Abstract
Mdm2 and Mdm4 are critical negative regulators of the p53 tumor suppressor. Mdm4-null mutants are severely anemic and exhibit impaired proliferation of the fetal liver erythroid lineage cells. This phenotype may indicate a cell-intrinsic function of Mdm4 in erythropoiesis. In contrast, red blood cell count was nearly normal in mice engineered to express low levels of Mdm2, suggesting that Mdm2 might be dispensable for red cell production. Here, we further explore the tissue-specific functions of Mdm2 and Mdm4 in the erythroid lineage by intercrossing conditional Mdm4 and Mdm2 alleles to an erythroid-specific Cre (Er-GFP-Cre) knock-in allele. Our data show that Mdm2 is required for rescuing erythroid progenitors from p53-mediated apoptosis during primitive erythropoiesis. In contrast, Mdm4 is only required for the high erythropoietic rate during embryonic definitive erythropoiesis. Thus, in this particular cellular context, Mdm4 only contributes to p53 regulation at a specific phase of the differentiation program.
Introduction
The p53 protein plays an important role in preventing cancer development by inducing apoptosis or inhibiting the proliferation of cells undergoing cellular stress.1 p53 performs these functions mainly by acting as a transcription factor, affecting the transcription of a plethora of genes.2 Hence, it is essential to restrain p53 activities during normal growth and development. Central to this process are the 2 structurally related proteins, Mdm2 and Mdm4.3 Germline inactivation of either Mdm2 or Mdm4 leads to embryonic mortality that is completely rescued by concomitant deletion of p53.4–8 Interestingly, Mdm2-null mice die before implantation due to excessive cell death,9 whereas Mdm4-null mice die at midgestation. Mdm4-null mice exhibit cell proliferation defects, particularly in the fetal liver erythroid progenitor cells, and apoptosis specifically in the neuroepithelium,8 suggesting that Mdm4 has cell-intrinsic functions in early brain development and erythropoiesis. In contrast, recent data indicate that Mdm2 might be dispensable for red cell production. The red blood cell count was indeed nearly normal in mice with one null and one hypomorphic Mdm2 allele.10 However, frequency of p53-dependent apoptosis was increased in a subset of homeostatic tissues in these mice. These data combined suggest that Mdm2 and Mdm4 are nonoverlapping critical regulators of p53 that may exert their functions in a temporal and tissue-specific manner. To examine this possibility we have specifically inactivated Mdm2 and Mdm4 in the erythroid lineage in vivo
Materials and methods
Mice
The Mdm2 and Mdm4 floxed alleles, and the GFPcre knock-in allele, controlled by the endogenous erythropoietin receptor (EpoR) promoter, were genotyped as described.11–13
Histology, immunohistochemistry, and in situ end-labeling staining
Pregnant females were injected intraperitoneally with 100 μg of BrdU per gram of body weight and killed 2 hours later. Assays were performed as previously described.14 Detection of phosphorylated histone H3 (1:30; Calbiochem, San Diego, CA) and BrdU-positive cells (1:200; Boehringer Mannheim, Mannheim, Germany) was performed as described.8,15 Light microscopy was performed using a BX51 Discussion microscope (BX51, Olympus) equipped with the following objectives: 10 ×/0.25 numerical aperture (NA), 20 ×/0.4 NA, 40 ×/0.65 NA, and 100 ×/1.25 NA (oil). Images were captured with a color camera cooled interline transfer CCD (Coolsnap color camera, Roper Scientific, Tucson, AZ) (1392 × 1040 pixel resolution; Olympus, Hamburg, Germany). RSI image software (Roper Scientific, Tucson, AZ) version 1.7.3 was used for image acquisition.
Immunohistofluorescence
Embryos were fixed overnight in 0.2% paraformaldehyde/1 × PBS, incubated in 2 mM MgCl2/30% sucrose/1 × PBS overnight, and embedded in OCT; 6 μm cryosections were prepared. Immunohistologic analysis was performed as described16 using anti-p53 (1:100, CM5; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom), anti–cleaved caspase-3 (1:100; Cell Signaling Technology, Beverly, MA), and anti–Ter-119 (1:100, clone Ter-119; eBioscience, San Diego, CA). Microscopy was performed with an Axiophot fluorescence microscope (Axiophot; Zeiss, Jena, Germany) equipped with a 40 ×/1.3 NA oil objective and a color camera (RTE/CCD-1300-Y/HS; Roper Scientific) cooled interline transfer CCD (1030 × 1300 pixel resolution; Carl Zeiss, Jena, Germany). Metamorph online software (Molecular Devices, Downingtown, PA) version 5.0 was used for image acquisition.
Hematocrit and phenylhydrazine stress test
Mice were injected subcutaneously on days 0, 1, and 3 with 35 mg/kg of phenylhydrazine in PBS. Blood was collected on days 0, 3, 6, and 9 for measurement of hematocrit and reticulocyte count as described.17
Purification of erythroid cells
Erythroid cells expressing the cellular marker Ter119 were purified from bone marrow by magnetic-activated cell sorting (MACS) as described.13
Colony assays
Results and discussion
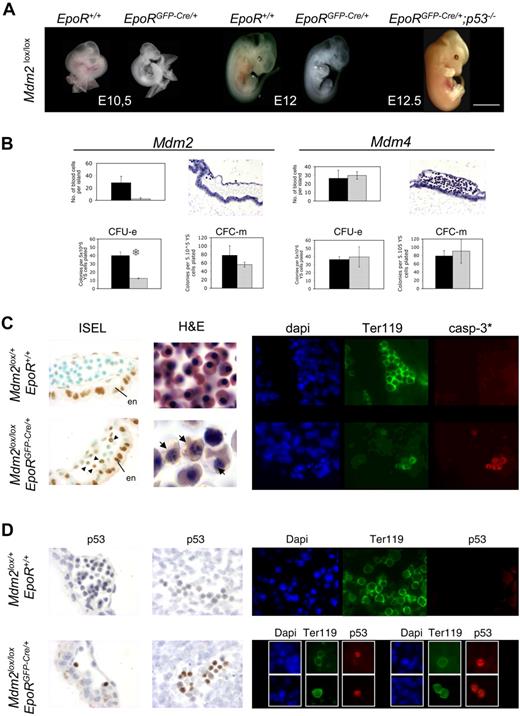
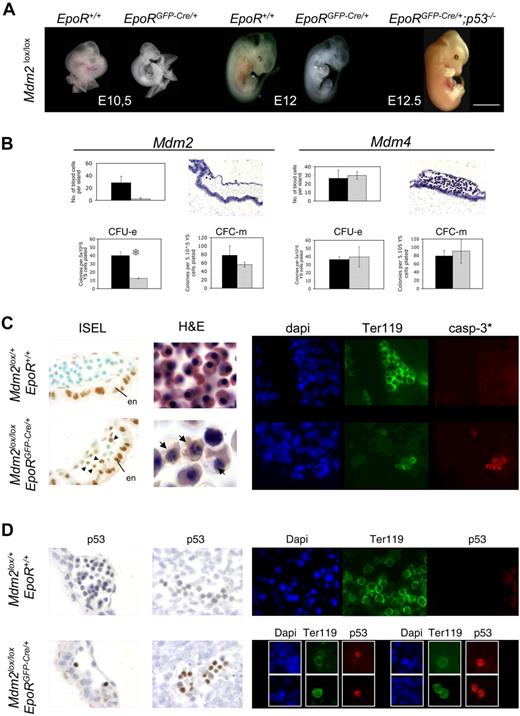
Whereas no Mdm2lox/lox; EpoRGFP-Cre/+ pups were found at weaning age, about 60% of the expected Mdm4lox/lox; EpoRGFP-Cre/+ mice were viable (Tables S1–S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). Examination of embryos at successive stages of embryonic development revealed that Mdm2lox/lox; EpoRGFP-Cre/+ embryos die at about embryonic day (E) 13. This phenotype is p53 dependent, because Mdm2lox/lox; EpoRGFP-Cre/+; p53−/− mice showed no erythropoietic defects and were viable (Figure 1A; Figure S1). At E12, Mdm2lox/lox; EpoRGFP-Cre/+ embryos appeared very pale (Figure 1A). Only rare erythroid cells were found in the heart, dorsal aorta, and liver (not shown) of these embryos, and most of them (> 90%) exhibited pyknotic nuclei, characteristic of apoptotic cells (Figure 1C). In contrast, numerous erythropoietic foci are found in the heart and liver of E12 Mdm4lox/lox; EpoRGFP-Cre/+ embryos (not shown), and at E13, these embryos are undistinguishable from control littermates (Figure 2A). Consistent with the anatomic and histologic analyses, the number of nucleated cells in livers was lower in Mdm2lox/lox; EpoRGFP-Cre/+ embryos, but not in Mdm4lox/lox; EpoRGFP-Cre/+ embryos, compared with control embryos.
Mdm2, but not Mdm4, is required for primitive erythropoiesis. (A) Mdm2lox/lox embryos that are either positive (EpoRGFP-Cre/+) or negative (EpoR+/+) for the EpoR-GFP-cre transgene at various stages of embryonic development. Mdm2lox/lox; EpoRGFP-Cre/+ embryos are devoid of any red cells as early as E10.5, whereas E12.5 Mdm2lox/lox; EpoRGFP-Cre/+;p53−/− embryos appear normal. Scale bar equals 15 μm. (B) A representative blood island in sections of E10.5 Mdm2lox/lox and Mdm4lox/lox ; EpoRGFP-Cre/+ embryos (top panels). The presence of blood cells is severely compromised in Mdm2lox/lox mutants. Data from controls (Mdm2lox/+ or Mdm4lox/+; EpoR+/+) and mutants are represented by the black and gray bars, respectively. The yolk sac sections are stained with hematoxylin and eosin. Magnification, × 20. Number of CFU-Es and monocyte-macrophage colony-forming cells (CFC-m's) detected in methylcellulose cultures supplemented with Epo or a mixture of interleukin-3 (IL-3), IL-6, and stem cell factor (SCF), respectively (bottom panels). Numbers are expressed per 5 × 105 yolk sac cells. Data from controls (Mdm2lox/+ or Mdm4lox/+; EpoR+/+) and mutants are represented by the black and gray bars, respectively. The data represent the means (± SD) of 6 independent experiments. There is a significant difference between the mean numbers of CFU-Es in the mdm2 mutants compared with their control littermates by the Student test (P < .001; S). (C) Mdm2 inactivation in erythroid progenitors leads to apoptotic cell death. In situ end labeling (ISEL) in blood islands of E10.5 embryos with the indicated genotypes (left panels). Note that strong background staining is observed in the visceral endoderm (en) of both control and mutant embryos. Within the blood islands, specific staining is only observed in sections from Mdm2lox/lox; EpoRGFP-cre/+ embryos (arrowheads). Hematoxylin and eosin–stained sections of heart from E12 fetuses (middle panels). In contrast to controls, only a few nucleated erythrocytes were found in Mdm2lox/lox; EpoRGFP-Cre/+ sections, and many apoptotic figures are seen (arrows). Magnification, ×100. Cleaved caspase-3 expression (casp-3*; red) in Ter119-positive erythroid progenitors (green) in the dorsal aorta of embryos with the indicated genotypes (right panels). DNA (blue) is stained with 4,6-diamidino-2-phenylindole (dapi). Magnification, ×40. (D) Mdm2 inactivation in erythroid progenitor cells leads to increased p53 protein levels. Immunohistochemistry for p53 in the blood islands (left panels) and the dorsal aorta (middle panels) and immunohistofluorescence for p53 and Ter-119 in the heart (right panels) of embryos with the indicated genotypes. p53 expression is only detected in the nuclei of most (> 90%) erythroid cells of Mdm2lox/lox; EpoRGFP-Cre/+ embryos. DNA (blue) is stained with dapi. Magnification, ×40.
Mdm2, but not Mdm4, is required for primitive erythropoiesis. (A) Mdm2lox/lox embryos that are either positive (EpoRGFP-Cre/+) or negative (EpoR+/+) for the EpoR-GFP-cre transgene at various stages of embryonic development. Mdm2lox/lox; EpoRGFP-Cre/+ embryos are devoid of any red cells as early as E10.5, whereas E12.5 Mdm2lox/lox; EpoRGFP-Cre/+;p53−/− embryos appear normal. Scale bar equals 15 μm. (B) A representative blood island in sections of E10.5 Mdm2lox/lox and Mdm4lox/lox ; EpoRGFP-Cre/+ embryos (top panels). The presence of blood cells is severely compromised in Mdm2lox/lox mutants. Data from controls (Mdm2lox/+ or Mdm4lox/+; EpoR+/+) and mutants are represented by the black and gray bars, respectively. The yolk sac sections are stained with hematoxylin and eosin. Magnification, × 20. Number of CFU-Es and monocyte-macrophage colony-forming cells (CFC-m's) detected in methylcellulose cultures supplemented with Epo or a mixture of interleukin-3 (IL-3), IL-6, and stem cell factor (SCF), respectively (bottom panels). Numbers are expressed per 5 × 105 yolk sac cells. Data from controls (Mdm2lox/+ or Mdm4lox/+; EpoR+/+) and mutants are represented by the black and gray bars, respectively. The data represent the means (± SD) of 6 independent experiments. There is a significant difference between the mean numbers of CFU-Es in the mdm2 mutants compared with their control littermates by the Student test (P < .001; S). (C) Mdm2 inactivation in erythroid progenitors leads to apoptotic cell death. In situ end labeling (ISEL) in blood islands of E10.5 embryos with the indicated genotypes (left panels). Note that strong background staining is observed in the visceral endoderm (en) of both control and mutant embryos. Within the blood islands, specific staining is only observed in sections from Mdm2lox/lox; EpoRGFP-cre/+ embryos (arrowheads). Hematoxylin and eosin–stained sections of heart from E12 fetuses (middle panels). In contrast to controls, only a few nucleated erythrocytes were found in Mdm2lox/lox; EpoRGFP-Cre/+ sections, and many apoptotic figures are seen (arrows). Magnification, ×100. Cleaved caspase-3 expression (casp-3*; red) in Ter119-positive erythroid progenitors (green) in the dorsal aorta of embryos with the indicated genotypes (right panels). DNA (blue) is stained with 4,6-diamidino-2-phenylindole (dapi). Magnification, ×40. (D) Mdm2 inactivation in erythroid progenitor cells leads to increased p53 protein levels. Immunohistochemistry for p53 in the blood islands (left panels) and the dorsal aorta (middle panels) and immunohistofluorescence for p53 and Ter-119 in the heart (right panels) of embryos with the indicated genotypes. p53 expression is only detected in the nuclei of most (> 90%) erythroid cells of Mdm2lox/lox; EpoRGFP-Cre/+ embryos. DNA (blue) is stained with dapi. Magnification, ×40.
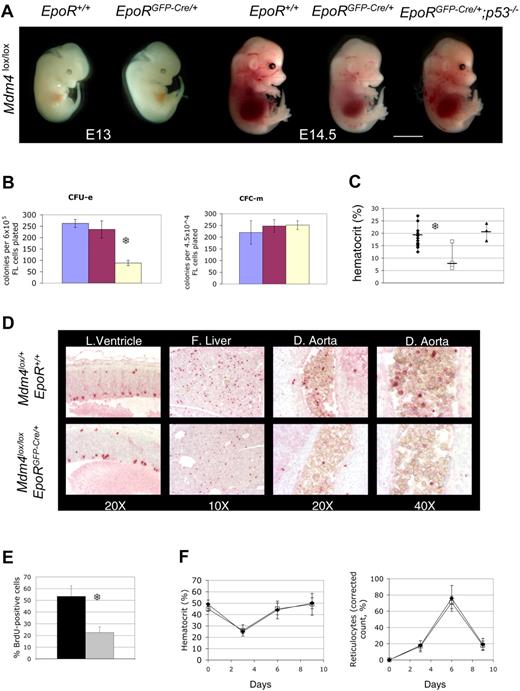
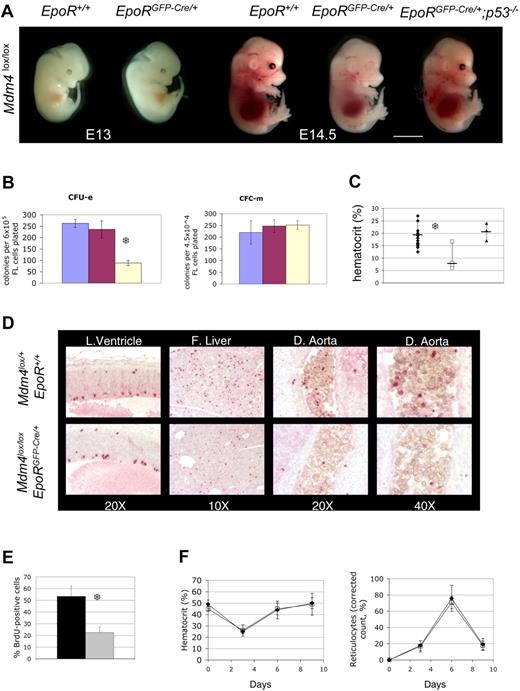
Mdm4 is required for fetal definitive, but not for adult, erythropoiesis. (A) Mdm4lox/lox embryos that are either positive (EpoRGFP-Cre/+) or negative (EpoR+/+) for the EpoR-GFP-cre transgene at various stages of embryonic development. Mdm4lox/lox; EpoRGFP-Cre/+ embryos are significantly paler than control littermates at E14.5, whereas Mdm4lox/lox; EpoRGFP-Cre/+; p53−/−embryos appear normal. Scale bar equals 15 μm. (B) Number of CFU-Es and CFC-m's detected in methylcellulose cultures supplemented with Epo or a mixture of IL-3, IL-6, and SCF, respectively. Numbers are expressed per 6 × 105 nucleated fetal liver cells for the CFU-E count and 4.5 × 104 cells for the CFC-m count. Data from controls (blue indicates Mdm4lox/l+; EpoR+/+; and red, Mdm4lox/+; EpoRGFP-Cre/+) and mutants (yellow indicates Mdm4lox/lox; EpoRGFP-Cre/+) represent the means (± SD) of 6 independent experiments. There is a significant difference between the mean numbers of CFU-Es in the Mdm4 mutants compared with their control littermates by the Student test (P < .001; S). (C) Hematocrits of Mdm4lox/+; EpoR+/+ (n = 15), Mdm4lox/lox; EpoRGFP-Cre/+ (n = 7), and Mdm4lox/lox; EpoRGFP-Cre/+; p53−/− (n = 3) E14.5 littermates. □, ♦, and ▴ represent the data from the Mdm4lox/lox; EpoRGFP-Cre/+ mice, control mice, and Mdm4lox/lox; EpoRGFP-Cre/+; p53−/− mice, respectively. Differences between control and Mdm4lox/lox; EpoRGFP-Cre/+ mice are statistically significant by the Student t test (P < .001; S). (D-E) inactivation of Mdm4 in erythroid progenitors leads to decreased cell proliferation. (D) Immunohistologic staining of phosphorylated histone H3 in the lateral ventricle (L. Ventricle; used here as a control), fetal liver (F. Liver), and dorsal aorta (D. Aorta) of E14.5 embryos with the indicated genotypes. Magnifications are indicated. (E) Percentage of BrdU-positive cells among 200 erythroid cells located in the dorsal aorta of E14.5 embryos. Cells (3 × 200) were analyzed in 2 different embryos for each genotype. Data from controls (Mdm4lox/+; EpoR+/+) and mutants (Mdm4lox/lox; EpoRGFP-Cre/+) are represented by the black and gray bars, respectively. Error bars denote standard error. There is a significant difference between the mean numbers by the Student t test (P < .001; S). (F) Five adult control (Mdm4lox/+; EpoR+/+) and Mdm4lox/lox; EpoRGFP-Cre/+ mice were injected with phenylhydrazine on days 0, 1, and 3. The corrected reticulocyte count allows assessment of erythropoietic rate, and was calculated assuming a normal hematocrit of .45, as follows: Corrected reticulocyte count (%) = reticulocyte count (%) × (hematocrit × 0.01/0.45). □ and ♦ represent the data from the Mdm4lox/lox; EpoRGFP-Cre/+ mice and control mice, respectively.
Mdm4 is required for fetal definitive, but not for adult, erythropoiesis. (A) Mdm4lox/lox embryos that are either positive (EpoRGFP-Cre/+) or negative (EpoR+/+) for the EpoR-GFP-cre transgene at various stages of embryonic development. Mdm4lox/lox; EpoRGFP-Cre/+ embryos are significantly paler than control littermates at E14.5, whereas Mdm4lox/lox; EpoRGFP-Cre/+; p53−/−embryos appear normal. Scale bar equals 15 μm. (B) Number of CFU-Es and CFC-m's detected in methylcellulose cultures supplemented with Epo or a mixture of IL-3, IL-6, and SCF, respectively. Numbers are expressed per 6 × 105 nucleated fetal liver cells for the CFU-E count and 4.5 × 104 cells for the CFC-m count. Data from controls (blue indicates Mdm4lox/l+; EpoR+/+; and red, Mdm4lox/+; EpoRGFP-Cre/+) and mutants (yellow indicates Mdm4lox/lox; EpoRGFP-Cre/+) represent the means (± SD) of 6 independent experiments. There is a significant difference between the mean numbers of CFU-Es in the Mdm4 mutants compared with their control littermates by the Student test (P < .001; S). (C) Hematocrits of Mdm4lox/+; EpoR+/+ (n = 15), Mdm4lox/lox; EpoRGFP-Cre/+ (n = 7), and Mdm4lox/lox; EpoRGFP-Cre/+; p53−/− (n = 3) E14.5 littermates. □, ♦, and ▴ represent the data from the Mdm4lox/lox; EpoRGFP-Cre/+ mice, control mice, and Mdm4lox/lox; EpoRGFP-Cre/+; p53−/− mice, respectively. Differences between control and Mdm4lox/lox; EpoRGFP-Cre/+ mice are statistically significant by the Student t test (P < .001; S). (D-E) inactivation of Mdm4 in erythroid progenitors leads to decreased cell proliferation. (D) Immunohistologic staining of phosphorylated histone H3 in the lateral ventricle (L. Ventricle; used here as a control), fetal liver (F. Liver), and dorsal aorta (D. Aorta) of E14.5 embryos with the indicated genotypes. Magnifications are indicated. (E) Percentage of BrdU-positive cells among 200 erythroid cells located in the dorsal aorta of E14.5 embryos. Cells (3 × 200) were analyzed in 2 different embryos for each genotype. Data from controls (Mdm4lox/+; EpoR+/+) and mutants (Mdm4lox/lox; EpoRGFP-Cre/+) are represented by the black and gray bars, respectively. Error bars denote standard error. There is a significant difference between the mean numbers by the Student t test (P < .001; S). (F) Five adult control (Mdm4lox/+; EpoR+/+) and Mdm4lox/lox; EpoRGFP-Cre/+ mice were injected with phenylhydrazine on days 0, 1, and 3. The corrected reticulocyte count allows assessment of erythropoietic rate, and was calculated assuming a normal hematocrit of .45, as follows: Corrected reticulocyte count (%) = reticulocyte count (%) × (hematocrit × 0.01/0.45). □ and ♦ represent the data from the Mdm4lox/lox; EpoRGFP-Cre/+ mice and control mice, respectively.
The number of erythrocytes and erythroid colony-forming units (CFU-Es) in the blood vessels of the yolk sac of Mdm2lox/lox; EpoRGFP-Cre/+ embryos was dramatically reduced compared with control embryos (Figure 1B). In contrast, primitive erythropoiesis appears unaffected in Mdm4lox/lox; EpoRGFP-Cre/+ mutants (Figure 1B). Importantly, the efficiency of recombination was comparable at the Mdm2 and Mdm4 loci of the EpoRGFP-Cre/+ mutants (Figure S2).
Most erythroid cells were apoptotic in mdm2lox/lox; EpoRGFP-Cre/+ embryos, both at E10.5 in the blood islands of the yolk sac and at E12 in the heart and dorsal aorta (Figure 1C). In contrast, apoptosis was not detected in the erythroid lineage of mdm4lox/lox; EpoRGFP-Cre/+ mice (not shown). Importantly, whereas no increase in p53 protein levels was detected in erythrocytes from mdm4lox/lox; EpoRGFP-Cre/+ embryos (not shown), more than 90% of the nuclei from mdm2lox/lox; EpoRGFP-Cre/+ erythrocytes showed marked p53 immunoreactivity (Figure 1D).
Whereas Mdm4 is dispensable for primitive erythropoiesis, it is required for the high erythropoietic rate during definitive erythropoiesis. Mdm4lox/lox; EpoRGFP-Cre/+ fetal livers contain fewer CFU-Es (Figure 2B) and erythroid burst-forming units (BFU-Es; not shown) erythroid progenitors than controls. E14.5 mdm4lox/lox; EpoRGFP-Cre/+ embryos were paler (Figure 2A), and their hematocrits were 50% lower than that of controls (Figure 2C). This anemia did not cause embryonic death before E12.5 (Table S2); however, about 40% of mutants die, presumably from anemia, between E12.5 and day 21 after birth (P). Importantly, mdm4lox/lox; EpoRGFP-Cre/+; p53−/− did not show any erythropoietic defects (Figure 2A, C). Together, Mdm4 controls, in a p53-dependent manner, a rate-determining step during fetal liver erythropoiesis.
We next examined the cause of fetal anemia in Mdm4lox/lox; EpoRGFP-Cre/+ embryos. Because there was no significant increase in apoptosis in Mdm4-deficient erythroid progenitors, we examined their ability to proliferate. The number of phosphorylated histone H3-positive erythroid progenitors was strikingly reduced in Mdm4lox/lox; EpoRGFP-Cre/+ embryos (Figure 2D). Moreover, a statistically significant reduction in the percentage of S-phase cells was observed in the dorsal aorta of Mdm4lox/lox; EpoRGFP-Cre/+ embryos (Figure 2E). The data indicate that the anemia observed in these embryos is a consequence of decreased cell proliferation. In agreement, expression of p21 and Ptprv, 2 key mediators of p53-induced cell-cycle exit, was slightly but reproducibly increased in fetal liver cells from Mdm4lox/lox; EpoRGFP-Cre/+ embryos (not shown).
To determine whether Mdm4 continues to function beyond fetal life, we measured hematocrit, reticulocyte count, and the number of bone marrow–erythroid progenitors in surviving adult Mdm4lox/lox; EpoRGFP-Cre/+ mice. Surprisingly, these mice have normal hematocrit (Figure 2F), and similar numbers of bone marrow CFU-E and BFU-E erythroid progenitors compared with control mice (Figure S3). Moreover, after administration of phenylhydrazine, an agent that induces hemolytic anemia,20 the magnitudes and kinetics of reticulocyte counts and hematocrit fluctuations were similar in controls and Mdm4 mutants (Figure 2F). Thus, adult Mdm4lox/lox; EpoRGFP-Cre/+ mice are not deficient in generating high erythropoietic rates in response to erythropoietic stress. Importantly, the deleted (loxP) allele is largely predominant over the nondeleted (floxed) allele in Ter119-positive cells sorted from Mdm4lox/lox; EpoRGFP-Cre/+ bone marrow (Figure S3D). This result strongly argues against a specific selection of Mdm4-competent erythoid cells in these mice. Together, these data indicate that Mdm4 is specifically required for fetal erythropoiesis, but not for production of adult red cells.
We show in this study that Mdm2 has an essential function during primitive erythropoiesis in rescuing erythroid progenitors from p53-mediated apoptosis. In contrast, Mdm4 is dispensable for primitive erythropoiesis, but is required for generating high erythropoietic rates during definitive fetal erythropoiesis. Several recent reports have described the conditional inactivation of Mdm2 and Mdm4 in other cell types, including cardiomyocytes, progenitor and postmitotic neuronal cells, and smooth muscles of the gastrointestinal (GI) tract.12,14,21,22 As in our present study, inactivation of Mdm2 consistently led to a more pronounced activation of p53 function and consequently to phenotypes more remarkable than those caused by loss of Mdm4. Depending on the cell type, loss of Mdm4 leads to phenotypes ranging from not obvious, as in cardiomyocytes or smooth muscle cells of the GI tract, to minor, as in proliferating and postmitotic neuronal cells.23 So far, the data have been consistent with a model in which Mdm2 is the master regulator of p53, with Mdm4 functioning to assist Mdm2 only in specific cell types. The data herein are partly consistent with this model, but they also highlight the ability of Mdm4 to function, in a given tissue, specifically at a particular phase of the differentiation program. Mdm4 is indeed required only for the massive expansion phase of erythroid progenitor cells during definitive erythropoiesis. These cells undergo rapid, multiple DNA replication cycles with little time for DNA repair mechanisms to operate, conditions that favor p53 activation. In this context, it is not surprising that additional brakes on p53, such as that provided by Mdm4, are active in these cells. Notably, in adult mice Mdm4 is not required for steady-state erythropoiesis or for the increase in erythropoietic rate in response to stress. This observation further supports the existence of molecular differences between fetal and adult erythropoiesis.24,25
Authorship
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Jean-Christophe Marine, Laboratory for Molecular Cancer Biology, VIB Technologiepark, 927, B-9052 Ghent, Belgium; e-mail: chris.marine@dmbr.ugent.be.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dieter Defever and Ines Bonk for excellent technical assistance. S.F. and P.F. were supported by grants from FNRS-Télévie and Fondation pour la Recherche Médicale, respectively. This work was supported in part by grants from the Association for International Cancer Research, Belgian Foundation against Cancer (nonprofit organization), and by EC FP6 funding (ACTIVEP53, contract 503576). This publication reflects only the authors' views. The commission is not liable for any use that may be made of the information herein.