Abstract
Many stem cell types have been shown to differentiate into endothelial cells (ECs); however, their specification to arterial or venous endothelium remains unexplored. We tested whether a specific arterial or venous EC fate could be induced in human multipotent adult progenitor cells (hMAPCs) and AC133+ cells (hAC133+). In vitro, in the presence of VEGF165, hAC133+ cells only adopted a venous and microvascular EC phenotype, while hMAPCs differentiated into both arterial and venous ECs, possibly because hMAPCs expressed significantly more sonic hedgehog (Shh) and its receptors as well as Notch 1 and 3 receptors and some of their ligands. Accordingly, blocking either of those pathways attenuated in vitro arterial EC differentiation from hMAPCs. Complementarily, stimulating these pathways by addition of Delta-like 4 (Dll-4), a Notch ligand, and Shh to VEGF165 further boosted arterial differentiation in hMAPCs both in vitro and in an in vivo Matrigel model. These results represent the first demonstration of adult stem cells with the potential to be differentiated into different types of ECs in vitro and in vivo and provide a useful human model to study arteriovenous specification.
Introduction
The vascular system is a bipolar complex network of arteries that transport oxygen-rich blood to all tissues and veins that bring oxygen-deprived blood back to the heart.1 Because of this bipolar set-up, arteries and veins feature anatomic and physiological differences. Unlike venous endothelium, arterial endothelium is surrounded by several layers of smooth muscle cells (SMCs), separated by elastic laminae, and embedded in a thick layer of fibrillar collagen.2 Moreover, both vessel types have a differential susceptibility to atherosclerotic disease, possibly due to exposure to different levels of shear stress. Arterial and venous endothelial cells (ECs) also have a distinct molecular signature, and such molecular specification occurs before the onset of blood flow.3 Indeed, arteriovenous (AV) specification of ECs is accomplished early in development and is associated with the expression of a specific complement of factors: venous endothelium is characterized by the expression of EphB4,4 Lefty-1,5 Lefty-2,5 COUP-TFII,6 and MYO1-β,5 and arterial ECs express high levels of Notch 1 and 4,7 Dll-4,8 EphrinB1 and EphrinB2,4 Jagged-1 and -2,7 connexin-40, and Hey-2 (gridlock zebrafish ortholog).9,10 Studies in Xenopus, zebrafish, and mice have revealed that, besides blood flow,11 vessel-intrinsic cues and—later in development—signals from outside the vasculature12,13 are implicated in defining arterial or venous fate such as members of the TGF-β pathway,14,15 VEGF isoforms,13,16–18 neuropilins,17 angiopoietins,19 the Notch pathway,7,9,20–22 the patched pathway,20 and COUP-TFII, a member of the orphan nuclear receptor superfamily.6
Although it has been shown that some of these pathways are well conserved from zebrafish to mouse, less information is available on whether they have a similar role in humans. Because these molecular differences between arterial and venous ECs exist independently of blood flow and some of these factors work in an EC-intrinsic way,2 it should be possible to manipulate some or all of these to endow ECs with an arterial or venous fate. Consistent with this notion, recent studies have suggested that arterial markers can be induced in primary mature ECs.5,13,21,23,24
Many different stem cell populations, including bone marrow (BM) mononuclear cells, AC133+ endothelial progenitor cells, and embryonic stem cells have the potential to differentiate in vitro and in vivo into mature and functional ECs.4,25–28 We have recently described another stem cell population, multipotent adult progenitor cells (MAPCs), that differentiates into most somatic cell types, including functional ECs, in vitro and in vivo.29–33 The question of whether and how these stem cells can be coaxed into arterial or venous ECs has thus far not been addressed. In this study, we analyzed the in vitro and in vivo arterial and venous endothelial differentiation of human MAPCs (hMAPCs) and hAC133+ cells.
Materials and methods
Additional and extended descriptions of methods are included in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell populations
Samples were obtained after informed consent from donor or mother according to the guidelines from the Committee on the Use of Human Subjects in Research at the Clínica Universitaria, Pamplona, Spain. For hAC133+ cells, BM and umbilical cord blood mononuclear cells were separated by Ficoll Hypaque centrifugation (specific gravity, 1077; Sigma, St Louis, MO), and hAC133+ cells were selected using the autoMACS with the AC133 Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany).34 The purity of hAC133+ cells was more than 90% in all samples by flow cytometry. New hMAPC clones were established, based on protocols described previously,29–33 at the University of Navarra using BM from donors aged 18 to 54 years. BM mononuclear cells were depleted of CD45+ and glycophorin A–positive cells using micromagnetic beads (Miltenyi Biotec) or directly after Ficoll-Paque. Characterization of hMAPCs was done by fluorescence-activated cell sorter (FACS) phenotyping, pluripotency marker expression analysis, and 3-lineage differentiation (Document S1, Figure S1, Table S1). Primary human umbilical vein ECs (HUVECs) and human umbilical artery ECs (HUAECs) were prepared in-house from human umbilical cords.
Endothelial differentiation/specification of hAC133+ cells and hMAPCs
Human AC133+ cells.
For hAC133+ cells, a total of 105/cm2 were plated on fibronectin (50 μg/mL)–coated culture vessels in IMDM (Gibco BRL, Carlsbad, CA) supplemented with 20% fetal calf serum (FCS) (Gibco BRL), 50 ng/mL VEGF165 (R&D Systems, Minneapolis, MN), 10 ng/mL bFGF (Sigma), and 1% penicillin/streptomycin. Cultures were maintained by media exchange every 4 to 5 days.
Human MAPCs.
The hMAPCs were plated at 30 × 103 to 40 × 103/cm2 in growth media: 58% low-glucose DMEM (Gibco BRL), 40% MCDB-201 (Sigma), 2% FCS (Biochrom, Cambridge, United Kingdom), ITS+1 (Sigma), 10−8 M dexamethasone (Sigma), 10−4 M ascorbic acid 2-phosphate (Sigma), 1% penicillin/streptomycin (Gibco BRL), and 10 ng/mL each of PDGF-BB and EGF during 24 hours, and then media were exchanged with the same media without FCS, EGF, and PDGF-BB but now with 100 ng/mL VEGF165. Media were changed every 4 to 5 days. Arterial-specific differentiation was boosted by addition of different combinations of the following growth factors: VEGF165 in different combinations with Dll-4, Jagged-1, or Shh (all from R&D Systems at 100 ng/mL). Blocking of patched and Notch pathways was performed using cyclopamine35 (Biomol, Plymouth Meeting, PA) at 5 μM (added every 4 to 5 days with medium change) and 1 μM γ-secretase inhibitor L-685458 (Bachem, King of Prussia, PA),36 respectively.
FACS analysis
For fluorescence-activated cell sorter (FACS) analysis, cells were detached with 0.05% trypsin-EDTA and washed with phosphate-buffered saline (PBS). The following antibodies were used: CD31-PE, CD34-APC, αVβ3-PE, CD73-PE, CD45-PerCP, CD90-APC, HLA-DR–PE, HLA-DP–PE, HLA-DQ–PE, HLA-A–PE, HLA-B–PE, HLA-C–PE, CD44-PE, CD13-PE, CD36-FITC, CD16+CD56-PE, CD3-FITC, CD19-APC, CD11B-PE, CD11C-APC, CD14-APC, CD14 FITC (all from BD PharMingen, San Diego, CA), CD105-PE (Ancell, Bayport, MN), and CD133/1-PE and CD133/1APC (Miltenyi Biotec), and their corresponding isotype controls (all from BD PharMingen). From 50 000 to 200 000 cells were incubated with primary antibody for 15 minutes in the dark at room temperature. Cells were fixed with 4% paraformaldehyde at 4°C. Syto was used to determine cell viability when necessary. Samples were analyzed using a FACS Calibur (BD PharMingen) cell sorter and Cellquest (BD PharMingen) software.
Immunofluorescent and histochemistry staining and analysis
A list of primary antibodies is provided in Table S2. Secondary antibodies coupled to FITC or PE were from Molecular Probes (Eugene, OR). For immunofluorescent staining, samples were fixed with 4% paraformaldehyde at 20°C and, in case of intracellular molecules, permeabilized with 0.1% Triton X-100. Blocking solution consisted of PBS, 1% bovine serum albumin, and 10% donkey serum. Primary antibodies were diluted in blocking solution and applied overnight at 4°C. After incubation, nonspecific binding was washed with a solution of PBS and 0.1% Tween 20. Secondary antibody at a dilution 1:1000 in PBS was applied for 1 hour at 4°C. Nonadherent antibody was washed with PBS and 0.1% Tween 20, after which samples were mounted using DAPI (Vector Laboratories, Burlingame, CA) or TO-PRO-3 iodide (Molecular Probes, Leiden, The Netherlands) as nuclear marker. For controls, cells were labeled with unspecific immunoglobulins (Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with the secondary antibody. For immunohistochemistry staining, the Envision (DAKO, Glostrup, Denmark) and ABC (Vector Laboratories) systems were used. Sirius red37 and orcein38 staining were performed as described. To quantify the percentage of cultured cells expressing arterial or venous markers, the number of positive cells in 20 randomly selected fields were scored and divided by the total number of cells.
Images were generated using a Zeiss microscope (Zeiss, Jena, Germany) connected to a monochrome fluorescence camera (Coolsnap SF; Photometrics, ____, Germany) and a CCD camera (SPOT; Diagnostic Instruments, Sterling Heights, MI), equipped with 20×/0.75 NA dry, 40×/0.75 NA dry, and 63×/1.40 NA oil objectives (Zeiss). Morphometric analyses were performed using MetaMorph software (version 6.3r6; Molecular Devices, Downington, PA). Immersion oil was purchased from Zeiss.
RNA isolation and real-time and quantitative RT-PCR
Total RNA was obtained using the RNeasy Mini extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. A more detailed description of methods and the primers used for reverse transcriptase–polymerase chain reaction (RT-PCR) and real-time PCR are shown in Document S1 and Table S3, respectively.
In vitro EC functional tests
To analyze acetylated-LDL uptake, cells were washed and 10 μg/mL acetylated-LDL-DiI (Biomedical Technologies, Stoughton, MA) was added in IMDM or MAPC differentiation medium (described under “Human MAPCs,” above). Cells were incubated for 2 hours at 37°C, washed, fixed, and viewed under a fluorescent microscope using DAPI as nuclear marker. For the in vitro Matrigel assay, 1 mm cold (4°C) Matrigel (BD Pharmingen) was incubated for 30 minutes at 37°C. After gelification, 30 × 103 to 50 × 103 cells differentiated for 14 days were plated in differentiation media on Matrigel. After 24 to 48 hours, tube formation was analyzed.
Electron microscopy
For ultrastructural studies, samples were washed in PBS and fixed with 2% glutaraldehyde. Samples were postfixed with 1% osmium, rinsed, dehydrated, and embedded in Araldite (Fluka, Buchs, Switzerland). Semithin sections (1.5 μm) were cut with a diamond knife and stained lightly with 1% toluidine blue. Then, semithin sections were reembedded in an araldite block and detached from the glass slide by repeated freezing (liquid nitrogen) and thawing. The block with semithin sections was cut into ultrathin (50 to 70 nm) sections, stained with lead citrate, and examined under a Jeol (Tokyo, Japan) JEM-1010 electron microscope.
ELISA
To assess cytokine production of undifferentiated cells, hMAPCs were plated in triplicate at 30 × 103 to 40 × 103/cm2 at day 0 in cytokine-less expansion media, and supernatant was collected 60 hours later and frozen. To assess cytokine production in differentiated cells, cells were plated in triplicate for endothelial differentiation as described above and media were collected after 7 and 14 days and frozen. Enzyme-linked immunosorbent assay (ELISA) kits were from R&D Systems, and the procedure was performed according to the manufacturer's recommendations.
In vivo Matrigel model
For the in vivo Matrigel plug assay, 10-week-old nude mice were injected subcutaneously in the back with 0.5 mL cold growth factor–reduced Matrigel containing 300 ng/mL VEGF165 alone, or 300 ng/mL VEGF165 + 100 ng/mL Shh + 100 ng/mL Dll-4, combined or not with 0.5 × 106 undifferentiated hMAPCs or hAC133+ cells (unlabeled or labeled with carboxyfluorescein succinimidyl ester [CFSE; Molecular Probes] or Resovist [Schering, Berlin, Germany] as described39 ). In some animals, 0.1 × 106 or 2.5 × 106 cells were injected. Ten days after injection, animals were perfusion fixed and Matrigel plugs were removed and processed for paraffin or OCT embedding. Tissue sections were examined and photographed under a fluorescence microscope (Zeiss) or a confocal microscope. Ultrastructural analysis was performed as described above. In vivo live imaging was performed under anesthesia using a Leica (Weztlar, Germany) dissection microscope.
Results
Isolation and qualification of hMAPCs
For the current studies, 8 new clones of hMAPCs very similar in their characteristics were used. They were established at the University of Navarra using methods as previously described32,33 and maintained for more than 50 to 80 population doublings (Table S1). The phenotype of most cells within these clones was CD90+, CD13+, CD44low, MHC-I−, αvβ3−, CD73−, AC133−, CD105−, MHC-II−, CD36−, CD45−, and CD34− (Figure S1A), largely consistent with their initial characterization.33 RT-PCR demonstrated presence of the transcription factors Oct3/4, Rex-1, and nanog as well as hTERT (Figure S1B), and cells stained positive for SSEA-4, Oct3/4, and nanog but not SSEA-1 proteins (Figure S1C-F). We further qualified the cells by showing differentiation into mesodermal (ECs, Figures S2J-R and S3; SMCs, Figure S1G), endodermal (hepatocytes, Figure S1H), and ectodermal (neurons, Figure S1I) cell types.
VEGF165 induces hAC133+ cells and hMAPCs to differentiate into functional ECs
First, we compared the ability of hMAPCs and cord blood– or BM-derived hAC133+ cells, a cell population previously shown to be enriched for endothelial, neuronal, and hematopoietic progenitors,25,26 to differentiate into functional mature endothelium. Culture of hAC133+ cells in the presence of VEGF165 induced down-regulation of AC133 and hematopoietic markers CD45 and CD34 and up-regulation of mature EC markers, CD105 and αvβ3 (Figure S2A-B), as described.26 Most of the cells (about 80%; Table S4) in 21-day cultures (which we further designate “hAC133-ECs”) expressed von Willebrand factor (VWF), angiopoietin receptors Tie-1 and -2, and VEGF receptors 1 and 2 (Flt-1 and KDR, respectively) (Figure S2C-G) and were functional as demonstrated by acetylated-LDL uptake (Figure S2H; Table S4) and vascular tube formation on Matrigel (Figure S2I). A fraction (about 20%) of hAC133+ cells differentiated to hematopoietic cells, indicated by coexpression of the CD45+ antigen and other myeloid-monocytic antigens (Figure S4). Unlike human EC controls, which mostly showed a stellate shape in culture (Figure S5), hAC133-ECs were mainly rounded (FigS2C-H). Most (more than 80%) hMAPCs cultured in the presence of VEGF165 acquired EC markers, including KDR, Flt-1, Tie-1, Tie-2, CD105, VE-cadherin, CD31, VWF, and αvβ3, while expression of hematopoietic markers CD34 and CD45 was very low or absent (Figures S2J-P and S3; Table S4). The resultant hMAPC-ECs were functional as shown by acetylated-LDL uptake (Figure S2Q; Table S4) and vascular tube formation on Matrigel (Figure S2R). While no SMC α-actin–positive cells were observed in hAC133+ cell–derived cultures, hMAPCs gave rise to SMC α-actin–positive cells representing 5% of the differentiated cells (not shown). For hMAPCs, the increase in mRNA expression varied between different endothelial genes from 5-fold (CD31) to more than 600-fold (Flt-1) (Figure S3).
VEGF165 supports arterial differentiation of hMAPCs but not hAC133+ cells
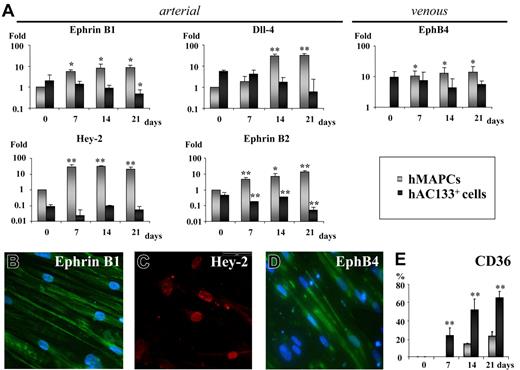
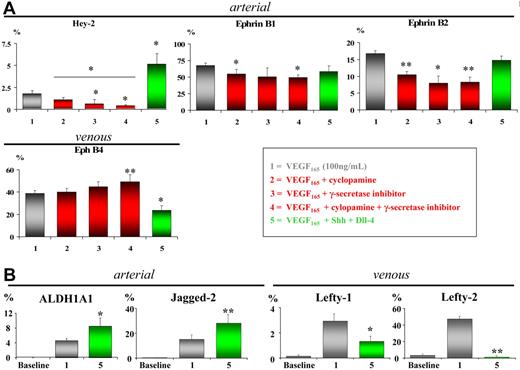
Studies in zebrafish and mice have suggested that VEGF165 would be part of a cascade involving Shh, Notch, and EphrinB2 that induces arterial differentiation in vivo and that VEGF165 alone may be sufficient to induce an arterial phenotype in vivo.20,40 To study whether VEGF165 was able to support arterial EC differentiation from stem cells in vitro, we determined using quantitative (Q)–RT-PCR and/or immunofluorescence if the arterial markers Hey-2, Dll-4, EphrinB2, and EphrinB1 and the venous marker EphB4 were expressed in hMAPC-ECs and hAC133-ECs generated in the presence of VEGF165. Low transcripts levels for arterial- and venous-specific genes were detected in either cell population before differentiation. In hAC133+ cells, VEGF165 treatment decreased arterial markers while venous markers remained stable. In contrast, VEGF165 treatment induced significant levels of the arterial markers Hey-2, Dll-4, EphrinB1, and EphrinB2 as well as the venous marker EphB4 in hMAPCs (Figures 1A and S6). Ten- to 100-fold differences were observed in arterial gene expression between hAC133-ECs and hMAPC-ECs. At the protein level, determined by immunofluorescence, expression of arterial Hey-2 (48.3% ± 3.5% of the cells were Hey2+), EphrinB1 (65.8% ± 4%), and venous EphB4 (31.2% ± 2.8%) was found in hMAPC-ECs at day 14 (Figure 1B-D), while no protein expression was detected at baseline (not shown). Interestingly, while most hAC133-ECs expressed CD36, suggesting a microvascular phenotype, hMAPC-ECs were mostly CD36−, suggesting a macrovascular phenotype41–43 (Figure 1E). Together, this suggests the ability of hMAPCs but not hAC133+ cells to differentiate into arterial ECs in addition to venous ECs.
VEGF165 induces arterial specification of hMAPCs but not hAC133+ cells. (A) Q-RT-PCR for arterial (EphrinB1, Dll-4, Hey-2, EphrinB2) and venous markers (EphB4) on hAC133+ cell–derived ECs (▪) or hMAPC-derived ECs (⊡) at different time points (0, 7, 14, and 21 days) after the start of the differentiation process. While hMAPCs up-regulated arterial and venous markers during the differentiation process, hAC133+ cell–derived ECs showed reduced arterial marker expression. Expression levels are presented as fold increase (in logarithmic scale) in comparison with baseline levels and were normalized by using GAPDH as housekeeping gene. The mRNA levels in undifferentiated hMAPCs were considered as 1. Expression between baseline levels (day 0) and day 7, 14, and 21 for each cell population was compared (*P < .05; **P < .01). (B-D) Immunofluorescent staining of hMAPC-derived ECs. After 14 days, hMAPCs were positive for arterial markers EphrinB1 (B), Hey-2 (C), and venous marker EphB4 (D) (see text for percentage of positive cells). A representative example from 3 different clones is shown. (E) Comparative expression, plotted as percentage of total number of cells, based on FACS analysis, of the microvascular-specific marker CD36 in hMAPC (⊡) and hAC133+ cell–derived ECs (▪) (**P < .01 versus hMAPC-ECs). The mean (± SEM) of 3 (A) or 5 (E) different experiments in triplicate is shown. Magnification ×40.
VEGF165 induces arterial specification of hMAPCs but not hAC133+ cells. (A) Q-RT-PCR for arterial (EphrinB1, Dll-4, Hey-2, EphrinB2) and venous markers (EphB4) on hAC133+ cell–derived ECs (▪) or hMAPC-derived ECs (⊡) at different time points (0, 7, 14, and 21 days) after the start of the differentiation process. While hMAPCs up-regulated arterial and venous markers during the differentiation process, hAC133+ cell–derived ECs showed reduced arterial marker expression. Expression levels are presented as fold increase (in logarithmic scale) in comparison with baseline levels and were normalized by using GAPDH as housekeeping gene. The mRNA levels in undifferentiated hMAPCs were considered as 1. Expression between baseline levels (day 0) and day 7, 14, and 21 for each cell population was compared (*P < .05; **P < .01). (B-D) Immunofluorescent staining of hMAPC-derived ECs. After 14 days, hMAPCs were positive for arterial markers EphrinB1 (B), Hey-2 (C), and venous marker EphB4 (D) (see text for percentage of positive cells). A representative example from 3 different clones is shown. (E) Comparative expression, plotted as percentage of total number of cells, based on FACS analysis, of the microvascular-specific marker CD36 in hMAPC (⊡) and hAC133+ cell–derived ECs (▪) (**P < .01 versus hMAPC-ECs). The mean (± SEM) of 3 (A) or 5 (E) different experiments in triplicate is shown. Magnification ×40.
Notch/patched pathway members are differentially expressed in hMAPCs and hAC133+ cells
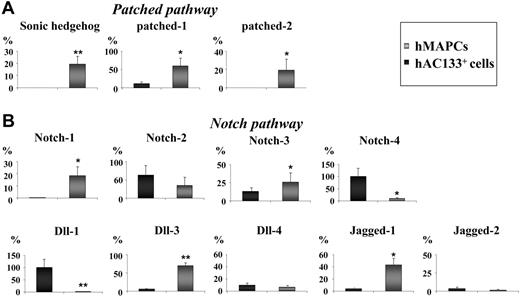
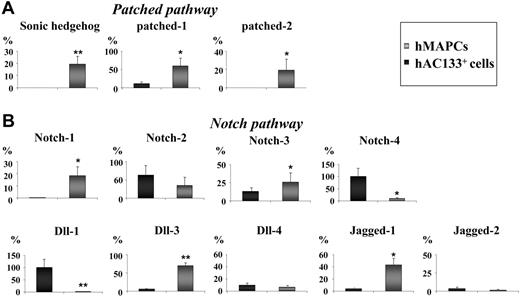
Because AV specification during zebrafish and mouse embryogenesis is in large part mediated by Notch and Shh in a cell-intrinsic manner,2 we compared the expression of Notch and its ligands, Jagged and Dll, and Shh and its receptor patched in undifferentiated hMAPCs and hAC133+ cells by Q-RT-PCR. Expression of Shh was restricted to hMAPCs (Figure 2A), and the expression of its receptors patched 1 and patched 2 was significantly higher in hMAPCs compared with hAC133+ cells (Figure 2A). Likewise, Notch-1 was uniquely expressed in hMAPCs (Figure 2B), and Dll-3, Jagged-1, and Notch-3 were more highly expressed in hMAPCs than hAC133+ cells (Figure 2B), while Dll-1 and Notch-4 were expressed preferentially in hAC133+ cells (Figure 2B). Expression of Jagged-2, Notch-2, and Dll-4 was not significantly different between the 2 undifferentiated cell populations (Figure 2B). To determine whether the different response to VEGF165 in hMAPCs and hAC133+ cells could be due to differences in VEGF receptor or endogenous VEGF165 expression, we compared their expression levels by Q-RT-PCR. Baseline endogenous VEGF165 expression was similar in both cell populations as well as expression of Flt-1 and KDR (not shown). Thus, the expression of Shh and patched, several of the Notch ligands, and receptors in hMAPCs may at least in part account for the ability of hMAPCs but not hAC133+ cells to differentiate along the arterial EC lineage.
Notch and patched pathway members are differentially expressed in hMAPCs and hAC133+ cells. Q-RT-PCR analysis of members of the patched pathway (Shh, patched-1, and patched-2) (A) and the Notch pathway (Notch-1, -2, -3, and -4 and ligands Dll-1, -3, -4, and Jagged-1 and -2) (B) known to be involved in AV specification. Note differences in expression (see “Results”) between hMAPCs compared with hAC133+ cells. The mRNA levels in all panels are expressed in percentage versus a positive control (total RNA) and were normalized by using GAPDH as housekeeping gene. The mean (± SEM) of 3 different experiments in triplicate is shown. *P < .05; **P < .01 versus hAC133+ cells.
Notch and patched pathway members are differentially expressed in hMAPCs and hAC133+ cells. Q-RT-PCR analysis of members of the patched pathway (Shh, patched-1, and patched-2) (A) and the Notch pathway (Notch-1, -2, -3, and -4 and ligands Dll-1, -3, -4, and Jagged-1 and -2) (B) known to be involved in AV specification. Note differences in expression (see “Results”) between hMAPCs compared with hAC133+ cells. The mRNA levels in all panels are expressed in percentage versus a positive control (total RNA) and were normalized by using GAPDH as housekeeping gene. The mean (± SEM) of 3 different experiments in triplicate is shown. *P < .05; **P < .01 versus hAC133+ cells.
Shh or Notch pathway blocking attenuates arterial EC differentiation in hMAPCs
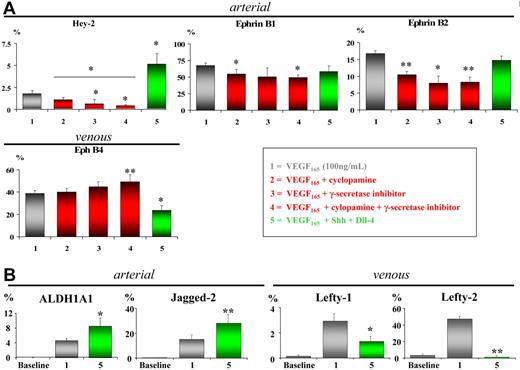
To investigate the causal involvement of Notch and/or Shh/patched pathways in arterial EC differentiation from hMAPCs, we manipulated each of them separately. Specific blocking of Shh signaling by cyclopamine-mediated inhibition of the patched receptor complex35 significantly decreased expression of arterial EC markers Hey-2, EphrinB1, and EphrinB2 and simultaneously slightly (but not statistically significant) increased expression of the venous marker EphB4 (Figure 3A). A more pronounced attenuation of arterial EC marker expression was observed by blocking the Notch pathway using an inhibitor for γ-secretase, essential for Notch receptor activation36 (Figure 3A). Compared with either inhibitor alone, combining cyclopamine and γ-secretase inhibitor further significantly decreased expression of Hey-2 (P < .05) but not of EphrinB1 or EphrinB2 (P > .05) (Figure 3A). These results suggest that arterial specification in hMAPCs in the presence of VEGF165 is at least in part mediated by the patched and Notch pathways.
Blockage of Notch/patched pathway attenuates while stimulation increases arterial EC differentiation of hMAPCs. (A) Q-RT-PCR analysis for arterial (EphrinB1, Hey-2, and EphrinB2) and venous markers (EphB4) on hMAPC-derived ECs (gray bars) after 14 days of differentiation using blocking (red bars) or activating (green bars) treatments (as indicated in the box). Shh blocking, Notch blocking, or a combination of both significantly reduced expression of arterial EC markers paralleled by an increase in venous marker expression compared with VEGF165 alone (gray bars). Conversely, addition of Shh and Dll-4 to VEGF165 further significantly increased expression of arterial marker Hey-2 while significantly decreasing venous marker EphB4. *P < .05; **P < .01 versus VEGF165 alone; *P < .05 condition 4 versus condition 2. (B) Q-RT-PCR analysis of additional arterial (ALDH1A1, Jagged-2) and venous markers (Lefty-1, Lefty-2) in hMAPC-derived ECs cultured in VEGF165 alone (gray bars) or combined with Shh and Dll-4 (green bars). Note the significant up-regulation of arterial markers and simultaneous down-regulation of venous markers in the combination cocktail as compared with VEGF165 alone. The mRNA levels in all panels are expressed as mean percentage of HUAECs (arterial markers) and HUVECs (venous markers) and were normalized using GAPDH as housekeeping gene. Baseline corresponds to undifferentiated hMAPCs. The mean (± SEM) of 3 different experiments is shown. *P < .05; **P < .01 versus VEGF165 alone.
Blockage of Notch/patched pathway attenuates while stimulation increases arterial EC differentiation of hMAPCs. (A) Q-RT-PCR analysis for arterial (EphrinB1, Hey-2, and EphrinB2) and venous markers (EphB4) on hMAPC-derived ECs (gray bars) after 14 days of differentiation using blocking (red bars) or activating (green bars) treatments (as indicated in the box). Shh blocking, Notch blocking, or a combination of both significantly reduced expression of arterial EC markers paralleled by an increase in venous marker expression compared with VEGF165 alone (gray bars). Conversely, addition of Shh and Dll-4 to VEGF165 further significantly increased expression of arterial marker Hey-2 while significantly decreasing venous marker EphB4. *P < .05; **P < .01 versus VEGF165 alone; *P < .05 condition 4 versus condition 2. (B) Q-RT-PCR analysis of additional arterial (ALDH1A1, Jagged-2) and venous markers (Lefty-1, Lefty-2) in hMAPC-derived ECs cultured in VEGF165 alone (gray bars) or combined with Shh and Dll-4 (green bars). Note the significant up-regulation of arterial markers and simultaneous down-regulation of venous markers in the combination cocktail as compared with VEGF165 alone. The mRNA levels in all panels are expressed as mean percentage of HUAECs (arterial markers) and HUVECs (venous markers) and were normalized using GAPDH as housekeeping gene. Baseline corresponds to undifferentiated hMAPCs. The mean (± SEM) of 3 different experiments is shown. *P < .05; **P < .01 versus VEGF165 alone.
Simultaneous Notch and patched activation boosts arterial EC fate in hMAPCs
To further evaluate the role of Notch and patched in arterial specification of hMAPCs, we evaluated the effect of VEGF165 (alone or combined with either Dll-4 or Jagged-1) and Shh (alone or in combination) (Figure S6). Of all combinations tested, addition of Dll-4 and Shh most efficiently increased expression of Hey-2 along with down-regulation of venous marker EphB4, indicating a preferential differentiation toward arterial endothelium (Figures 3A and S6). We analyzed the latter condition (VEGF165 + Shh + Dll-4) in more detail by examining the expression of additional arterial specific genes, showing that in addition to Hey-2, Jagged-2 and ALDH1A1 were also up-regulated, in agreement with previous work with HUVECs.5 Increased expression of arterial markers was associated with decreased levels of additional venous-specific Lefty-1 and Lefty-2 transcripts (Figure 3B). While Notch and patched activation enhanced arterial markers in hMAPCs, a similar effect was not observed in hAC133+ cells (Figure S6).
Shh and Dll-4 boost arterial EC differentiation of hMAPCs and arterial-like vessel growth in vivo
To determine whether the same factors could also induce hMAPC differentiation into arterial endothelium in vivo, we injected 0.5 × 106 undifferentiated hMAPCs in growth factor–reduced Matrigel containing either VEGF165 (“standard media”) or VEGF165 + Shh + Dll-4 (“arterial media”) under the skin of nude mice (n = 6 per group). To account for effects of the admixed cytokines on host cells, we also included the corresponding “cytokine-alone” groups. To track the cells following implantation, hMAPCs were labeled with CFSE or iron particles (Resovist)39,44 before injection. Irrespective of the cytokine cocktail used, localized areas of CFSE-labeled cells (Figure 4A) and single Resovist-labeled hMAPC-derived cells (Figure 4B) persisted for at least 10 days in the Matrigel plug as determined by in vivo live imaging and electron microscopy, respectively. Most implanted cells expressed (human) CD31 and (human) VE-cadherin (Figure 4C-D) and Fli-1 (not shown), indicating their EC identity. The hMAPC-ECs contributed to 2.3% ± 0.7% of the vessels in the Matrigel plugs with standard media and 3.1% ± 1.3% in arterial media, indicating that most cells of blood vessels in the Matrigel plugs were host derived. Dose-response studies revealed that engraftment strongly correlated with cell dose and cell dose with vascularity (Figure S7; Table S5).
Shh and Dll-4 boost functional arterial hMAPC-EC differentiation in vivo. (A) Live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation. Note the localized CFSE-labeled area (outlined by a dashed white line) located in the Matrigel in the vicinity of a large vascular tree (arrowheads) from the overlying host skin. (B-J) Histologic analysis on cross-sections through Matrigel plugs containing hMAPCs and VEFG165 (B-D) or hMAPCs and VEGF165 + Shh+ Dll-4 (“arterial cytokine mix” [E-J]). (B) Electron microscopy showing a capillary composed of a Resovist-labeled hMAPC-derived EC in Matrigel plugs. A semithin section (B, top panel), an ultrathin section (B, bottom panel), and a detail of iron particles (insets in lower panel of B) are shown. (C-F) Immunohistochemical staining of 3 μm paraffin cross-sections through Matrigel plugs for human-specific CD31 (C) and human-specific VE-cadherin (D) (both indicating their EC identity) and human-specific Hey-2 (E) and human-specific EphrinB1 (F) (both indicating their arterial EC identity). Arrows indicate red blood cells in vessel lumen. (G-H) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and Hey-2 (red) (G) or UEA (green) and Ephrin B1 (red) (H). Topro (blue) was used for nuclear staining. (I) High-resolution live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation and 30 minutes after intravenous injection of UEA lectin. Note colocalization (yellow; indicated by arrowheads) of CFSE-labeled cells (green) and UEA lectin (red) area, indicating that the vessels containing CFSE-labeled cells were connected to the host vascular system. (J) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and α-actin (red), showing hMAPC-ECs (arrowheads) coated by α-actin–positive SMCs. Topro (blue) was used for nuclear staining. “L” in panels B and D-F indicates the vessel lumen. Magnifications ×63 (E-F), ×40 (C-D,J), and ×20 (G-I). Scale bars in panel B: 10 μm (semithin); 2.5 μm (ultrathin); 1 μm (upper inset); 0.5 μm (lower inset).
Shh and Dll-4 boost functional arterial hMAPC-EC differentiation in vivo. (A) Live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation. Note the localized CFSE-labeled area (outlined by a dashed white line) located in the Matrigel in the vicinity of a large vascular tree (arrowheads) from the overlying host skin. (B-J) Histologic analysis on cross-sections through Matrigel plugs containing hMAPCs and VEFG165 (B-D) or hMAPCs and VEGF165 + Shh+ Dll-4 (“arterial cytokine mix” [E-J]). (B) Electron microscopy showing a capillary composed of a Resovist-labeled hMAPC-derived EC in Matrigel plugs. A semithin section (B, top panel), an ultrathin section (B, bottom panel), and a detail of iron particles (insets in lower panel of B) are shown. (C-F) Immunohistochemical staining of 3 μm paraffin cross-sections through Matrigel plugs for human-specific CD31 (C) and human-specific VE-cadherin (D) (both indicating their EC identity) and human-specific Hey-2 (E) and human-specific EphrinB1 (F) (both indicating their arterial EC identity). Arrows indicate red blood cells in vessel lumen. (G-H) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and Hey-2 (red) (G) or UEA (green) and Ephrin B1 (red) (H). Topro (blue) was used for nuclear staining. (I) High-resolution live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation and 30 minutes after intravenous injection of UEA lectin. Note colocalization (yellow; indicated by arrowheads) of CFSE-labeled cells (green) and UEA lectin (red) area, indicating that the vessels containing CFSE-labeled cells were connected to the host vascular system. (J) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and α-actin (red), showing hMAPC-ECs (arrowheads) coated by α-actin–positive SMCs. Topro (blue) was used for nuclear staining. “L” in panels B and D-F indicates the vessel lumen. Magnifications ×63 (E-F), ×40 (C-D,J), and ×20 (G-I). Scale bars in panel B: 10 μm (semithin); 2.5 μm (ultrathin); 1 μm (upper inset); 0.5 μm (lower inset).
Following transplantation with all cell doses (0.5 × 106 to 2.5 × 106 cells), several hMAPC-ECs generated in the presence of the arterial cytokine combination—while arterial marker expression was only detected with the highest cell dose with VEGF165 alone (Figure S8)—expressed both human-specific lectin UEA and human-specific arterial markers Hey-2 and EphrinB1, as shown by immunohistochemistry (Figure 4E-F) and double immunofluorescence confocal microscopy (Figure 4G-H), demonstrating differentiation of hMAPCs in vivo to arterial endothelium. Importantly, these arterial hMAPC-ECs contributed to vessels functionally connected to the host vasculature as demonstrated by the presence of erythrocytes in their lumen (Figure 4F). Also, in some animals, we injected TRITC-labeled UEA lectin (that specifically binds to human ECs) in the tail vein 30 minutes before killing. Colabeling of TRITC-UEA with CFSE (with which the hMAPCs were labeled) confirmed that the hMAPC-EC–containing vessels were connected to the host vasculature (Figure 4I). Although transplanted hAC133+ cells gave rise to CD31+ UEA lectin-positive ECs, the latter did not express arterial markers, nor were they associated with larger vessels (Figure S9). Thus, as in vitro, unlike hAC133+ cells, hMAPCs—and more so with the arterial cytokine mix—could be specified to functional arterial ECs in vivo.
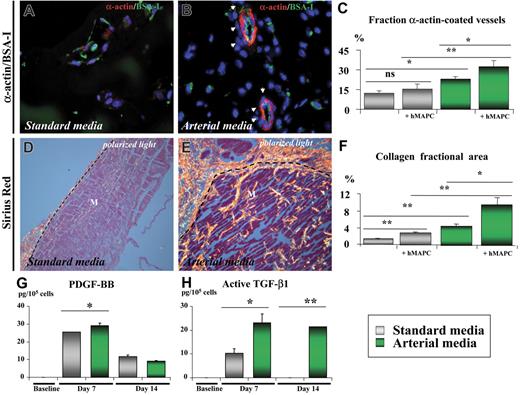
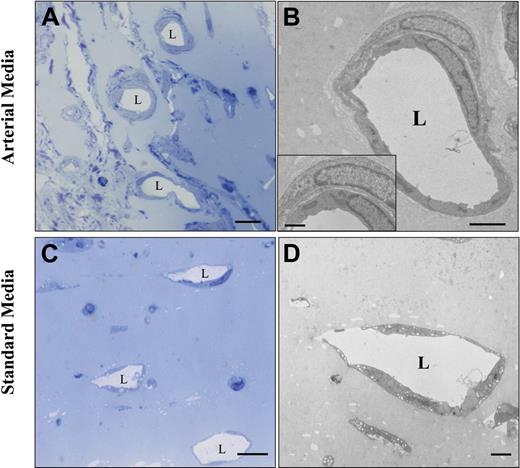
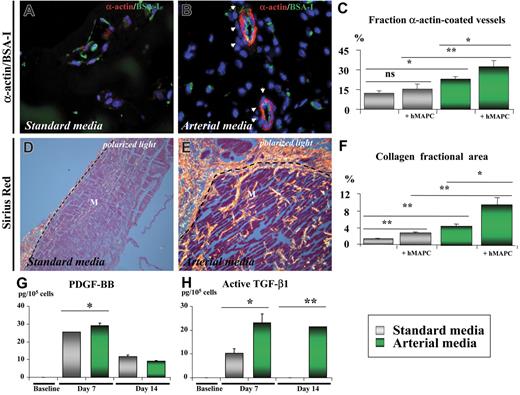
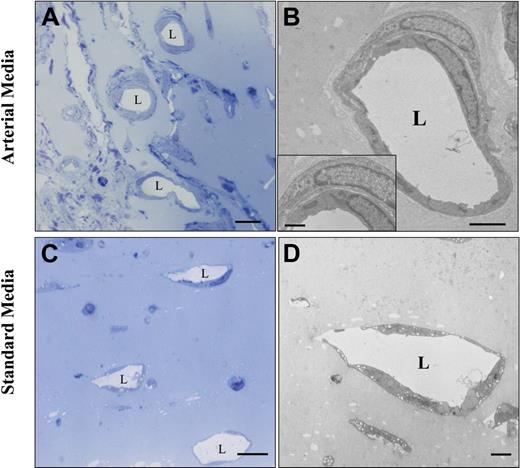
In addition to its arterial differentiation effect on hMAPC-ECs, the arterial cytokine mix also induced the formation of arterial-like vessels in which both implanted and host cells participated. Indeed, coating with host α-actin–positive SMCs of human EC-containing vessels was frequently observed in the Matrigel plugs containing the arterial cytokine combination, as shown by double confocal immunofluorescence (Figure 4J). Moreover, in addition to a significant increase in total vessel number (number of lectin-positive vessels: 124 ± 16/mm2 in arterial media versus 74 ± 10/mm2 in standard media; P < .05), the arterial mix also significantly increased the fraction of vessels coated with SMCs (32% ± 5% in arterial media versus 15% ± 4% in standard media; P < .05; Figure 5A-C) as well as the diameter of these vessels (20.1 ± 4.2 μm versus 14.7 ± 3.7 μm; P = .01). Consistent with an effect of the cytokine mix on host cells, without addition of hMAPCs more α-actin coated vessels were also seen in Matrigels containing arterial versus standard media. However, when hMAPCs were coimplanted, they had an additive effect on SMC coating (Figure 5C), in part by in situ differentiation to SMCs (not shown). Significantly more deposition of Sirius red–positive fibrillar collagen (Figure 5D-F) and orcein-positive elastin (not shown), both characteristics of arteries, could be detected surrounding the newly formed EC channels when hMAPCs and the arterial cytokine combination was used. Again, even in the absence of hMAPCs, there was more collagen deposition with the arterial mix than with standard media, although it was significantly higher when hMAPCs were coimplanted (Figure 5F). In addition to a direct contribution to arterial growth, we hypothesized that the additive effect of hMAPCs on SMCs and extracellular matrix (ECM) coating was due to trophic effects by secretion of arteriogenic cytokines (similar to what has been described for other stem cells45 ). In support of such a hypothesis, an increase in the production of PDGF-BB and TGF-β1, factors known to stimulate SMC proliferation and ECM production, was observed after in vitro culture of hMAPCs in the presence of the standard media and more so in the presence of the arterial cytokine combination (Figure 5G-H). Electron microscopic analysis further confirmed the differences in complexity and caliber between vessels formed in Matrigel plugs with standard versus arterial media (Figure 6). Together, these data indicate that the arterial cytokine combination induced the formation of vessels with arterial characteristics and that the presence of hMAPCs had an additive effect on this process.
Shh and Dll-4 together with hMAPCs additively increase formation of arterial-like vascular structures in vivo. (A-F) Histologic analysis on cross-sections through Matrigel plugs containing hMAPCs and VEFG165 (A,D) or hMAPCs and VEGF165 + Shh + Dll-4 (B,E). (A-B) Double immunofluorescent staining of 3 μm paraffin cross-sections through Matrigel plugs stained with SMC α-actin (red) and BS-I lectin (staining ECs in green) showing more SMC-coated (indicated by arrowheads) vessels when the arterial media were used (B) in comparison with standard media (A). (C) Diagram comparing the fraction of SMC-coated vessels (expressed as percentage ± SEM versus the total number of vessels) for the conditions outlined in the box; *P < .05; **P < .01. Note the additive effect of hMAPCs. (D-E) Sirius red staining (visualized by polarized light microscopy) indicating abundant and thick (orange-red birefringent) fibrillar collagen around vessels in Matrigels containing hMAPCs combined with the arterial mix (E) as compared with the less abundant and thinner collagen in Matrigels containing hMAPCs combined with VEGF165 alone (D); dashed lines indicate the edge of the Matrigel (M). (F) Diagram comparing the collagen fractional area (expressed as percentage ± SEM versus the total area) for the conditions outlined in the legend; *P < .05; **P < .01. Note the additive effect of hMAPCs. (G,H) ELISA for PDGF-BB (G) and active TGF-β1 (H) on cell supernatants of undifferentiated hMAPCs (“baseline”) or differentiated for 7 or 14 days to ECs either in standard media (gray bars) or arterial media (green bars). While PDGF-BB production was only slightly and temporarily higher, active TGF-β1 production was significantly higher in arterial media versus standard media. *P < .05; **P < .01. Data are expressed as picogram per 105 cells and represent the mean (± SEM) of 3 experiments performed in triplicate.
Shh and Dll-4 together with hMAPCs additively increase formation of arterial-like vascular structures in vivo. (A-F) Histologic analysis on cross-sections through Matrigel plugs containing hMAPCs and VEFG165 (A,D) or hMAPCs and VEGF165 + Shh + Dll-4 (B,E). (A-B) Double immunofluorescent staining of 3 μm paraffin cross-sections through Matrigel plugs stained with SMC α-actin (red) and BS-I lectin (staining ECs in green) showing more SMC-coated (indicated by arrowheads) vessels when the arterial media were used (B) in comparison with standard media (A). (C) Diagram comparing the fraction of SMC-coated vessels (expressed as percentage ± SEM versus the total number of vessels) for the conditions outlined in the box; *P < .05; **P < .01. Note the additive effect of hMAPCs. (D-E) Sirius red staining (visualized by polarized light microscopy) indicating abundant and thick (orange-red birefringent) fibrillar collagen around vessels in Matrigels containing hMAPCs combined with the arterial mix (E) as compared with the less abundant and thinner collagen in Matrigels containing hMAPCs combined with VEGF165 alone (D); dashed lines indicate the edge of the Matrigel (M). (F) Diagram comparing the collagen fractional area (expressed as percentage ± SEM versus the total area) for the conditions outlined in the legend; *P < .05; **P < .01. Note the additive effect of hMAPCs. (G,H) ELISA for PDGF-BB (G) and active TGF-β1 (H) on cell supernatants of undifferentiated hMAPCs (“baseline”) or differentiated for 7 or 14 days to ECs either in standard media (gray bars) or arterial media (green bars). While PDGF-BB production was only slightly and temporarily higher, active TGF-β1 production was significantly higher in arterial media versus standard media. *P < .05; **P < .01. Data are expressed as picogram per 105 cells and represent the mean (± SEM) of 3 experiments performed in triplicate.
Ultrastructural comparison between the vessel make-up in Matrigel plugs injected with hMAPCs in arterial or standard media. Ultrastructural analysis of Matrigel plugs injected subcutaneously with hMAPCs combined with arterial (A-B) or standard media (C-D). Semithin sections of a Matrigel plug (A,C) and an ultrathin section of an artery-like tube (B) with a detail showing an SMC around an EC (inset) and a veinlike tube (D). “L” indicates the vessel lumen. Scale bars: 10 μm (A,C); 2.5 μm (B); 2 μm (D); 1 μm (B, inset).
Ultrastructural comparison between the vessel make-up in Matrigel plugs injected with hMAPCs in arterial or standard media. Ultrastructural analysis of Matrigel plugs injected subcutaneously with hMAPCs combined with arterial (A-B) or standard media (C-D). Semithin sections of a Matrigel plug (A,C) and an ultrathin section of an artery-like tube (B) with a detail showing an SMC around an EC (inset) and a veinlike tube (D). “L” indicates the vessel lumen. Scale bars: 10 μm (A,C); 2.5 μm (B); 2 μm (D); 1 μm (B, inset).
Discussion
In the last decade, our knowledge about the molecular differences between arterial and venous ECs and the pathways underlying their specification during development has rapidly increased (reviewed by Lawson et al,20 Harvey and Oliver,46 Shawber and Kitajewski,47 and Iso et al48 ). That such specification is not only important during development but also in adult life is perhaps most clearly demonstrated by the occurrence of diseases restricted to arteries (eg, atherosclerosis) or veins (eg, varicose veins). Therefore, knowledge about the mechanisms that underlie EC specification may allow us to design more optimal therapeutic regimens for those diseases. Unfortunately, most of our understanding about AV specification is derived from animal studies. Therefore, the experiments presented here with human stem cells provide a valuable model for the elucidation of mechanisms involved in EC differentiation and AV specification in humans.
Developmental studies in zebrafish9,20 and Xenopus18 have demonstrated that AV specification occurs very early, at the level of the angioblast, the embryonic EC precursor. Currently, it is not known what initially determines arterial and venous angioblasts. Until recently, it was suggested that VEGF—the most widely explored angiogenic growth factor—equally affects proliferation and migration of all EC (precursor) types. However, evidence has now been presented that VEGF preferentially stimulates the growth of arterial ECs (and their precursors) during development (reviewed by Torres-Vazquez et al2 ) and adulthood.40 Several hypotheses have been put forward to explain VEGF's preference, but none of them has been unequivocally proven.2,20 Arterial angioblasts may respond differently because they are, as shown in Xenopus18 and zebrafish,9 the first ones to migrate and thus encounter the VEGF signal earlier. Alternatively, specific responses may be elicited by the need for a coreceptor for VEGF165 to engage the downstream Notch pathway. Recent evidence has shown that NP-1, which only binds the VEGF165 isoform, might be a candidate coreceptor.6,17 Although our in vitro system did not allow addressing the spatially different responses of arterial and venous angioblasts to VEGF, our findings support the notion that, like in zebrafish and mouse development, there are separate arterial and venous endothelial precursors in the adult human. Under conditions in which hMAPCs gave robust arterial EC differentiation, hAC133+ cells appeared to have lost that ability both in vitro and in vivo and therefore could be considered venous EPCs. In contrast, hMAPCs are less mature than EPCs32 and may constitute a cell population that precedes AV specification and can therefore differentiate to both arterial and venous ECs in the presence of VEGF165. Noteworthy in this context is the fact that hAC133+ cells gave rise to venous and CD36+ microvascular ECs, while hMAPCs differentiated mainly into CD36− macrovascular ECs.
During zebrafish development, VEGF is part of an arterial EC inductive sequence involving Shh (acting upstream from VEGF) and Notch (acting downstream),20 a cascade that is at least in part conserved in mouse development (reviewed by Harvey and Oliver46 ). Using hMAPCs, we demonstrate both by positive and negative manipulation that the Shh and Notch elements of this cascade are also active during arterial EC specification from adult human stem cells. Although we show that VEGF165, Shh, and Dll-4 may have an additive effect on arterial EC differentiation, additional mechanistic studies will help to determine whether they work in a similar cascade. The involvement of those pathways was further substantiated by the differential expression of many of the pathway members in hMAPCs and hAC133+ cells. Increased expression of the patched receptors in hMAPCs may be the result of an autocrine positive feedback loop49,50 driven by hMAPC-derived Shh. Intriguingly, while Notch-1 was exclusively expressed in hMAPCs, Notch-4 was significantly underexpressed in hMAPCs in comparison with hAC133+ cells. Recent studies have, however, demonstrated the redundancy between Notch-1 and -4, supporting the notion that Notch-1 may be sufficient to induce arterial EC differentiation in hMAPCs.51 The fact that not all arterial markers were elevated in hMAPCs under the influence of VEGF165 (combined or not with Notch and patched ligands) and that arterial differentiation was not completely abrogated by Notch and/or Shh blocking may reflect the existence of additional VEGF/Notch/patched-independent pathways in arterial differentiation. We did, for instance, not fully explore the potential involvement of TGF-β, COUP-TFII, adrenomedullin, or angiopoietins, known to play a role in AV fate decisions.7,14,22,40
During development and in primary ECs,52 Notch activation has been associated with endothelial-to-mesenchymal transformation. Despite the ability of hMAPCs to robustly differentiate into SMCs under other conditions (Figure S1; Document S1), the conditions used for EC differentiation induced SMC differentiation of hMAPCs in vitro and in vivo to a low degree (not shown). This lack of significant SMC (trans)differentiation, however, allowed us to conclude that changes in expression of certain Notch components (like Jagged-1 and Ephrin B2, which have been shown to be expressed also in arterial SMCs48,53 ) were EC specific in our system. However, we cannot exclude that activation of Notch in fully EC-differentiated MAPCs may induce such transformation. Dll-4–induced Notch activation in ECs was recently shown to down-regulate EC proliferation, maybe due to an attenuated responsiveness to VEGF.54 However, despite this inhibitory effect on EC proliferation, Notch activation was still compatible with up-regulation of arterial EC markers, such as Hey-2, similar to what we and others documented in in vitro studies.55,56 Shh has also been shown to support neuronal differentiation,57 yet we did not observe the formation of neurons in our system despite the intrinsic ability of hMAPCs for neuronal differentiation, the latter of which requires a different cytokine cocktail (Figure S1; Document S1).
In agreement with the in vitro data, hMAPCs but not hAC133+ cells were capable of arterial EC differentiation in vivo. Indeed, the combination of VEGF165, Shh, and Dll-4 boosted arterial EC differentiation from coimplanted hMAPCs. In addition, this arterial “cocktail” induced a different vascular pattern than VEGF165 alone (ie, vessels were larger in diameter, more frequently coated with host SMCs, and contained elastin and more fibrillar collagen—altogether features consistent with arterial vessels). Other studies have shown that in the setting of ischemia, vessels formed in the presence of Shh had similar characteristics as the ones described here (ie, larger-diameter vessels and more often coated with SMCs).50,58 In our study, injection of the arterial cocktail without hMAPCs also induced effects on the endogenous mouse vascular cells but, importantly, the effects were more pronounced in the presence of hMAPCs. We hypothesize that, in addition to their direct contribution to arterial endothelium, hMAPCs (similar to other stem cells45 ), and more so when cultured in arterial media, had trophic effects on endogenous cells through secretion of PDGF-BB and TGF-β1. Interestingly, as previously shown in tumors, Shh in the arterial mix or produced by hMAPCs could potentially enhance PDGF receptor expression on the host vasculature and thereby enhance its response to the hMAPC-derived PDGF-BB signal.59
Together, our data show that coaxing human stem cells in vitro and in vivo (along with endogenous vascular cells) specifically into an arterial phenotype is feasible, thereby providing a model to study AV specification in humans. Moreover, our findings may also be of importance for improvement and optimization of strategies for vascular regeneration in ischemic patients and for the design of EC-coated artificial arterial grafts.
Authorship
Contribution: X.L.A. and C.C. performed and designed research, collected data, and analyzed data; A.L., C.M.V., and F.P. designed research, analyzed data, and wrote the manuscript; C.M., G.A., M.A.B., B.P., M.U., M.A., A.E., M.S., E.J.A., and J.M. performed research and collected data; and J.M.G.-V. contributed analytical tools and collected and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
X.L.A., A.L., and C.C. contributed equally to this study.
Correspondence: Felipe Prósper, Hematology and Cell Therapy Area, Clínica Universitaria, University of Navarra, Av Pio XII 36, Pamplona 31009, Spain; e-mail: fprosper@unav.es.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work as supported in part by grants from the Health Department of the Government of Navarra (4/2004), Spanish Ministerio de Ciencia y Tecnología (SAF 2002-04574-C02), Ministerio de Sanidad PI050168, FEDER (INTERREG IIIA) the UTE project CIMA, the American Heart Association (A.L., B.P.), Excellentie financiering KULeuven (EF/05/013) (A.L.), and the Belgian American Educational Foundation (BAEF) (A.L.).


![Figure 4. Shh and Dll-4 boost functional arterial hMAPC-EC differentiation in vivo. (A) Live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation. Note the localized CFSE-labeled area (outlined by a dashed white line) located in the Matrigel in the vicinity of a large vascular tree (arrowheads) from the overlying host skin. (B-J) Histologic analysis on cross-sections through Matrigel plugs containing hMAPCs and VEFG165 (B-D) or hMAPCs and VEGF165 + Shh+ Dll-4 (“arterial cytokine mix” [E-J]). (B) Electron microscopy showing a capillary composed of a Resovist-labeled hMAPC-derived EC in Matrigel plugs. A semithin section (B, top panel), an ultrathin section (B, bottom panel), and a detail of iron particles (insets in lower panel of B) are shown. (C-F) Immunohistochemical staining of 3 μm paraffin cross-sections through Matrigel plugs for human-specific CD31 (C) and human-specific VE-cadherin (D) (both indicating their EC identity) and human-specific Hey-2 (E) and human-specific EphrinB1 (F) (both indicating their arterial EC identity). Arrows indicate red blood cells in vessel lumen. (G-H) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and Hey-2 (red) (G) or UEA (green) and Ephrin B1 (red) (H). Topro (blue) was used for nuclear staining. (I) High-resolution live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation and 30 minutes after intravenous injection of UEA lectin. Note colocalization (yellow; indicated by arrowheads) of CFSE-labeled cells (green) and UEA lectin (red) area, indicating that the vessels containing CFSE-labeled cells were connected to the host vascular system. (J) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and α-actin (red), showing hMAPC-ECs (arrowheads) coated by α-actin–positive SMCs. Topro (blue) was used for nuclear staining. “L” in panels B and D-F indicates the vessel lumen. Magnifications ×63 (E-F), ×40 (C-D,J), and ×20 (G-I). Scale bars in panel B: 10 μm (semithin); 2.5 μm (ultrathin); 1 μm (upper inset); 0.5 μm (lower inset).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-06-030411/4/m_zh80060709720004.jpeg?Expires=1769475236&Signature=oLovOjrdQBMsHSahb6pLaexamzsnQlOQ67R9TKJH8vhcyfXrha4IVHrpvN86vXZS6SsmWfQFBKTL0yS3rMDBXYjrKQBESgToV5fXqmEsSlNH4y1orZkbUCDe7iapYuLpj8GuaM0GUptSewSaSX2QRDUzT-ZFKcYtVUZgBbVijgyQkgjKm~eUnxPk9yezaLsuTx3EJ6X97bkSPIqbCCvGjLX1JijF5hVGYM~iRs42bp8OSgJeKowAGC6sl~IflHsxQJTH7exM2~og-FQC~MklVfUSOFgecbW~ajrDauyqMgrmJOM8P2f9sDBVYxB9RXhPDLrQWvP~0reTF5ozDCvWhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Shh and Dll-4 boost functional arterial hMAPC-EC differentiation in vivo. (A) Live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation. Note the localized CFSE-labeled area (outlined by a dashed white line) located in the Matrigel in the vicinity of a large vascular tree (arrowheads) from the overlying host skin. (B-J) Histologic analysis on cross-sections through Matrigel plugs containing hMAPCs and VEFG165 (B-D) or hMAPCs and VEGF165 + Shh+ Dll-4 (“arterial cytokine mix” [E-J]). (B) Electron microscopy showing a capillary composed of a Resovist-labeled hMAPC-derived EC in Matrigel plugs. A semithin section (B, top panel), an ultrathin section (B, bottom panel), and a detail of iron particles (insets in lower panel of B) are shown. (C-F) Immunohistochemical staining of 3 μm paraffin cross-sections through Matrigel plugs for human-specific CD31 (C) and human-specific VE-cadherin (D) (both indicating their EC identity) and human-specific Hey-2 (E) and human-specific EphrinB1 (F) (both indicating their arterial EC identity). Arrows indicate red blood cells in vessel lumen. (G-H) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and Hey-2 (red) (G) or UEA (green) and Ephrin B1 (red) (H). Topro (blue) was used for nuclear staining. (I) High-resolution live in vivo imaging of a Matrigel plug containing VEGF165 and hMAPCs labeled with CFSE 10 days after subcutaneous implantation and 30 minutes after intravenous injection of UEA lectin. Note colocalization (yellow; indicated by arrowheads) of CFSE-labeled cells (green) and UEA lectin (red) area, indicating that the vessels containing CFSE-labeled cells were connected to the host vascular system. (J) Double confocal immunofluorescence staining of 40 μm cryopreserved cross-sections through Matrigel plugs with human endothelial-specific lectin UEA (green) and α-actin (red), showing hMAPC-ECs (arrowheads) coated by α-actin–positive SMCs. Topro (blue) was used for nuclear staining. “L” in panels B and D-F indicates the vessel lumen. Magnifications ×63 (E-F), ×40 (C-D,J), and ×20 (G-I). Scale bars in panel B: 10 μm (semithin); 2.5 μm (ultrathin); 1 μm (upper inset); 0.5 μm (lower inset).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-06-030411/4/m_zh80060709720004.jpeg?Expires=1769475237&Signature=F~QfC8KMXaUKci4UAGMkk3W97~pTbOTyeoOt37M9NL1-jIupG16zElUnq~WDswvxI8dFv5jJv9HtXkbNPR0sSfA43WddroB5I9kX-VHQYlY4hRPkof~1HJt9mx4nmzM7Yctkgc0FwDVaxel8d0Ic16btTYC8N6ZjXbGOp02YOV8vjHCStCONXC6ky0yNnJLT9fymxhBFoY7WCx-oEIhzp78okWjelf1YRp84WKgjtvHDQjSVoBaTdRnettN4oU5edkiiUo9bRx15puNCpuHD49mvnbSzvJke2gyq-laL2UTtErpeYedAwDteYvwLHgYdfWVHQb~odAsamhbd11f2jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)