Abstract
The generation of photoproducts of psoralen (POPs) might be relevant in cell death induced by psoralen plus UVA, namely PUVA, which is a recognized effective treatment for cutaneous T-cell lymphoma, chronic graft-versus-host disease, and psoriasis. We investigated the occurrence of POP-induced cell death and the underlying mechanisms. POPs were produced by irradiating a psoralen solution with UVA. Jurkat cells treated in the dark with these mixtures died mainly through an apoptotic mechanism. POPs were separated by high-performance liquid chromatography (HPLC), and cells were added with each of these fractions. A total of 2 dimers of psoralen and 6-formyl-7-hydroxycoumarin (FHC) were identified in the apoptogenic fractions. Apoptosis was preceded by mitochondrial dysfunction caused by the opening of the mitochondrial permeability transition pore (PTP). In fact, both mitochondrial depolarization and cell death were prevented by the PTP inhibitor cyclosporin A (CsA). PTP opening was also documented in isolated mitochondria added with POP, suggesting that apoptosis is caused by a direct effect of POP on mitochondria. In fact, FHC alone induced PTP opening and CsA-inhibitable cell death of Jurkat cells, whereas nontransformed T lymphocytes were resistant. Along with identifying novel apoptogenic molecules, the present results indicate that POP generation directs transformed cells to apoptosis.
Introduction
The combination of psoralens and UVA irradiation, which is commonly referred to as PUVA, represents a therapeutic approach useful in the treatment of cutaneous T-cell lymphoma (CTCL)1 as well as skin diseases such as psoriasis and vitiligo.2 In particular, the combination of interferon α-2a with oral photochemotherapy (PUVA) resulted in 70% complete remission in patients with CTCL.1 A modified PUVA protocol is called extracorporeal photopheresis. This treatment involves the mechanical separation of mononuclear white cells, which are treated with psoralen and exposed to UVA light, and then returned to the patient.3 Besides CTCL, extracorporal photopheresis is a recognized effective treatment modality in chronic graft-versus-host disease.4
Different mechanisms have been related to PUVA cytotoxicity.5 Besides the generation of reactive oxygen species, psoralen has been shown to bind covalently to and cross-link DNA.6 Laskin and coworkers demonstrated that mammalian cells have specific high-affinity receptor sites for psoralens, distinct from DNA, but their molecular characterization has been not elucidated yet.7 More recently, PUVA treatment has been reported to cause cell death by apoptosis,8 and we have demonstrated that the underlying mechanism involves mitochondrial dysfunction caused by opening of the permeability transition pore (PTP).9 On the other hand, upon UVA irradiation, psoralen photoproducts (POPs) are generated, which are likely to contribute to PUVA cytotoxicity.10 In fact, POP were reported to elicit a wide variety of effects, such as oxidation of proteins and unsaturated lipids, modulation of the immunologic response,10,11 and inhibition of the growth of an EL4 tumor, which represents an experimental murine model of human CTCL.11
The effect of the exposure to light of a photosensitizer prior its use in biological systems was also referred to as “preactivation” by Gulliya et al.12–14 In the case of photoactive compounds that were irradiated with visible light, such as merocyanine 540, naphthalimide, and Photofrin II, the “preactivation” protocol was successfully exploited for antiviral and antitumor therapeutic approaches.15–18
Here we show that POPs caused apoptosis in Jurkat cells, thus highlighting the relevance of long-lasting toxic molecules generated by psoralen photodegradation.9 The apoptogenic potency of the mixture was reduced—but not suppressed—by irradiating the psoralen solution in the absence of oxygen, suggesting that both oxygen-dependent and oxygen-independent reactions are relevant during the process of POP generation.9
The present study was aimed at both investigating the mechanism of cell death induced by POP treatment and identifying the novel apoptogenic compounds which are generated by the photodegradation of psoralen. Among POPs, 3 cytotoxic molecules were purified and characterized by nuclear magnetic resonance (NMR) and mass spectrometry (MS), namely a pyrone-pyrone and a furan-pyrone dimer of psoralen, and 6-formyl-7-hydroxycoumarin (FHC). Furthermore, apoptosis was also caused by 6-formyl-7-hydroxy-8-methoxycoumarin (methoxy-FHC), which is a photoproduct generated from another psoralen, 8-methoxypsoralen,19,20 widely used in PUVA therapy.
Materials and methods
Chemicals
Psoralen was a kind gift from Franco-Indian Chemical (Bombay, India). Methoxy-FHC belongs to the collection of the Department of Pharmaceutical Sciences, University of Padova (Italy). Both compounds were checked for purity by thin-layer chromatography (TLC) and NMR analysis. Psoralen stock solutions in absolute ethanol (10 mM) were stored in the dark. 7-Hydroxycoumarin, hexamethylenetetramine, and trifluoroacetic acid were purchased from Janssen (Beerse, Belgium), Carlo Erba (Milano, Italy), and Sigma-Aldrich (Milano, Italy), respectively. Monoclonal antibody against poly(ADP-ribose) polymerase (PARP) was purchased from Biomol International (Exeter, United Kingdom), antibody against cytochrome c was purchased from BD Biosciences (San Jose, CA), and antimouse immunoglobulins were purchased from DAKO (Glostrup, Denmark). TMRM (tetramethylrhodamine methyl ester) was purchased by Molecular Probes (Eugene, OR). All other chemicals and the antibody against β-actin were purchased from Sigma-Aldrich.
Cell isolation and culture
The human T lymphoblastoid Jurkat cell line was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Paisley, Scotland) in 5% CO2–95% air at 37°C in a humidified atmosphere.
Peripheral blood mononuclear cells (PBMCs) were obtained from freshly heparinized blood following centrifugation on Ficoll-Hypaque gradient and washing with phosphate-buffered saline. The cells were then resuspended in RPMI 1640 medium. For the assessment of cell viability, PB T lymphocytes were further enriched following rosetting of PBMCs with neuraminidase-treated sheep red blood cells.21 More than 98% of PB lymphocytes obtained with this procedure were represented by T lymphocytes.
Treatment protocols
A water-ethanol (2:1) psoralen solution (2.7 mM) was irradiated with 2 Philips HPW 125 lamps (Philips, Eindhoven, The Netherlands), mainly emitting at 365 nm. The fluence rate at 365 nm at the sample level was 4 mW/cm2. The solution in quartz cuvettes was irradiated with increasing UVA doses (0-112 J/cm2), and then added to Jurkat cells in serum-free medium in the absence or presence of 1 μM CsA preincubated for 1 hour. The mixture of POPs was analyzed by high-performance liquid chromatography (HPLC). The eluate was collected into 6 fractions. Acetonitrile was removed under reduced pressure, and water was removed by lyophilization. The residues were dissolved in DMSO (dimethylsulphoxide) and applied to cells in serum-free medium. FHC was added to Jurkat cells and T lymphocytes incubated in serum-free medium. After 15 minutes, FBS was added to reach a final concentration of 5%.
Purification and characterization of POPs
POPs were separated by a Perkin Elmer Series 200 HPLC system (Shelton, CT) using a Chromolith column, 100 × 4 mm (E. Merck, Darmstadt, Germany). The UV Diode Array detector (Perkin Elmer) was set at 230 nm and operated at a flow rate of 2 mL/minute. The eluent was a gradient of water-acetonitrile mixtures (5 minutes at 15% acetonitrile, then gradually increased up to 90% over a period of 26 minutes). TLC was performed on precoated silica gel plates (60 F254; E. Merck). After elution with the appropriate solvents, the plates were visualized under 365- and 254-nm light. The bands were scraped, extracted with warm chloroform and ethanol, and evaporated under reduced pressure.
MSs were performed by means of an API-TOF Mariner 5220 instrument (PerSeptive Biosystems, Stratford, TX). Injection was performed directly with a micrometric pump (mod. 11; Harvard Apparatus, Holliston, MA). 1H NMR spectra were recorded on a Bruker AMX300 spectrometer (Bruker Spectrospin Italiana, Cernusco, Italy).
Synthesis of FHC
7-Hydroxycoumarin (0.5 g, 3 mmol) and hexamethylenetetramine (0.7 g, 5 mmol) were dissolved in trifluoroacetic acid (5 mL), heated at reflux for 10 minutes, cooled, and poured onto ice water while stirring. The solution was alkalinized with NaHCO3 and extracted with diethyl ether. Evaporation of the solvent gave a yellow residue that was applied onto TLC plates and eluted with chloroform-methanol at a ratio of 96:4. FHC was identified as the band migrating at Rf = 0.85 displaying yellow fluorescence.
Assessment of cell viability
Jurkat cells (106 cells/mL) were stained with 10 μM Hoechst 33258 and 1 μM propidium iodide (PI) for 5 minutes.22 Cells were then washed with Hanks balanced salt solution supplemented with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4) and visualized with an Olympus IMT-2 inverted microscope (Olympus Optical, Tokyo, Japan) equipped with a xenon lamp and a 12-bit digital cooled charge-coupled device (CCD) camera (Micromax; Princeton Instruments, Monmouth Junction, NJ) as previously described.22 Excitation/emission cubes of 340/440 ± 25 nm and 568/585 ± 25 nm longpass filter were used for Hoechst 33258 and PI, respectively. A total of 3 randomly selected fields were acquired for each treatment. The corresponding bright-field images were also acquired, and the 3 channels were overlaid using the appropriate function of the Metamorph software (Universal Imaging, West Chester, PA). For staining with annexin V, each cover slip was incubated for 15 minutes at 25°C in 0.25 mL of a solution containing 140 mM NaCl, 5 mM CaCl2, and 10 mM HEPES/NaOH (pH 7.4), 1 μM PI, and annexin V–FLUOS (Roche Molecular Biochemicals, Indianapolis, IN) to a final dilution of 1:25 (vol/vol). Cells were washed twice with phosphate-buffered saline before analysis. A total of 3 randomly selected fields were acquired from each cover slip stained with both annexin V–FLUOS and PI, using excitation/emission cubes of 488/525 ± 25 nm bandpass and 568/585 longpass, respectively.23
Detection of PARP cleavage
Cell lysates (15 μg) were separated on a 10% SDS/polyacrylamide minigel and electrotransferred onto 0.45 μm nitrocellulose membranes (Bio-Rad, Hercules, CA), as previously described.24 The blots were probed with anti-PARP antibodies and antimouse peroxidase-conjugated secondary antibodies, and PARP was detected by chemoluminescence. Each assay was performed at least 3 times.
Mitochondrial cytochrome c release measurement
Release of cytochrome c from mitochondria to cytosol was analyzed by Western blot as described by Gajate et al.25 Briefly, Jurkat cells (106 cells/mL) were incubated in the absence or presence of 50 μM FHC for 20 hours in serum-free medium. Then, cells (4 × 106) were harvested by centrifugation, washed once with ice-cold Hanks balanced salt solution, and gently lysed for 30 seconds in 50 μL ice-cold buffer containing 250 mM sucrose, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.05% digitonin, 25 mM Tris (tris(hydroxymethyl)aminomethane; pH 6.8), 1 mM DTT (dithiothreitol), 1 μg/mL leupeptin, 1 μg/mL pepstatin, and 0.1 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 12 000g at 4°C for 2 minutes. Supernatants (40 μg protein) were electrophoresed on SDS 15% polyacrylamide gels and analyzed by Western blot. The blots were probed with anti–cytochrome c antibodies and antimouse peroxidase-conjugated secondary antibodies, and cytochrome c was detected by chemoluminescence.
TMRM staining and imaging
At the end of the incubation with POPs, Jurkat cells were washed with Hanks balanced salt solution supplemented with 10 mM HEPES (pH 7.4) and then incubated for 15 minutes at 37°C with 25 nM TMRM. Cyclosporin H (1.6 μM) was also added to inhibit multidrug-resistance pumps, which can affect TMRM loading.26 Cellular fluorescence images were acquired with the fluorescence microscope as previously described.22 For detection of TMRM fluorescence, 568 ± 25 nm excitation and 585 nm longpass emission filter settings were used. Data were acquired and analyzed using the Metamorph software. Mitochondria were identified as regions of interest, and at least 30 regions were considered for each experiment. The decrease in fluorescence intensitiy induced by FCCP (carbonylcyanide-p-trifluoromethoxyphenylhydrazone) was expressed as the difference of the values obtained before and after the addition of the uncoupler. The values of these differences obtained in the treated cells were normalized to the difference values of the untreated cells (control).
Measurements on isolated mitochondria
Rat liver mitochondria from albino Wistar rats weighing about 300 g were prepared by standard centrifugation techniques as described previously.27 Swelling was monitored as the changes in absorbance at 540 nm as previously described.23 Incubations were carried out at 25°C with 0.5 mg mitochondrial protein/mL in the medium containing 0.2 M sucrose, 10 mM MOPS (3-[N-morpholino]propanesulphonic acid)–Tris, 10 μM EGTA (ethyleneglycoltetraacetic acid)–Tris, 1 mM phosphate-Tris, 5 mM succinate-Tris, and 2 μM rotenone or 5 mM glutamate/2.5 mM malate, 0.5 μg/mL oligomycin (pH 7.4). PTP opening was induced by the addition of 80 μM Ca2+. Oxygen consumption was measured polarographically with a Clark oxygen electrode (Yellow Spring Instruments, Yellow Springs, OH) equipped with a Teflon membrane in a closed vessel equipped with magnetic stirring and thermostated at 25°C.28
Statistical analysis
Data are reported as means ± SD. The Student t test was used, and results were considered to be significant when P was less than .01.
Results
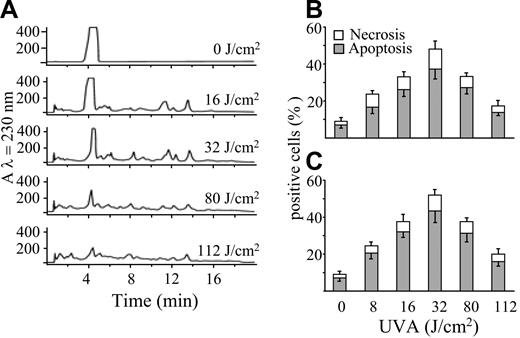
The apoptogenic potency of POPs was investigated after irradiation of a water-ethanol solution of psoralen (2.7 mM) with UVA light (0-112 J/cm2). This procedure was also aimed at characterizing the UVA dose necessary to obtain the highest yield of POPs. Aliquots of solutions irradiated at various fluencies were both analyzed by HPLC and incubated in the dark for 20 hours with Jurkat cells to assess their capacity to induce cell death. Figure 1A shows that psoralen undergoes extensive photolysis, as shown by the decrease of its peak in reverse-phase HPLC. Increasing UVA doses (up to 32 J/cm2) resulted in the formation of several additional peaks that decreased at the highest UVA doses used (80 and 112 J/cm2), indicating that the photoproducts themselves are unstable to light. Figure 1B-C shows that POPs induce cell death prevailing by apoptosis as detected by chromatin hypercondensation and phosphatidylserine exposure. This effect reached its maximal extent when the psoralen solution was preirradiated with a UVA dose of 32 J/cm2. In fact, the highest UVA doses decreased significantly the apoptogenic potency of the mixture, along with the disappearance of POPs.
POPs generated by UVA and separated by HPLC induce cell death. (A) RP-HPLC elution profiles of a psoralen solution (2.7 mM) irradiated with increasing UVA doses (0-112 J/cm2). Jurkat cells (106/mL) were incubated at 37°C in the presence of POPs (50 μM) generated by increasing UVA doses, as reported in panel A. After 20 hours, apoptosis was evaluated by appropriate changes of nuclei stained with Hoechst 33258 (B) or by staining with annexin V (C), whereas nuclear staining by PI was utilized for the evaluation of necrosis. Values are means ± SD of at least 4 experiments.
POPs generated by UVA and separated by HPLC induce cell death. (A) RP-HPLC elution profiles of a psoralen solution (2.7 mM) irradiated with increasing UVA doses (0-112 J/cm2). Jurkat cells (106/mL) were incubated at 37°C in the presence of POPs (50 μM) generated by increasing UVA doses, as reported in panel A. After 20 hours, apoptosis was evaluated by appropriate changes of nuclei stained with Hoechst 33258 (B) or by staining with annexin V (C), whereas nuclear staining by PI was utilized for the evaluation of necrosis. Values are means ± SD of at least 4 experiments.
POP induced apoptosis by means of mitochondrial dysfunction
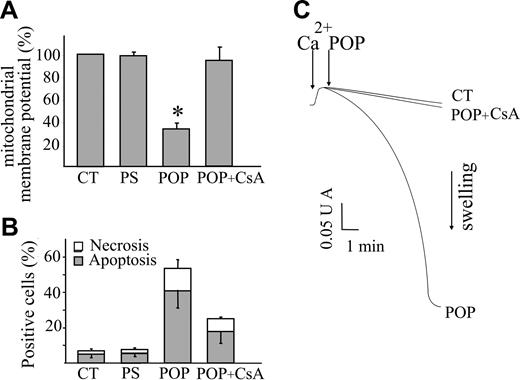
Since mitochondrial dysfunction is considered a crucial step in the commitment of the cell to apoptosis,26 we focused our attention on these organelles to investigate the mechanism(s) activated by POPs and responsible for apoptosis. To this aim, the changes in mitochondrial function were assessed in Jurkat cells by loading with TMRM, a lipophilic cation that accumulates within polarized mitochondria and that is commonly used for the assessment of mitochondrial membrane potential (Δψm).22 To exclude artefacts due to the different loading capacity of the various cells which can be erroneously interpreted as Δψm differences, after each treatment the cells were added with an uncoupler of oxidative phosphorylation (FCCP) that abolishes Δψm.9 Thus, in each cell the difference of fluorescence intensities obtained before and after FCCP provided a reliable assessment of Δψm. At 4 hours after POP treatment an extensive decrease of Δψm was measured (Figure 2A). Conversely, cell death was barely detectable after 4 hours (not shown) and significantly increased when the incubation was prolonged to 20 hours, as documented in Figure 2B. This result pointed out that mitochondrial dysfunction precedes nuclear fragmentation and demonstrated that mitochondrial depolarization is an early event in the apoptotic pathway. The mechanism of cell death appeared to be due to the opening of the PTP since its inhibitor, cyclosporin A, prevented both the decrease in Δψm and the loss of cell viability, as shown in Figure 2A-B. This demonstrates the causal relationship between PTP opening and cell death in POP-treated cells.
Inhibition of the opening of the PTP antagonizes POP effects on mitochondrial function and cell viability. Jurkat cells (106/mL) were treated with POP (50 μM) in the absence or presence of the PTP inhibitor cyclosporin A (CsA; 1 μL) or with nonirradiated psoralen (PS; 50 μM). CT indicates untreated cells. (A) After 4 hours of incubation, cells were stained with 25 nM TMRM to detect changes in mitochondrial membrane potential (Δψm). The images were acquired before and after the addition of the mitochondrial uncoupler FCCP (2 μM). The differences between the fluorescence values of each cell reflect the actual extent of Δψm. The values obtained in treated cells were normalized to those obtained in untreated cells at time 0. (B) After 20 hours of incubation, apoptosis and necrosis were evaluated by means of Hoechst 33 258 and PI fluorescence, respectively. Values are means ± SD of at least 4 experiments. (C) Mitochondrial swelling, which reflects PTP opening, was monitored as the decrease in light absorbance at 540 nm. RLM (0.5 mg protein/mL) were incubated with 80 μM Ca2+, which triggered PTP opening only upon the further addition of POP (10 μM). Since Ca2+ did not cause mitochondrial swelling in untreated mitochondria (CT), POPs caused an increased sensitivity of the PTP to Ca2+. Mitochondrial swelling was prevented by CsA (1 μM) demonstrating the involvement of the PTP.
Inhibition of the opening of the PTP antagonizes POP effects on mitochondrial function and cell viability. Jurkat cells (106/mL) were treated with POP (50 μM) in the absence or presence of the PTP inhibitor cyclosporin A (CsA; 1 μL) or with nonirradiated psoralen (PS; 50 μM). CT indicates untreated cells. (A) After 4 hours of incubation, cells were stained with 25 nM TMRM to detect changes in mitochondrial membrane potential (Δψm). The images were acquired before and after the addition of the mitochondrial uncoupler FCCP (2 μM). The differences between the fluorescence values of each cell reflect the actual extent of Δψm. The values obtained in treated cells were normalized to those obtained in untreated cells at time 0. (B) After 20 hours of incubation, apoptosis and necrosis were evaluated by means of Hoechst 33 258 and PI fluorescence, respectively. Values are means ± SD of at least 4 experiments. (C) Mitochondrial swelling, which reflects PTP opening, was monitored as the decrease in light absorbance at 540 nm. RLM (0.5 mg protein/mL) were incubated with 80 μM Ca2+, which triggered PTP opening only upon the further addition of POP (10 μM). Since Ca2+ did not cause mitochondrial swelling in untreated mitochondria (CT), POPs caused an increased sensitivity of the PTP to Ca2+. Mitochondrial swelling was prevented by CsA (1 μM) demonstrating the involvement of the PTP.
The occurrence of PTP opening was directly tested in isolated rat liver mitochondria by means of the conventional swelling assay.23 These organelles were loaded with a small Ca2+ pulse that did not cause PTP opening per se, and then added with POPs (10 μM), which caused the absorbance decrease that is indicative of swelling. Figure 2C clearly documents that mitochondrial swelling was largely prevented by pretreatment with 1 μM CsA, thus indicating that POPs directly caused PTP opening.
Identification and apoptogenic properties of photoproducts
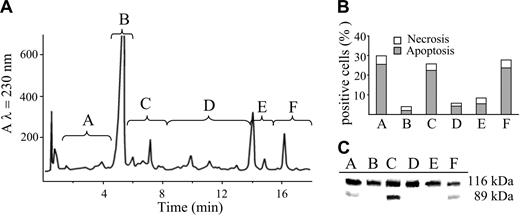
In order to identify the molecules causing apoptosis, POP were separated by reverse-phase HPLC. The eluate was collected into 6 different fractions as reported in Figure 3A. Each fraction was dried out, and the residue dissolved in DMSO and administered to Jurkat cells. The apoptogenic effect of the fractions was evaluated by double staining with Hoechst 33258 and PI. Only 3 fractions were able to elicit apoptosis as documented in Figure 3B. The occurrence of apoptosis was confirmed by the cleavage of PARP, a well-known substrate of caspase-3 (Figure 3C).
Effects of the different fractions of POP obtained by HPLC separation on the occurrence of cell death. (A) HPLC elution profile of a UVA-irradiated solution of psoralen (32 J/cm2). The eluate was divided in 6 different fractions (A-F) that were evaluated for their apoptogenic activity. (B) Jurkat cells (106/mL) were incubated in the dark with the different HPLC fractions of POP for 20 hours at 37°C. Necrosis and apoptosis were assessed as described in “Materials and Methods” and in Figure 1B. (C) Western blot analysis of PARP from cells incubated under the same conditions described for panel B. PARP cleavage provides further evidence of apoptosis.
Effects of the different fractions of POP obtained by HPLC separation on the occurrence of cell death. (A) HPLC elution profile of a UVA-irradiated solution of psoralen (32 J/cm2). The eluate was divided in 6 different fractions (A-F) that were evaluated for their apoptogenic activity. (B) Jurkat cells (106/mL) were incubated in the dark with the different HPLC fractions of POP for 20 hours at 37°C. Necrosis and apoptosis were assessed as described in “Materials and Methods” and in Figure 1B. (C) Western blot analysis of PARP from cells incubated under the same conditions described for panel B. PARP cleavage provides further evidence of apoptosis.
POPs were also separated by TLC. The irradiated solution was partly evaporated and applied onto silica gel plates. After elution with chloroform, many bands appeared. The plate was divided into 8 zones, the gel was scraped and extracted, and the solvent was removed. The residues were dissolved in DMSO and applied to cells. Only 3 out of the 8 samples were active in inducing apoptosis, namely those containing a dark spot at Rf = 0.90 (compound 1), a yellowish fluorescent band at Rf = 0.62 (compound 2), and a violet-fluorescent band at Rf = 0.15 (compound 3) (not shown). In these conditions, intact psoralen appears as an azure-fluorescent spot at Rf = 0.58. The 3 products were analyzed by HPLC. Compounds 1, 2, and 3 were assigned to the peaks eluting at 16.8 minutes (thus belonging to fraction F), 3.1 minutes (fraction A), and 7.7 minutes (fraction C), respectively. The 3 active products were analyzed by NMR and MS to identify their molecular structure.
Compound 1.
1H-NMR (CD3COCD3; 300 MHz), δ 7.97 (s, 2H, H-8); 7.95 (d, 2H, H-5′, J = 2.5 Hz); 7.42 (s, 2H, H-5); 7.01 (broad s, 2H, H-4′), 5.60 (d, 2H, H-4, J = 2.2 Hz); 4.36 (d, 2H, H-3), J = 2.2 Hz). MS (electrospray), m/z 391 [M+Na]+; 373 [M+H]+.
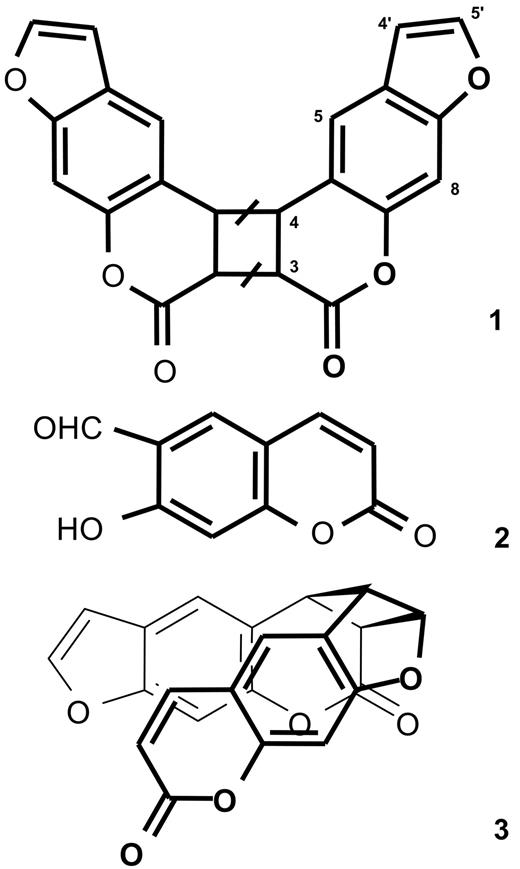
The mass spectrum suggests that compound 1 is a dimer of psoralen (mw 186). The NMR spectrum only shows 6 signals, indicating that the dimer is symmetrical. Protons 3 and 4 of psoralen undergo downfield shift with respect to the parent molecule, indicating saturation of the 3,4 double bond of both monomers through a [2 + 2] cycloaddition reaction. Compound 1 is therefore a pyrone-pyrone dimer of psoralen (Figure 4). Such a dimer can exist in 4 different isomeric forms: cis-syn, cis-anti, trans-syn, and trans-anti.29 Syn and anti indicate that protons 3 of the 2 monomers are vicinal or diagonal in the cyclobutane, respectively. Cis and trans indicate that the 2 psoralen moieties are on the same side, or on the opposite side of the plane defined by the cyclobutane, respectively. Of note, since all these structures display an element of symmetry, the NMR analysis cannot provide regio- and stereochemical information.
Molecular structure of the photolysis products of psoralen. Compound 1, pyrone-pyrone dimer; compound 2, 6-formyl-7-hydroxycoumarin; and compound 3, pyrone-furan dimer. As detailed in the “Results,” the bars on the cyclobutane ring of structure 1 indicate that the stereochemistry of the dimer has not been determined.
Molecular structure of the photolysis products of psoralen. Compound 1, pyrone-pyrone dimer; compound 2, 6-formyl-7-hydroxycoumarin; and compound 3, pyrone-furan dimer. As detailed in the “Results,” the bars on the cyclobutane ring of structure 1 indicate that the stereochemistry of the dimer has not been determined.
Compound 2.
1H-NMR (CD3OD, 300 MHz): δ 10.09 (s, 1H, CHO); 8.47 (s, 1H, H-8); 7.72 (d, 1H, H-4, J = 8.5 Hz); 7.67 (s, 1H, H-5); 5.98 (d, 1H, H-3, J = 8.5 Hz). MS (electrospray): m/z 191 [M+H]+.
Figure 1A shows that compound 2, which eluted at 3.1 minutes, was barely detectable. Based on both its fluorescence on the TLC plate and its UV absorption spectrum, we hypothesized that compound 2 was the aldehyde resulting from the oxidative cleavage of the furan ring of psoralen (ie, FHC).
In order to confirm this hypothesis and to obtain amounts of 2 adequate for NMR and MS analysis and for biological tests, FHC was synthesised ex novo by using hexamethylenetetramine as the formylating agent. Among the several products formed, 1 compound coeluted with compound 2 in both TLC and HPLC and displayed identical UV absorption and fluorescence properties. Since this compound was identified as FHC by NMR and MS spectra, compound 2 is indeed FHC.
Compound 3.
1H-NMR (CD3COCD3, 300 MHz): δ 7.46 (d, 1H, H-5′(r), J = 2.2 Hz); 7.19 (d, 1H, H-4(f), J = 9.5 Hz); 7.08 (s, 1H, H-5(r)); 6.46 (d, 1H, H-4′(f), J = 2.2 Hz); 6.41-6.43 (not resolved signal, 3H, H-8(r), H-8 (f), and H-5(f)); 5.61 (d, 1H, H-3(f); J = 9.5 Hz); 5.32 (m, 1H, H-5′(f)); 4.11 (m, 1H, H-3(r) or H-4′(f)); 3.99 (m, 1H, H-4(r)); 3.78 (m, 1H, H-4′(f) or H-3(r)). “f” refers to the monomer in the foreground of the structure 3 of scheme 1, and “r” to the other. MS (electrospray): m/z 373 [M+H]+.
The mass spectrum also indicates that compound 3 is a dimer of psoralen. The NMR spectrum is more complex than that of compound 1, showing that the molecule is not symmetrical. Homodecoupling and nuclear Overhauser effect spectroscopy experiments established that compound 3 is the cis-syn pyrone-furan dimer depicted in Figure 4.
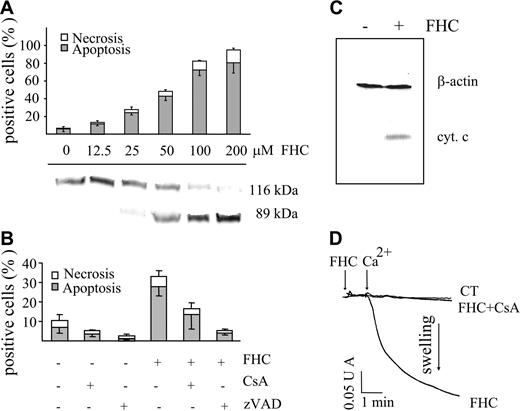
Since fraction A (eluted from HPLC) was found to be apoptogenic, we investigated whether FHC (compound 2) could induce apoptosis as well. Figure 5A shows that in a dose-dependent manner FHC caused (1) cell death represented almost exclusively by apoptosis and (2) caspase-3 activation, as detected by PARP cleavage. The relevance of caspase activation was also supported by the inhibition of apoptosis afforded by zVAD (Z-Val-Ala-Asp-fluoromethyl-ketone; Figure 5B). Confirming well-established evidence, caspase activation was likely due to cytochrome c release from mitochondria into the cytosol (Figure 5C). On the other hand, the inhibition of apoptosis by CsA suggested the involvement of PTP opening (Figure 5B). FHC mimicked not only the ability of POPs to induce mitochondrial dysfunction in intact cells but also the direct effect in mitochondria. Indeed, the addition of FHC to isolated rat liver mitochondria resulted in a CsA-inhibitable mitochondrial swelling indicating PTP opening (Figure 5D). Of note, at the concentration used for the swelling assay, both POPs and FHC did not modify state 4 and uncoupled respiration in mitochondria using succinate or glutamate/malate as respiratory substrates (data not shown).
Effect of FHC on the occurrence of cell death and on mitochondrial swelling. (A) Jurkat cells (106/mL) were incubated with concentrations of FHC ranging from 12.5 to 100 μM for 20 hours at 37°C. Necrosis and apoptosis were assessed as described in “Materials and Methods” and in Figure 1B (top panel). PARP cleavage was determined by Western blot analysis (bottom panel). (B) Jurkat cells (106/mL) were incubated with FHC (25 μM) for 20 hours at 37°C in the absence or presence of zVAD (50 μM) or CsA (1 μM). Values are means ± SD of at least 4 experiments. Necrosis and apoptosis were assessed as described in panel A. (C) Western blot analysis of cytochrome c release. Jurkat cells were incubated in the absence or in the presence of 50 μM FHC for 20 hours at 37°C. Cell lysates were centrifuged, and the supernatants were analyzed for their cytochrome c content as detailed in “Materials and Methods.” β-actin monoclonal antibody (Clone AC-15) was used as a loading control. (D) Mitochondrial swelling was assayed as reported in Figure 2C. FHC (1 μM) was incubated 1 minute before the addition of 30 μM Ca2+. Mitochondrial swelling was attributed to PTP opening by means of CsA inhibition.
Effect of FHC on the occurrence of cell death and on mitochondrial swelling. (A) Jurkat cells (106/mL) were incubated with concentrations of FHC ranging from 12.5 to 100 μM for 20 hours at 37°C. Necrosis and apoptosis were assessed as described in “Materials and Methods” and in Figure 1B (top panel). PARP cleavage was determined by Western blot analysis (bottom panel). (B) Jurkat cells (106/mL) were incubated with FHC (25 μM) for 20 hours at 37°C in the absence or presence of zVAD (50 μM) or CsA (1 μM). Values are means ± SD of at least 4 experiments. Necrosis and apoptosis were assessed as described in panel A. (C) Western blot analysis of cytochrome c release. Jurkat cells were incubated in the absence or in the presence of 50 μM FHC for 20 hours at 37°C. Cell lysates were centrifuged, and the supernatants were analyzed for their cytochrome c content as detailed in “Materials and Methods.” β-actin monoclonal antibody (Clone AC-15) was used as a loading control. (D) Mitochondrial swelling was assayed as reported in Figure 2C. FHC (1 μM) was incubated 1 minute before the addition of 30 μM Ca2+. Mitochondrial swelling was attributed to PTP opening by means of CsA inhibition.
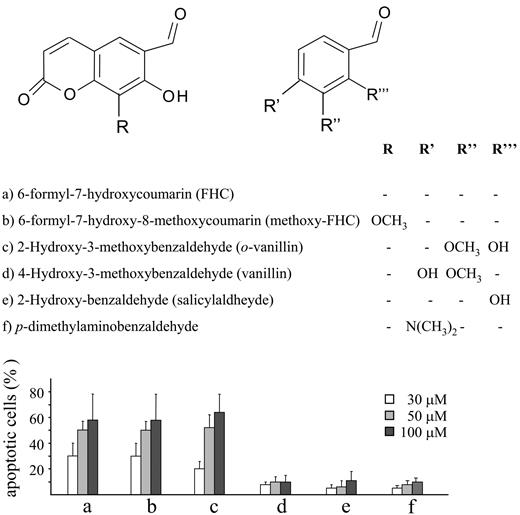
A similar deleterious effect on cell viability was obtained with methoxy-FHC, the hydroxyaldehyde arising from the oxidative fission of the furan ring of 8-methoxypsoralen19 (Figure 6). Furthermore, some model compounds, including 7-hydroxycoumarin and some aromatic aldehydes, were tested to characterize the structural determinants required for cytotoxic effects. Among them, only o-vanillin was found to cause programmed cell death (Figure 6).
Structures and apoptotic activity for the analyzed compounds. Jurkat cells (106/mL) were incubated for 20 hours at 37°C with increasing concentrations (30-100 μM) of the different compounds shown in the top panel. Necrosis and apoptosis were assessed as reported as described in “Materials and Methods” and in Figure 1B. Necrosis was not shown in the bar graph since it remained below 5% regardless of the compounds tested. The results obtained with FHC (compound a) are as same as those in Figure 5 and are reported in the present graph to provide the proper term of comparison. Values are means ± SD of at least 4 experiments.
Structures and apoptotic activity for the analyzed compounds. Jurkat cells (106/mL) were incubated for 20 hours at 37°C with increasing concentrations (30-100 μM) of the different compounds shown in the top panel. Necrosis and apoptosis were assessed as reported as described in “Materials and Methods” and in Figure 1B. Necrosis was not shown in the bar graph since it remained below 5% regardless of the compounds tested. The results obtained with FHC (compound a) are as same as those in Figure 5 and are reported in the present graph to provide the proper term of comparison. Values are means ± SD of at least 4 experiments.
As far as compounds 1 and 3 are concerned, they both caused apoptosis without significant necrosis, yet we were not able to establish a dose-response relationship, due to their low stability (not shown).
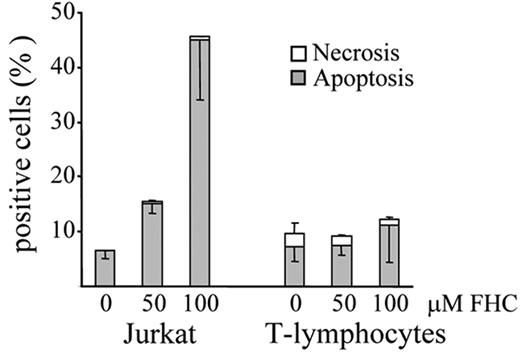
Finally, FHC was tested on T lymphocytes freshly isolated from healthy individuals. The viability of normal T lymphocytes was not affected by treatment with FHC concentrations that were cytotoxic in Jurkat cells (Figure 7). Therefore, the apoptogenic effect of FHC appears to be specific for transformed T lymphocytes.
Loss of cell viability induced by FHC in Jurkat cells and normal T lymphocytes. Jurkat cells and T lymphocytes (106/μL) were incubated for 16 hours at 37°C with increasing concentrations (50-100 μM) of FHC. Necrosis and apoptosis were assessed as described in “Materials and Methods” and in Figure 1B.
Loss of cell viability induced by FHC in Jurkat cells and normal T lymphocytes. Jurkat cells and T lymphocytes (106/μL) were incubated for 16 hours at 37°C with increasing concentrations (50-100 μM) of FHC. Necrosis and apoptosis were assessed as described in “Materials and Methods” and in Figure 1B.
Discussion
The present results demonstrate that POPs activate the apoptotic program in Jurkat cells and that mitochondrial dysfunction is a causative event in the commitment of the cell to die. Therefore, POP formation plays a relevant role in PUVA-induced apoptosis. At variance from PUVA or UVA irradiation, which cause both necrosis and apoptosis in this same experimental model,9 the extent of necrosis was barely detectable in cells treated with the entire POP mixture or with the different isolated photoproducts. PUVA-induced necrosis might be related to the generation of short living reactive oxygen species, which is inevitably associated with the irradiation of photosensitizers after their intracellular accumulation. Therefore, it is likely that reactive oxygen species accumulation is not required for ensuing apoptosis, yet it likely amplifies and/or accelerates PUVA toxicity, causing necrosis besides higher extent of apoptosis.
The reduced extent of necrosis, which is likely to decrease the inflammatory response, might represent a therapeutic advantage that adds to other potential benefits related to preactivation procedures. In fact, the efficacy of a given photosensitizer can be undermined by inactivation due to excessive photo-oxidation within the irradiated tissue in situ. Perhaps more important, in the preactivation approach, tissues are not exposed to uncontrolled formation of reactive oxygen species, which attack every cellular structure with nonspecific mechanisms, so that the therapeutic efficacy can be referred to generation of biologically active molecules which might recognize specific targets. Therefore, it is imperative to identify the moieties generated upon photoirradiation. Accordingly, a major goal of the present study was the identification of molecules generated by psoralen irradiation and responsible for cell death. In particular, we demonstrated that FHC (ie, the hydroxyaldehyde generated by the oxidative fission of the furan ring of psoralen) mimicked POP cytotoxicity's ability to induce apoptosis in a dose-dependent manner, without significant necrosis. In particular, both POP- and FHC-induced apoptosis appear to depend on PTP opening. In fact, in both cases, apoptosis is largely prevented by the PTP inhibitor CsA. However, this compound can elicit several effects that are not related to PTP.30 To address the specificity of POPs and FHC, experiments were performed on isolated mitochondria. The results clearly showed that these substances are PTP agonists. Therefore, PTP opening is causally related to POP- and FHC-induced apoptosis.
Apoptosis was similarly induced by methoxy-FHC which is generated by photodegradation of 8-methoxypsoralen.19,20 On the contrary, 7-hydroxycoumarin was found to be completely inactive.
Although these results highlight the relevance of the formyl moiety, additional findings of the present study indicate that cell death does not depend on the aldehyde residue per se. The present data showed that several aromatic aldehydes, such as salicylaldehyde, 4-hydroxy-3-methoxybenzaldehyde (vanillin), and p-dimethylaminobenzaldehyde, did not affect cell viability. The lack of activity of salicylaldehyde demonstrated also that the o-hydroxybenzaldehyde arrangement is not sufficient to explain the apoptotic potency of FHC and methoxy-FHC. Interestingly, 2-hydroxy-3-methoxybenzaldehyde (o-vanillin) caused apoptosis at an extent comparable to that obtained with methoxy-FHC. Thus, the o-hydroxybenzaldehyde arrangement seems to be necessary but not sufficient for eliciting apoptosis. On a more general note, these findings suggest that only precise locations of the aldehyde moiety on specific molecular scaffolds are associated with cytotoxicity. For instance, previous reports demonstrated that mitochondrial dysfunction is only caused by a particular set of aldehydes.31 The concept that cell death depends on specific structures more than on unspecific reactivity of chemical groups is further supported by the identification of other cytotoxic molecules (ie, compounds 1 and 3). Indeed, our results showed that upon psoralen irradiation, only 2 of the 7 dimers generated caused cell death, indicating that molecules with similar chemical structures display strikingly different biological activities. Further studies are in progress to identify the structure-activity relationships of these molecules. Since cytotoxic effects were elicited at micromolar concentrations, it is likely that POPs interact with specific cellular structures, such as proteins. Therefore, it might be worth using POPs as tools in ligand-binding studies to identify proteins involved in apoptosis. This might be especially relevant at the mitochondrial level, since despite the conspicuous amount of evidence indicating the role of PTP opening in causing cell death, its molecular nature is still elusive.30
Besides apoptosis, POP formation could play other roles contributing to salutary effects. For instance, one of the photolysis products of 8-methoxypsoralen in MeOH, namely 4′,5′-dihydro-8,5′-dimethoxy-4′-hydroxypsoralen, at concentrations as low as 2.5 × 10−8, has been shown to bind anaphylatoxin C5a. Consequently, the chemotactic activity of polymorphonuclear neutrophils toward this proinflammatory factor is inhibited.32 On the other hand, aldehyde-containing compounds are useful immunomodulatory agents by forming Schiff bases with amines expressed on antigen-presenting cell and T-cell surfaces.33 Among these aldehydes, tucaresol is currently in phase 1/2 trial against HIV infections.34 Interestingly, this drug contains the same o-hydroxybenzaldehyde moiety typical of the active compounds examined in this study.
In conclusion, the present study identifies novel apoptogenic molecules and highlights the ability of photoirradiation to generate cytotoxic products that are likely to play a relevant role in PUVA therapy. As far as the underlying mechanisms are concerned, PTP opening emerges as a central mechanism in the sequence of events set in motion by POPs that commit Jurkat cells to apoptosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
F.D.L. was the recipient of grants from the Ministero dell'Instruzione (MIUR), the Consiglio Nazionale delle Ricerche (CNR), and the Fondo per gli Investimenti della Ricerca di Base (FIRB).
Authorship
Author contributions: S.C. and F.D.A. isolated and characterized the tested compounds; F.B., M.F., and M.C. performed research and analyzed data; and S.C., F.D.L., G.S., and M.C. designed research and wrote the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Marcella Canton, Department of Biological Chemistry, Viale G. Colombo 3, I-35121 Padova, Italy; e-mail: marcella.canton@unipd.it.