BMMCs (Figure 8A), also suggesting that mast cells are one of the important sources of p40. However, since the survival rate is not completely normalized by the transfer, the role of mast cells as a source of IL-12 may be distinguished from those of macrophages and dendritic cells. Alternatively, the transferred mast cells could not fully recover the production of enough IL-12 in the peritoneal cavity of IL-12–deficient mice. In addition, treatment of IL-12 partially rescued the reduced survival rate of mast cell–deficient mice (Figure 8B). This observation indicates that deficiency of mast cells is not fully complemented by presence of IL-12, and that TNF-α produced from mast cells is one of the candidates to explain the partial recovery of mast cell functions. In brief, the lack of TNF-α produced from mast cells probably causes the impaired migration of neutrophils, which results in the partial rescue against infection even in the presence of IL-12. Although we have injected 25 ng recombinant IL-12 according to the previous reports, higher dose of IL-12 might be required for full rescue in this experimental condition. In addition, IFN-γ level affected by the IL-12 produced from mast cells is suggested to be critical for bacterial clearance in this study. Measurement of IFN-γ concentration in the peritoneal cavity and/or injection of recombinant IFN-γ in CLP experiments using W/Wv and p40−/− mice may be helpful to further clarify the correlation between IFN-γ level and protective effect. Regardless, further detailed analysis is required to clarify the difference between IL-12 produced from mast cells and from others.
In infectious diseases, IL-23 also plays an important role in host defense, although this role(s) has not been fully clarified.18,58 We were unable to define the presence and/or the amount of IL-23 in the culture media of mast cells because of the absence of an IL-23 ELISA system, and, therefore do not rule out the involvement of IL-23 produced by mast cells in the resistance to CLP-induced acute septic peritonitis in this investigation. In CLP experiment, IL-23 treatment partially rescues survival rate of W/Wv mice (Figure 8B), suggesting that IL-23 may have a protective effect on the bacterial clearance reduced by mast cell deficiency. Further study is required to elucidate the involvement of IL-23. In any case, this study demonstrates that mast cells as well as dendritic cells and macrophages are an important source of p40 during polymicrobial infections, and that they contribute to bacterial clearance through the production of p40. Although mast cells had been characterized as a source of Th2 cytokines such as IL-4, IL-5, and IL-13, our results indicate that mast cells have bilateral characteristics that are able to give the signal to induce the production of either Th1 or Th2 cytokines depending on the environment of the cells. This hypothesis is not only important for understanding innate immune responses to bacterial infections, but is also useful in clarifying the roles of mast cells in other skin diseases caused by Th1 dominancy, including psoriasis and contact sensitivity, in which p40 plays a pivotal role in the pathogenesis,64,65 and in the infiltration of mast cells in skin lesions.66
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by a Grant-in-aid for Scientific Research (c) (C.N.) and a Grant-in-aid for Young Scientists (N.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We are grateful to members of the Atopy Research Center, Department of Immunology, and Department of Dermatology for helpful discussions, especially Drs H. Yagita and S. Kamijo (Department of Immunology), Dr A. Nakao (Department of Immunology, Faculty of Medicine, University of Yamanashi), and Drs K. Maeda and T. Kuhara (Atopy Research Center). We thank Drs H. Kawada, T. Ito, A. Takagi, W. Ng, and T. Fukai as well as Ms K. Fukuyama, Mr H. Yokoyama, Ms Q. Wang, Ms M. Hara, and Ms T. Tokura for their technical support, and Ms M. Matsumoto for secretarial assistance.
Authorship
Contribution: N.N. performed research, analyzed data, and wrote the paper; S.K., Y.N., and N.S. performed research; H.U. contributed analytical tools; M.N. designed research and wrote the paper; K.O. and H.O. designed research; and C.N. designed research, performed research, and the wrote paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chiharu Nishiyama, Atopy (Allergy) Research Center, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo, 113-8421, Japan; e-mail: chinishi@med.juntendo.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal