Abstract
In 15% to 30% of patients with acute myeloid leukemia (AML), aberrant proliferation is a consequence of a juxtamembrane mutation in the FLT3 gene (FMS-like tyrosine kinase 3–internal tandem duplication [FLT3-ITD]), causing constitutive kinase activity. ABT-869 (a multitargeted receptor tyrosine kinase inhibitor) inhibited the phosphorylation of FLT3, STAT5, and ERK, as well as Pim-1 expression in MV-4-11 and MOLM-13 cells (IC50 approximately 1-10 nM) harboring the FLT3-ITD. ABT-869 inhibited the proliferation of these cells (IC50 = 4 and 6 nM, respectively) through the induction of apoptosis (increased sub-G0/G1 phase, caspase activation, and PARP cleavage), whereas cells harboring wild-type (wt)–FLT3 were less sensitive. In normal human blood spiked with AML cells, ABT-869 inhibited phosphorylation of FLT3 (IC50 approximately 100 nM), STAT5, and ERK, and decreased Pim-1 expression. In methylcellulose-based colony-forming assays, ABT-869 had no significant effect up to 1000 nM on normal hematopoietic progenitor cells, whereas in AML patient samples harboring both FLT3-ITD and wt-FLT3, ABT-869 inhibited colony formation (IC50 = 100 and 1000 nM, respectively). ABT-869 dose-dependently inhibited MV-4-11 and MOLM-13 flank tumor growth, prevented tumor formation, regressed established MV-4-11 xenografts, and increased survival by 20 weeks in an MV-4-11 engraftment model. In tumors, ABT-869 inhibited FLT3 phosphorylation, induced apoptosis (transferase-mediated dUTP nick-end labeling [TUNEL]) and decreased proliferation (Ki67). ABT-869 is under clinical development for AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive, heterogeneous disease with numerous cytogenetic abnormalities and mutations within key signaling pathways involved in cell differentiation, proliferation and survival.1 One of these key signaling molecules is FLT3 (FMS-like tyrosine kinase 3), a receptor tyrosine kinase (RTK) expressed and activated in most patients with AML, and also expressed in some normal hematopoietic cell types.1,2 Despite the success of initial chemotherapy, there is a relatively high relapse rate for patients with AML.3 These patients ultimately become refractory to traditional chemotherapies and succumb to the disease. The failure of initial chemotherapy or the refractory nature of AML has been associated with the acquisition of mutations that constitutively activate kinases essential for AML cell survival: internal tandem duplication (ITD) mutations in the juxtamembrane domain (15%-30% of patients) and point or short-length mutations in the activation loop of the kinase domain (about 7% of patients),4 and various similar mutations.5-7 The FLT3-ITD induces ligand-independent dimerization, autophosphorylation and constitutive activation of these receptors, and is able to transform hematopoietic cells.1 Generation of a constitutively active FLT3 also activates downstream phosphorylation events (eg, STAT5, AKT, and ERK), which regulate the FLT3 dependent survival of these cells.8 The ITD effectively activates STAT5 phosphorylation and the induction of STAT5 target genes (eg, CIS and Pim-2), whereas the D835 mutations behave similarly to the wt-FLT3 with only a weak activation of STAT5 phosphorylation and no induction of STAT5 target genes.8 Clinically, the FLT3-ITD is an important independent negative prognostic factor in AML and is associated with increased blast count, increased relapse rate, and poor overall survival.9 Inhibition of FLT3, especially the mutant forms responsible for the refractory nature of this disease, has made this an attractive target for the treatment of AML.10-14
ABT-869 (Figure 1; Table 1) is a structurally novel multitargeted RTK inhibitor that potently inhibits all members of the vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptor families, but has much less activity (IC50 values > 1 μM) against unrelated RTKs, cytoplasmic tyrosine kinases, or Ser/Thr kinases.15 The ability of ABT-869 to inhibit RTKs is also evident in cellular assays of RTK phosphorylation and VEGF-induced endothelial cell proliferation; however, ABT-869 is not a general antiproliferative agent since, in most cells, more than 1000-fold higher concentrations of ABT-869 are required to inhibit proliferation. In preclinical tumor growth studies, ABT-869 exhibits efficacy in human fibrosarcoma, breast, colon, and small-cell lung carcinoma xenograft models, as well as in orthotopic breast, prostate, and glioma models.15
Chemical structure of ABT-869 (N-[4-(3-amino-1H-indazol-4-yl)phenyl]-N′-(2-fluoro-5-methylphenyl)urea).
Chemical structure of ABT-869 (N-[4-(3-amino-1H-indazol-4-yl)phenyl]-N′-(2-fluoro-5-methylphenyl)urea).
Herein, we report the characterization of ABT-869 against AML cell lines harboring RTK mutations that result in constitutively activated RTKs or signaling pathways; these cells appear to be more sensitive to the effects of ABT-869. These results demonstrate the efficacy of ABT-869 in both the in vitro, spiked blood model and the in vivo leukemia model, and that phosphorylation of FLT3 and STAT5 appear to be feasible biomarkers for the assessment of clinical activity of ABT-869 in AML.
Materials and methods
Cell culture and reagents
Cell-culture media were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Hyclone (defined, heat inactivated; Logan, UT) or from Invitrogen (Carlsbad, CA). MV-4-11, RS4;11, Kasumi-1, KG-1, U937, K562, NB 4, SUP-B15, HL60, and Jurkat human cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA). MOLM-13 cells were purchased from Deutsche Sammlung von Microorganismen und Zellkulturen GmbH (DSMZ) (Braunschweig, Germany). All cells were cultured according to ATCC or DSMZ guidelines.
Viability and cell proliferation assays
For cell lines treated with ABT-869, live and dead cells were counted 24, 48, and 72 hours after treatment using trypan blue exclusion assay. All experiments were performed in triplicate. Percentage of viability was calculated and compared with the control cells treated with DMSO (0.1%). Cell proliferation was assessed with alamarBlue (Biosource, Camarillo, CA; final solution 10%) as described in Glaser et al.16 Data represent 2 separate experiments, with each data point carried out in duplicate in each experiment.
Colony formation assays with normal and AML patient samples
Institutional review board (IRB) approval for human bone marrow and blood samples were obtained at both UCLA and Abbott Laboratories (Abbott Park, IL) to perform this work. All animal studies were completed under the Institutional Animal Care and Use Committee (IACUC)–approved protocols for animal welfare. Human bone marrow cells from healthy controls and patients with AML were resuspended in a volume of 0.3 mL of Iscove medium with 2% FBS at 5 × 105 cells/mL and mixed with varying concentrations of the drug (10 pM to 10 μM suspended in 0.1% DMSO final) or DMSO control. This was then added to 3 mL of methylcellulose-containing growth factors interleukin-3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, erythropoietin, and stem cell factor (SCF) (Methocult GF-H4434; Stem Cell Technology, Vancouver, BC, Canada). The mixture of 1.1 mL was plated in culture dishes. The colonies were observed each day and counted on day 14. The total number of colonies and types of colonies were scored. For the normal samples, total colony numbers and the numbers of granulocyte macrophage–colony-forming units (CFU-GMs), CFU–granulocyte erythrocyte macrophage megakaryocyte (GEMM), erythroid blast-forming units (BFU-Es), CFU-Es, and CFU-Ms were analyzed. For the AML samples, the colonies were less differentiated and ranged from nice clusters with lots of cells to small clusters of 8 to 10 cells. Since we were starting with a heterogeneous population of cells, we also observed some CFU-GM and very few BFU-E colonies. We counted all the colonies, undifferentiated colonies, clusters, and differentiated colonies in our study.
Electrophoresis and western blotting
SDS-PAGE samples were electrophoresed, transferred to PVDF membranes (Invitrogen, Carlsbad, CA), and visualized by enhanced chemiluminescence with the Pierce Dura SuperSignal substrate (Pierce, Rockford, IL) according to Glaser et al.16
Blood spiked model and bone marrow for FLT3 phosphorylation
MV-4-11 cells (1 × 107 cells) were treated with compound (final 0.1% DMSO), immediately added to 1 mL blood from healthy human donors, incubated at 37°C for 2 hours with gentle shaking, then added to 2 vol of lysis buffer (20 mM Tris [pH 7.5], 137 mM NaCl, 10% glycerol, protease inhibitor cocktail, and phosphatase inhibitor cocktails [Sigma, St Louis, MO]). The sample was precleared then immunoprecipitated with anti-FLT3 (Sc480; Santa Cruz Biotechnology, Santa Cruz, CA) and 50 μL of protein G–agarose, incubated overnight, and washed 5 times with 1 mL of PBS containing 1 mM vanadate and 1 × protease inhibitor cocktail (16000g for 2 minutes). Phosphorylated FLT3 was detected with 4G10 (Upstate Biotechnology, Lake Placid, NY) and blots were reprobed for total FLT3 (sc-480). Each experiment was carried out in duplicate, and a representative result from 1 of these studies is presented.
Analysis of ERK and STAT5 phosphorylation and Pim-1 expression in the human blood spiked model
Peripheral blood mononuclear cells (PBMCs) were isolated from spiked human blood in hypotonic buffer (155 mM NH4Cl, 10 nM KHCO3, and 0.1 mM EDTA), centrifuged at 260g for 10 minutes at 20°C, washed 1 to 2 times in 10 mL PBS (260g for 10 minutes at 20°C). Lysis buffer (M-PER; Pierce), protease inhibitor cocktail, and phosphatase inhibitor cocktails were added to the PBMC pellet (3 vol/cell pellet vol) and sonicated. Lysates were clarified (20000g for 20 minutes at 4°C) and electrophoresed. Phospho-ERK and total ERK were detected using phospho-ERK (Thr 202/Try 204; Upstate Biotechnology) and total ERK (p44/42; Cell Signaling Technology, Beverly, MA). Phospho-specific STAT5 was from Upstate Biotechnology, and β-actin was used as the load control for STAT5 (Sigma). Pim-1 antibody was from Bethyl Laboratories (Montgomery, TX).
Analysis of apoptosis in MV-4-11 cells
MV-4-11 cells treated for 72 hours with ABT-869 were fixed with 80% ethanol, washed with PBS, and incubated with propidium iodide (50 mg/mL). DNA content was determined by fluorescence cell analysis using a FACSCalibur (Becton Dickinson, San Diego, CA) flow cytometer, and cell-cycle distribution was analyzed with CellQuest software (BD, Franklin Lakes, NJ). MV-4-11 or MOLM-13 cells (1 × 105 cells/mL) were treated with ABT-869 for 48 hours and analyzed for apoptosis using annexin V–FITC (Apo-alert apoptosis kit; BD Biosciences, San Diego, CA) and propidium iodide. Flow cytometry was performed at the UCLA Flow Cytometry core facility. Cleavage of poly-ADP-ribose polymerase (PARP) or activation of caspase-3 and caspase-9 was determined from cell lysates made 48 hours after treatment. Western blot was performed as previously described.17,18 Anti–caspase-3 was from Upstate Biotechnology, anti-PARP from Cell Signaling Technology, and anti–β-tubulin and anti–caspase-9 from Santa Cruz Biotechnology.
In vivo models
AML cell line flank and engraftment model in SCID mice.
MV-4-11 or MOLM-13 cells (5 × 106 cells) were mixed with matrigel and injected subcutaneously into the hind flank of the mice on day 0. For MV-4-11 tumors, each cohort of mice (15 mice) was randomized into 5 groups and dosed with compound or vehicle. ABT-869 (5, 10, 20, and 40 mg/kg/day) dissolved in 100% pure refined corn oil was delivered orally by gavage. Tumor growth was measured every 2 days using vernier calipers. Tumor volume was calculated as the product of length times width times height. The tumors were allowed to grow to 400 mm3 before the mice were grouped randomly after outliers were culled and treated with ABT-869.
Histology.
The animals were killed and tumors and organs were removed, fixed in formalin, and embedded in paraffin. Sections were prepared and stained with hematoxylin-eosin (H&E). Proliferation was assessed by immunostaining with Ki67 antisera (DAKO Laboratories, Glostrup, Denmark). Apoptosis was determined using transferase-mediated dUTP nick-end labeling (TUNEL) staining. These experiments were performed by the UCLA Immuno-Histochemistry Core facility, Department of Pathology. Images were acquired on an Olympus BX41 microscope (Olympus America, Center Valley, PA) using an Olympus Uplan F1 objective lens with a numeric aperture of 40× or 100× plus oil, giving a magnification of 40×/0.13 and 10×/0.3. Imaging medium used was oil, and H&E stain was used for tissues. Images were captured with an Olympus C-300 zoom digital 3.3 megapixel camera and imported into Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) for processing.
Analysis of tumor FLT3 phosphorylation.
MV-4-11 tumors (7 days) received a single 10 mg/kg dose of ABT-869 administered orally. At 3 and 6 hours after dosing, the mice were killed and the tumors were resected and snap-frozen in liquid nitrogen. Tumors were homogenized in ice-cold RIPA buffer using a Polytron (Brinkmann, Westbury, NY). Phosphorylated proteins were immunoprecipitated overnight at 4°C using PY20-agarose beads (sc-508; Santa Cruz Biotechnology). Immunoprecipitation from 2 mg of tumor lysate was loaded in each gel lane and visualized on Western blots using total FLT3 antibody sc-480 (Santa Cruz Biotechnology).
Bone marrow engraftment model.
Nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice were pretreated with cyclophosphamide by intraperitoneal injection of 150 mg/kg/day for 2 days, followed by 24 hours of rest prior to intravenous injection of 5 × 106 MV-4-11 cells via the tail vein.19 Bone marrow cell suspensions were prepared by flushing mouse femurs with cold, sterile PBS; cell suspensions were fixed, stained with anti–human CD45, and analyzed by flow cytometry. ABT-869 or its vehicle (ABT-869 was dissolved in 2% ethanol, 5% Tween-80, 20% PEG 400, and 73% hydroxypropyl methylcellulose [0.2% HPMC]) was dosed orally daily by gavage (10-12 animals per dose of ABT-869). Survival was determined by observation when the animals demonstrated hind-limb paralysis and became moribund (in control animals, this occurred between days 42 and 50 after engraftment).
Pharmacokinetic analysis of ABT-869 in mice.
ABT-869 was administered orally to nontumor-bearing mice at doses of 3 and 10 mg/kg; the vehicle used was 2.5% ethanol, 5% Tween-80, and 25% PEG 400 in PBS, and for the 40 mg/kg dose, pure refined corn oil was used as vehicle. Blood was sampled at the appropriate times by either orbital bleed or cardiac puncture into heparinized tubes. Plasma was prepared and frozen until protein precipitation with acidified methanol and analyzed by liquid chromatography–mass spectrometry (LC/MS) on an YMC-ODS-AQ column (Waters, Milford, MA) with a mobile phase of 45% acetonitrile and 0.1% acetic acid. ABT-869 was detected as m/z = 376.1 on a Finnigan LCQ Duo LC/MS (Thermo Fisher Scientific, Waltham, MA).
Results
In vitro assessment of ABT-869
ABT-869 inhibits proliferation of cells expressing mutant, constitutively active RTKs.
The activity of ABT-869 was evaluated in cell lines that harbored either wt-FLT3 (RS4;11 and KG1), a mutated kinase or signaling pathway (MV-4-11, MOLM-13, Kasumi-1, KG1, and K562), or were FLT3-null (Jurkat and HL-60). Cell lines dependent on mutated kinases, FLT3-ITD in MV-4-11 and MOLM-13, or c-KIT–activating mutations in Kasumi-1 cells,20-22 for survival are more sensitive to the antiproliferative effects of ABT-869 (IC50 values of 4, 6, and 16 nM, respectively; Table 2). Cell lines that are not dependent on these mutations harboring wt-FLT3 (RS4;11) or FLT3-null cells (Jurkat and HL-60) are far less sensitive to ABT-869 (Table 2). Consistent with its kinase activity profile (Table 1), ABT-869 does not appear to affect the proliferation of KG-1 cells harboring a fibroblast growth factor (FGF)–FGF receptor (FGFR) autocrine loop.23
ABT-869 inhibits the phosphorylation of FLT3 and downstream signaling molecules.
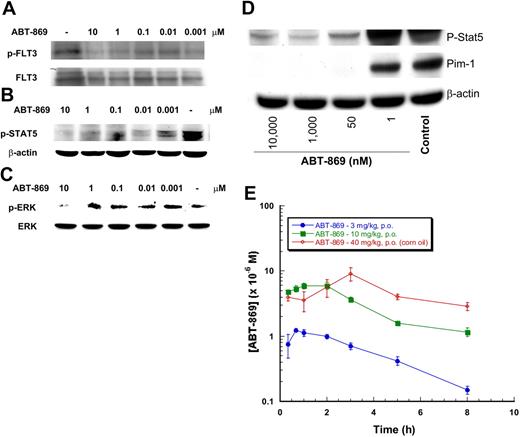
In MV-4-11 cells, the FLT3 is constitutively phosphorylated (Figure 2A). The ligand-independent phosphorylation of FLT3-ITD was inhibited by ABT-869 (IC50 approximately1 nM) after a 2-hour exposure (Figure 2A). ABT-869 did not modulate the expression of FLT3 protein during the 2-hour incubation period (data not shown; for in vitro cell culture, β-actin was used as a load control).
ABT-869 inhibits phosphorylation of FLT3, STAT5, and ERK in MV-411 cells. MV-4-11 cells were cultured in the presence of varying concentrations of ABT-869 or vehicle control (0.1% DMSO) for 2 hours. Cells were lysed and lysates analyzed by Western blot using (A) anti–phospho-FLT3, (B) anti–phospho-STAT5, (C) anti–phospho-ERK for MV-4-11 cells, and (D) anti–phospho-STAT5 and anti–Pim-1 for MOLM-13 cells. For cell culture, β-actin was used as the load control for FLT3 and STAT5, whereas total ERK was used for ERK. The results from at least 2 independent experiments demonstrate similar inhibition of FLT3, STAT5, and ERK phosphorylation with ABT-869, a representative blot is shown.
ABT-869 inhibits phosphorylation of FLT3, STAT5, and ERK in MV-411 cells. MV-4-11 cells were cultured in the presence of varying concentrations of ABT-869 or vehicle control (0.1% DMSO) for 2 hours. Cells were lysed and lysates analyzed by Western blot using (A) anti–phospho-FLT3, (B) anti–phospho-STAT5, (C) anti–phospho-ERK for MV-4-11 cells, and (D) anti–phospho-STAT5 and anti–Pim-1 for MOLM-13 cells. For cell culture, β-actin was used as the load control for FLT3 and STAT5, whereas total ERK was used for ERK. The results from at least 2 independent experiments demonstrate similar inhibition of FLT3, STAT5, and ERK phosphorylation with ABT-869, a representative blot is shown.
Similar to its potency against phosphorylation of FLT3, the observed IC50 was approximately 1 nM for inhibition of phosphorylation of STAT5 (Figure 2B) and approximately 10 to 100 nM for phosphorylation of ERK (Figure 2C). In MOLM-13 cells, ABT-869 potently inhibited STAT5 phosphorylation (IC50 = 1-10 nM) and, as expected, decreased the expression of a STAT5-responsive gene, Pim-1 (IC50 ≤ 1 nM) (Figure 2D). Consistent with the effect of ABT-869 on c-KIT and Kasumi-1 cell proliferation, ABT-869 also inhibited the phosphorylation of KIT and STAT5 in Kasumi-1 cells with an IC50 of approximately 100 nM (data not shown).
ABT-869 induces apoptosis in MV-4-11 and MOLM-13 cells.
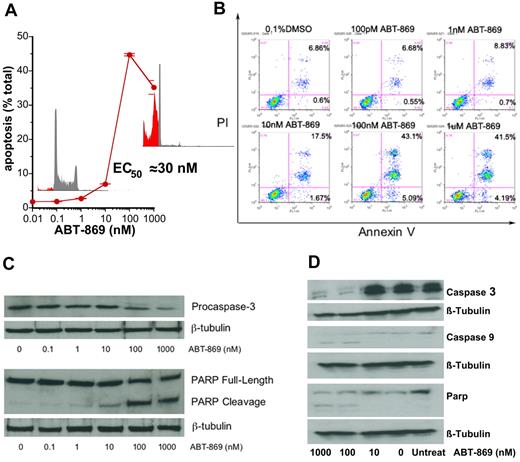
ABT-869 induced an apoptotic response in MV-4-11 cells as determined by an increase in the sub-G0/G1 cell population upon fluorescence-activated cell sorter (FACS) analysis (EC50 = 30 nM; Figure 3A). Annexin V staining demonstrated a concentration-dependent apoptosis of cells, with the greatest increase in annexin V staining occurring with between 10 and 100 nM ABT-869 (Figure 3B). With increasing concentrations of ABT-869 there was significant cleavage of PARP with decreasing amounts of pro–caspase-3, indicating that caspase-3 was activated, resulting in PARP cleavage (Figure 3C). Decreases in pro–caspase-3, and cleavage of pro–caspase-9 and PARP were also observed for ABT-869 between 10 and 100 nM in MOLM-13 cells (Figure 3D).
ABT-869 induces apoptosis of MV-411 cells. (A) MV-4-11 cells were treated for 72 hours with ABT-869, stained with propidium iodide (50 mg/mL), and analyzed on a FACS Calibur flow cytometer. Sub-G0/G1 population was determined by CellQuest software and designated as apoptotic cells (% apoptotic cells ± SEM). (B) MV-4-11 cells (1 × 106) were harvested at 48 hours after treatment with various drug concentrations. Cells were stained with anti–annexin V–FITC antibody and propidium iodide and analyzed by flow cytometry. Increase in apoptosis was observed with increasing concentrations of ABT-869. Percentages in bottom right quadrants are cells in early apoptosis; annexin V+ and PI−. Percentages in bottom right quadrants are cells in late apoptosis: annexin V+ and PI−. (C) Western blot analysis for PARP cleavage and caspase-3 to assess apoptosis. (D) Representative Western blot analysis of caspase-3 and caspase-9 activation and PARP cleavage in MOLM-13 cells treated with ABT-869. The results from 3 independent experiments show decrease in pro–caspase-3 levels and cleavage of PARP with increasing concentrations of ABT-869.
ABT-869 induces apoptosis of MV-411 cells. (A) MV-4-11 cells were treated for 72 hours with ABT-869, stained with propidium iodide (50 mg/mL), and analyzed on a FACS Calibur flow cytometer. Sub-G0/G1 population was determined by CellQuest software and designated as apoptotic cells (% apoptotic cells ± SEM). (B) MV-4-11 cells (1 × 106) were harvested at 48 hours after treatment with various drug concentrations. Cells were stained with anti–annexin V–FITC antibody and propidium iodide and analyzed by flow cytometry. Increase in apoptosis was observed with increasing concentrations of ABT-869. Percentages in bottom right quadrants are cells in early apoptosis; annexin V+ and PI−. Percentages in bottom right quadrants are cells in late apoptosis: annexin V+ and PI−. (C) Western blot analysis for PARP cleavage and caspase-3 to assess apoptosis. (D) Representative Western blot analysis of caspase-3 and caspase-9 activation and PARP cleavage in MOLM-13 cells treated with ABT-869. The results from 3 independent experiments show decrease in pro–caspase-3 levels and cleavage of PARP with increasing concentrations of ABT-869.
In vitro AML model: human blood spiked with AML cells
Modulation of FLT3, STAT5, and ERK phosphorylation in the AML model.
In normal human blood, the amount of FLT3 protein was too low to be detected by Western blot (data not shown). Therefore, an ex vivo model was developed where MV-4-11 or MOLM-13 cells were spiked into normal human blood to mimic the clinical setting. ABT-869 inhibited the phosphorylation of FLT3 in a concentration-dependent manner (IC50 approximately 100 nM; Figure 4A). In vitro, the IC50 value for ABT-869 was 1 nM in MV-4-11 cells, significantly lower than the concentration needed to inhibit phosphorylation in the blood model. This result suggested a differential of 100-fold relative to low protein conditions, consistent with the fact that ABT-869 is more than 99% protein bound in human plasma.15 ABT-869 also inhibited phosphorylation of STAT5 (IC50 approximately 1-10 nM) and ERK (IC50 approximately 1-10 μM) (Figure 4B–C, respectively). Inhibition of STAT5 phosphorylation occurs before inhibition of FLT3, while inhibition of MAPK phosphorylation occurs much after FLT3 inhibition. This effect is probably due to differential effects on other signaling pathways that effect the phosphorylation of downstream targets of FLT3. This is also evident in MOLM-13 cell–spiked blood by the inhibition of STAT5 phosphorylation and expression of the STAT5 responsive gene, Pim-1 (Figure 4D). As the effect of ABT-869 was weaker (approximately 100 nM) in Kasumi-1 cells in culture, when spiked into blood, ABT-869 inhibited KIT phosphorylation between 1 and 10 μM, also consistent with a 100-fold shift in potency relative to low protein conditions (data not shown).
ABT-869 inhibits phosphorylation of FLT3, STAT5, and ERK in the human blood AML model. MV-4-11 cells were spiked into normal human blood, 1 × 107 cells/mL, and treated with varying concentrations of ABT-869 for 2 hours. For FLT3 phosphorylation (A), blood was lysed and the FLT3 was immunoprecipitated from 1 mL of lysate with anti-FLT3 (sc-480; 10 mg/mL) and analyzed by Western blot. The results from at least 3 independent experiments demonstrate similar inhibition of phosphorylation of FLT3 by ABT-869; a representative blot is shown. (B) STAT5 and (C) ERK phosphorylation was analyzed from PBMCs prepared from blood treated as described above. Phospho-STAT5 and -ERK were determined directly in cell lysates. (D) Phospho-STAT5 and Pim-1 expressions were analyzed from PBMCs prepared from blood treated as described in “Materials and methods,” with the exception of spiking with MOLM-13 cells. The results from 2 independent experiments demonstrate similar inhibition of the phosphorylation of STAT5 and ERK and decreased Pim-1 expression by ABT-869; a representative blot is shown. (E) Pharmacokinetic analysis of ABT-869 plasma concentration from orally dosed mice with 3 and 10 mg/kg/day in 2.5% ethanol, 5% Tween-80, and 25% PEG 400 in PBS, and the 40 mg/kg dose in pure refined corn oil. ABT-869 was analyzed from plasma that had been protein precipitated with acidified methanol and concentration determined using LC-MS as described in “Materials and methods.” Data are shown as μM ± SEM.
ABT-869 inhibits phosphorylation of FLT3, STAT5, and ERK in the human blood AML model. MV-4-11 cells were spiked into normal human blood, 1 × 107 cells/mL, and treated with varying concentrations of ABT-869 for 2 hours. For FLT3 phosphorylation (A), blood was lysed and the FLT3 was immunoprecipitated from 1 mL of lysate with anti-FLT3 (sc-480; 10 mg/mL) and analyzed by Western blot. The results from at least 3 independent experiments demonstrate similar inhibition of phosphorylation of FLT3 by ABT-869; a representative blot is shown. (B) STAT5 and (C) ERK phosphorylation was analyzed from PBMCs prepared from blood treated as described above. Phospho-STAT5 and -ERK were determined directly in cell lysates. (D) Phospho-STAT5 and Pim-1 expressions were analyzed from PBMCs prepared from blood treated as described in “Materials and methods,” with the exception of spiking with MOLM-13 cells. The results from 2 independent experiments demonstrate similar inhibition of the phosphorylation of STAT5 and ERK and decreased Pim-1 expression by ABT-869; a representative blot is shown. (E) Pharmacokinetic analysis of ABT-869 plasma concentration from orally dosed mice with 3 and 10 mg/kg/day in 2.5% ethanol, 5% Tween-80, and 25% PEG 400 in PBS, and the 40 mg/kg dose in pure refined corn oil. ABT-869 was analyzed from plasma that had been protein precipitated with acidified methanol and concentration determined using LC-MS as described in “Materials and methods.” Data are shown as μM ± SEM.
As demonstrated in Figure 4E, at efficacious doses of ABT-869 (10 mg/kg/day in 2.5% ethanol, 5% Tween-80, and 25% PEG 400 in PBS vehicle or 40 mg/kg/day in pure refined corn oil vehicle), plasma concentration exceeded 1 μM for up to 8 hours after dosing. These levels are sufficient to cover the IC50 values for FLT3 and downstream signaling phosphorylation events observed in the in vitro spiked blood model.
ABT-869 does not inhibit growth of normal human bone marrow progenitor cells in vitro at concentrations less than 1μM.
FLT3 is expressed on immature hematopoietic progenitors and also on some mature myeloid and lymphoid cells.24 ABT-869 demonstrated a concentration-dependent inhibition of FLT3 ligand (FL)–stimulated FLT3 phosphorylation (estimated IC50 value of 10 nM), with complete inhibition being observed at 1 μM ABT-869 in normal human bone marrow (data not shown).
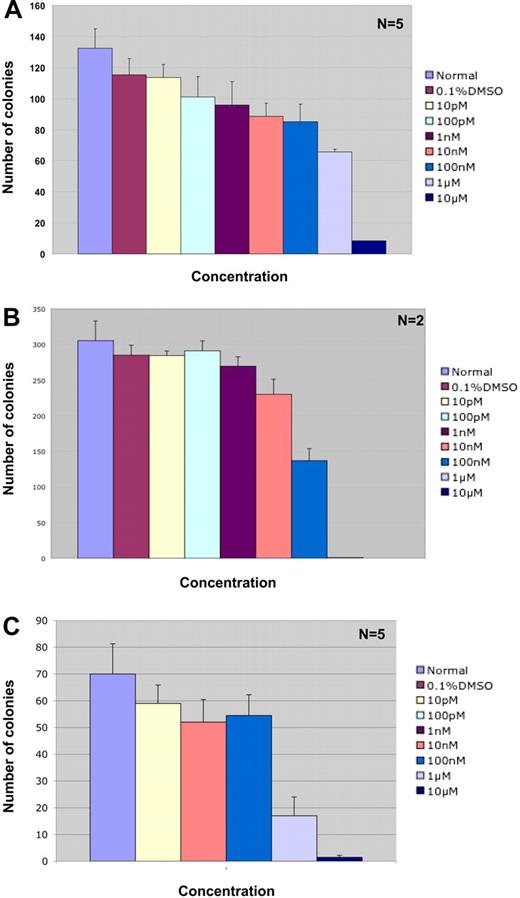
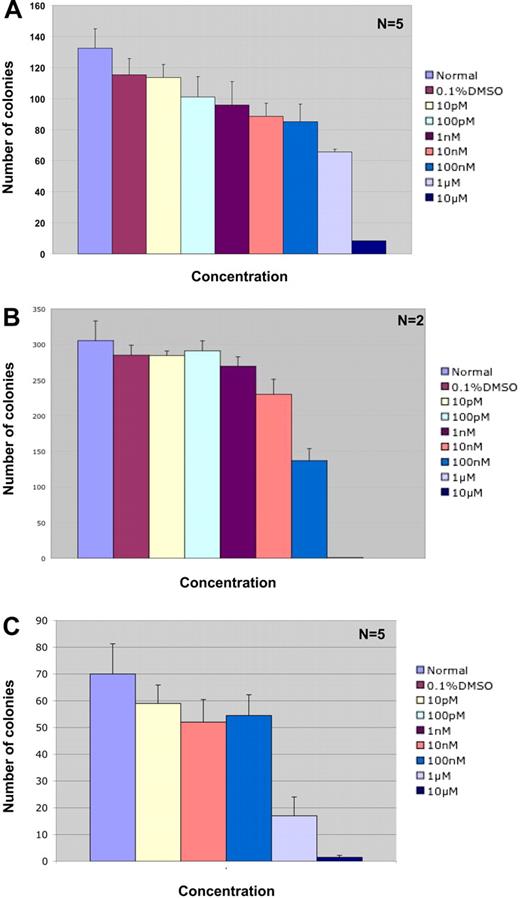
Colony-forming assays in methylcellulose containing IL-3, IL-6, and SCF demonstrate that ABT-869 does not have a significant effect on the proliferation and differentiation of human bone marrow cells up to a concentration of 1 μM (26% and 43% inhibition at 0.1 and 1.0 μM, respectively; Figure 5A). Above 1 μM there was significant decrease in the numbers of CFU-GM colonies (92% inhibition at 10 μM). These results indicate that ABT-869 is not toxic to normal human bone marrow, cell proliferation, and differentiation at the concentrations effectively inhibiting growth of AML cells in vitro.
Effect of ABT-869 on proliferation and differentiation of normal human bone marrow progenitors and AML patient samples. (A) Methylcellulose-based colony assay was performed with normal human bone marrow cells treated with varying concentrations of ABT-869 and analyzed 14 days after treatment. The experiment is an average of 5 samples with triplicate platings. The inhibitor was not toxic to the bone marrow cells up to a concentration of 1 μM. (B) Methylcellulose-based colony assay performed on AML patient samples containing the FLT3-ITD gene. The experiment is an average of 2 patients with triplicate platings. (C) Methylcellulose-based colony assay performed on FLT3-ITD–negative AML patient samples. The experiment is an average of 5 samples with triplicate platings. All data are expressed as number of colonies ± SEM. All human samples were obtained through an approved protocol from the UCLA IRB and uphold the tenets of the Helsinki protocol.
Effect of ABT-869 on proliferation and differentiation of normal human bone marrow progenitors and AML patient samples. (A) Methylcellulose-based colony assay was performed with normal human bone marrow cells treated with varying concentrations of ABT-869 and analyzed 14 days after treatment. The experiment is an average of 5 samples with triplicate platings. The inhibitor was not toxic to the bone marrow cells up to a concentration of 1 μM. (B) Methylcellulose-based colony assay performed on AML patient samples containing the FLT3-ITD gene. The experiment is an average of 2 patients with triplicate platings. (C) Methylcellulose-based colony assay performed on FLT3-ITD–negative AML patient samples. The experiment is an average of 5 samples with triplicate platings. All data are expressed as number of colonies ± SEM. All human samples were obtained through an approved protocol from the UCLA IRB and uphold the tenets of the Helsinki protocol.
ABT-869 effectively inhibits primary AML bone marrow cells in vitro.
To examine the effect of ABT-869 on primary AML patient samples, we performed in vitro colony assays with methylcellulose containing IL-3, IL-6, GM-CSF, G-CSF, erythropoietin, and SCF on bone marrow samples from patients with AML that expressed either the FLT3-ITD or no-FLT3 mutation. Our results (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) demonstrated that ABT-869 inhibits colony formation of FLT3-ITD–positive cells at an IC50 of 100 nM (Figure 5B). ABT-869 inhibited colony formation of AML cells without FLT3 mutations at an IC50 of 1μM (Figure 5C), suggesting that the drug may be inhibiting growth by targeting additional pathways. The effect of ABT-869 on CFU-Es and CFU-GMs in AML cells without FLT3 mutations (Figure S1) is similar to that shown in Figure 5C, where significant inhibition is observed between 100 nM and 1 μM.
In vivo assessment of ABT-869
ABT-869 inhibits growth of MV-4-11 tumor xenografts.
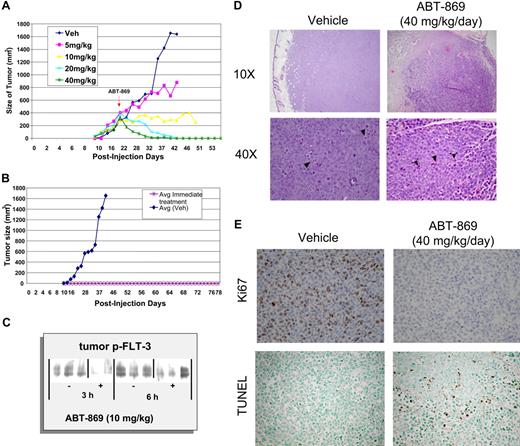
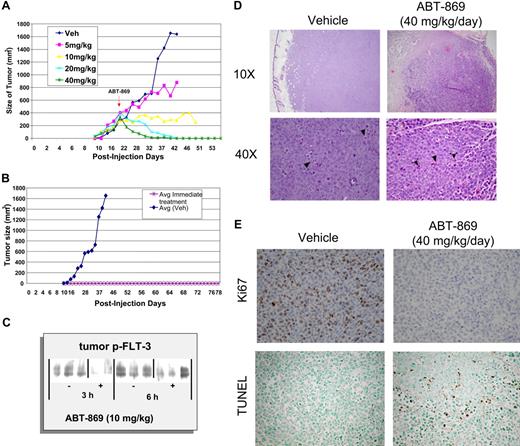
MV-4-11 cells were subcutaneously implanted into SCID mice, and solid tumors of approximately 400 mm3 grew within 3 weeks. Histology of the tumors showed a large number of mitotic figures indicative of active proliferation (Figure 6D). Immunohistochemical staining for Ki67 demonstrated a high proliferative index consistent with rapid tumor growth, in contrast to treated tumors, which showed no mitotic activity (Figure 6E).
ABT-869 exhibits dose-dependent efficacy and regression of established subcutaneous MV-4-11 tumors. (A) Daily oral administration of ABT-869 at concentrations of 5, 10, 20 and 40 mg/kg/day was initiated when the MV-411 tumors reached an average of 400 mm3 in volume. The graph shows the dosage response to the inhibitor compared with tumors treated with vehicle control (3 mice per group). (B). Mice injected with MV-411 cells were treated with 40 mg/kg/day ABT-869 or vehicle the day after the injection of cells. The graph shows that the mice receiving treatment never developed tumors in contrast to the vehicle control (3 mice per group). (C) After a single administration of ABT-869 (10 mg/kg), tumors were harvested 3 and 6 hours later, snap-frozen in liquid N2, homogenized in lysis buffer, immunoprecipitated with anti-PY20, and analyzed by Western blot with anti-FLT3. (D) H&E sections of tumors from day 12 of dosing treated with vehicle or 40 mg/kg/d of ABT-869 at low (× 10) and high (× 40) power. Vehicle control–treated tumors show increased mitotic activity (arrowheads, lower left panel). Treated tumors show areas of cell death and inflammatory cells (arrowheads, lower right panel). (E) Ki67 and TUNEL staining of tumor sections from day 12 of dosing were used to evaluate proliferation and apoptosis, respectively.
ABT-869 exhibits dose-dependent efficacy and regression of established subcutaneous MV-4-11 tumors. (A) Daily oral administration of ABT-869 at concentrations of 5, 10, 20 and 40 mg/kg/day was initiated when the MV-411 tumors reached an average of 400 mm3 in volume. The graph shows the dosage response to the inhibitor compared with tumors treated with vehicle control (3 mice per group). (B). Mice injected with MV-411 cells were treated with 40 mg/kg/day ABT-869 or vehicle the day after the injection of cells. The graph shows that the mice receiving treatment never developed tumors in contrast to the vehicle control (3 mice per group). (C) After a single administration of ABT-869 (10 mg/kg), tumors were harvested 3 and 6 hours later, snap-frozen in liquid N2, homogenized in lysis buffer, immunoprecipitated with anti-PY20, and analyzed by Western blot with anti-FLT3. (D) H&E sections of tumors from day 12 of dosing treated with vehicle or 40 mg/kg/d of ABT-869 at low (× 10) and high (× 40) power. Vehicle control–treated tumors show increased mitotic activity (arrowheads, lower left panel). Treated tumors show areas of cell death and inflammatory cells (arrowheads, lower right panel). (E) Ki67 and TUNEL staining of tumor sections from day 12 of dosing were used to evaluate proliferation and apoptosis, respectively.
Mice treated with 40 mg/kg/day ABT-869 showed complete regression of the tumors within 2 weeks of treatment (Figure 6A). Histology of the regressed tumors showed necrosis and a number of inflammatory cells. TUNEL staining of the sections from treated tumors indicated a number of apoptotic bodies (Figure 6D–E). Tumors treated with 20 mg/kg/day ABT-869 showed complete regression within a month of treatment (Figure 6A). Tumors treated with 10 mg/kg/day ABT-869 stopped growing and showed minimal regression (Figure 6A). Ki67 staining of these tumors indicates that there is no mitotic activity in these treated tumors (data not shown). These results indicated that ABT-869 causes regression of the tumors in a dose-dependent manner by inhibiting proliferation and inducing apoptosis. In established MV-4-11 tumors (approximately 1 g), ABT-869 demonstrated a dose-dependent inhibition of FLT3 phosphorylation in the MV-4-11 tumors at 3 and 6 hours after receiving a dose of ABT-869 (10 mg/kg; Figure 6C).
ABT-869 prevents tumor formation using a MV-4-11 xenograft.
To determine whether treatment of ABT-869 prevents MV-4-11 cells from forming tumors, treatment was started 24 hours after tumor cell injection with 40 mg/kg/day ABT-869. The group receiving ABT-869 did not develop tumors even after 3 months, in contrast to the control group, in which tumors grew rapidly (Figure 6B). Daily dosing of ABT-869 was well tolerated with no signs of toxicity.
ABT-869 inhibits and prevents tumor growth in MOLM-13 xenograft models.
In MOLM-13 xenograft tumors, representative studies demonstrate that ABT-869 was less efficacious than in MV-4-11 xenograft tumors (Figure 7A–B), but still produced profound effects on MOLM-13 tumor formation and growth. ABT-869 slowed the growth of MOLM-13 tumors from approximately day 18 onward (Figure 7A). ABT-869 did not produce frank regressions in the MOLM-13 xenograft model. In the tumor prevention model, ABT-869 was able to prevent MOLM-13 tumor growth up to day 26, after which tumor growth recommenced but at a slower rate than control untreated tumor growth for the 40 mg/kg group (Figure 7B).
ABT-869 inhibits the growth of and prevents MOLM-13 tumor formation. (A) Daily oral administration of ABT-869 at concentrations of 5, 10, 20, and 40 mg/kg/day was initiated when the MOLM-13 tumors reached an average of 400 mm3 in volume (1 mouse per dosage group). The graph shows the dosage response to the inhibitor compared with tumors treated with vehicle control. (B). Mice injected with MOLM-13 cells were treated with 40 mg/kg/day ABT-869 or vehicle the day after injection of cells (2 mice per dosage group). The graph shows that the mice receiving treatment developed tumors after 26 days of treatment in contrast to the vehicle control (tumor size ± SEM). (C) ABT-869 prolongs survival in MV-4-11 bone engraftment model. NOD-SCID mice were injected with 5 × 106 MV-4-11 cells after bone marrow ablation with cyclophosphamide. Treatment with ABT-869 began on day 40 after injection and continued for another 20 weeks. Survival was determined by observation when the animal demonstrated hind limb paralysis or became moribund.
ABT-869 inhibits the growth of and prevents MOLM-13 tumor formation. (A) Daily oral administration of ABT-869 at concentrations of 5, 10, 20, and 40 mg/kg/day was initiated when the MOLM-13 tumors reached an average of 400 mm3 in volume (1 mouse per dosage group). The graph shows the dosage response to the inhibitor compared with tumors treated with vehicle control. (B). Mice injected with MOLM-13 cells were treated with 40 mg/kg/day ABT-869 or vehicle the day after injection of cells (2 mice per dosage group). The graph shows that the mice receiving treatment developed tumors after 26 days of treatment in contrast to the vehicle control (tumor size ± SEM). (C) ABT-869 prolongs survival in MV-4-11 bone engraftment model. NOD-SCID mice were injected with 5 × 106 MV-4-11 cells after bone marrow ablation with cyclophosphamide. Treatment with ABT-869 began on day 40 after injection and continued for another 20 weeks. Survival was determined by observation when the animal demonstrated hind limb paralysis or became moribund.
ABT-869 enhances survival in a bone marrow engraftment model of leukemia.
In the engraftment model of MV-4-11 cells in mice with their endogenous bone marrow ablated by pretreatment with cyclophosphamide,19 human cells (7% of total cells) were detected by CD45 expression in bone marrow 25 days after cell inoculation. Within 42 to 50 days, the bone marrow content of CD45-positive cells from vehicle-treated animals had increased to 22%, and clinically the mice exhibited hind-limb paralysis, rough coat, and decreased activity. Treatment with ABT-869 (1, 3, and 10 mg/kg/day), initiated 25 days after MV-4-11 cell inoculation, decreased the proportion of CD45-positive cells detected in bone marrow 40 days after inoculation (16%, 16%, and 0.7%, respectively). Continued treatment resulted in a dose-dependent prolongation in survival (Figure 7C) from 3 weeks up to 20 weeks at 1 and 10 mg/kg/day, respectively. Preliminary flow cytometric analyses of the blood and bone marrow from these engraftment mice demonstrated a reduction in the amount of phosphorylated STAT5 and ERK in the MV-4-11–positive cells at 10 mg/kg/day (data not shown), and further supports the mechanism of ABT-869 in vivo.
Comparison of ABT-869 to other FLT3 and VEGFR/PDGFR inhibitors.
Comparison of the kinase inhibition profile of ABT-869 and other FLT3 inhibitors (MLN 518, CHIR 258, sunitinib [SU-11248] and sorafenib [BAY 439006]) is shown in the heatmap of Ki values determined for 79 different kinases (Figure S2), and provides 1 method of evaluating the potential clinical merit of new RTK inhibitors. MLN 518 is the apparently the most selective FLT3 inhibitor but is less potent against FLT3 than the VEGFR/PDGFR family inhibitors, including ABT-869. The IC50 values against FLT3 at 1 mM ATP are 4 nM for ABT-869, 98 nM for MLN 518, 1 nM for CHIR 258, 4 nM for sunitinib, and 46 nM for sorafenib. These IC50 values are consistent with the antiproliferative properties of these inhibitors in MV-4-11, MOLM-13, RS4;11, SUP-B15, and Kasumi-1 cells in vitro (Table 3)
Diverse effects of ABT-869 on activation loop mutants of FLT3.
ABT-869 inhibits the catalytic domain of wt-FLT3 with an IC50 value of 4 nM (Table 1). Different activation loop mutations of the FLT3 gene (D835H, D835Q, D835V, and D835Y) were expressed as catalytic domains and demonstrated diverse responses to ABT-869. The D835H and D835Q mutations were approximately 10-fold less sensitive to ABT-869 (IC50 values of 52 and 64 nM, respectively). The D835Y mutation was more than 1000-fold less sensitive to ABT-869 (IC50 = 4600 nM), and the least sensitive mutant was D835V, with an IC50 value of 19800 nM. The effect of ABT-869 on the growth of Baf3 cells transfected with these mutations is being investigated both in vitro and in vivo.
Discussion
In AML cell lines harboring mutated kinases, RTK inhibitors (RTKIs) of the VEGFR/PDGFR families inhibit proliferation and survival subsequent to the inhibition of FLT3 phosphorylation and phosphorylation of downstream targets of FLT3. In several instances this has also been translated into clinical samples from patients with AML.10,11,25,26 Strong inhibition of FLT3 phosphorylation by sunitinib was evident in all 5 mutant FLT3 patients (3 ITDs, 1 D835Y, and 1 G846S), suggesting that patients with AML who carry these mutations may be more sensitive to RTKIs.11,25 Similar results were also obtained for CEP-70110 and PTK41226 in patients with AML.
In hematologic malignancies the angiogenesis component has not been fully assessed; therefore, it would be essential for a potential new therapeutic agent not only to have an antiangiogenic component but to also have a direct antitumor effect, as demonstrated herein with ABT-869. Mutations in the growth factor signaling pathways that make survival dependent on that kinase activity provides this avenue for the RTKIs.3 Described herein, in AML cell lines harboring the FLT3-ITD mutation (MV-4-11 cells27 and MOLM-1328 ), ABT-869 inhibited proliferation by induction of apoptosis as well as phosphorylation of FLT3 and downstream effectors, STAT5 and ERK, as well as expression of the STAT5-responsive gene Pim-1. Kasumi-1 cells harboring the AML-ETO fusion gene, an autocrine SCF-KIT mutation,20 and an activating c-KIT N822K mutation21,22 were also potently inhibited by ABT-869. These antiproliferative results are consistent with the in vitro enzyme profile of ABT-869 (Table 1). In wt-FLT3 cells, RS4;11, and FLT3-negative Jurkat cells, the antiproliferative effect of ABT-869 was approximately 1000-fold less potent. The differential between mutated and wt-FLT3 is also consistent with the lack of effect of ABT-869 (> 1000 nM) on normal bone marrow progenitor cells in a colony-forming assay, even though ABT-869 inhibits ligand-induced FLT3 phosphorylation with an IC50 of approximately 10 nM (data not shown). Our data suggests that ABT-869 is significantly more active against cell lines that contain mutated or constitutively active RTKs targeted by ABT-869. The AML clinical studies with sunitinib, PKC412, and CEP-701 suggest that this may also be the case in patients with AML.10,11,25,26 Based on these results, the antileukemic activity of ABT-869 is dependent on (1) the expression of a mutated kinase or altered signaling pathway that is essential for cell survival and (2) the intrinsic potency of ABT-869 against that receptor tyrosine kinase.
Comparison of ABT-869 with other FLT3 inhibitors (Table 3) suggests that ABT-869 is superior to MLN 518 at the enzyme and cellular level and is essentially equivalent to the more nonselective inhibitors sunitinib, sorafenib, and CHIR 258. In a mechanism-based in vivo model, Albert et al15 have demonstrated that ABT-869 is more potent than other RTKIs (eg, sunitinib, sorafenib, AG013736, and ZD6474) in blocking VEGF-induced edema. These data, along with the overall in vivo antitumor assessment of ABT-86915 suggest that ABT-869 is a novel RTKI that has potential in the treatment of AML through direct effects on mutated kinases and as an antiangiogenic compound. The activity of ABT-869 on primary AML patient samples also demonstrates a selectivity for patients with the FLT3-ITD; however, the activity observed against wt-FLT3 AML patient samples also suggests that ABT-869 may be acting through additional pathways that mediate AML cell survival.
In flank tumors, ABT-869 is able to cause the complete regression of established tumors (Figure 6A) and in mice with large, established tumors (approximately 2 g), ABT-869 can also generate a significant tumor regression.15 When administered at the time of MV-4-11 cell inoculation, ABT-869 can prevent the outgrowth of a palpable tumor for more than 3 months and for approximately 26 days in MOLM-13 tumors. The inhibition of MV-4-11 flank tumor growth is associated with a decrease in FLT3 phosphorylation, an increase in apoptotic cells, and a decrease in proliferation index (Ki-67 staining) consistent with the in vitro effects of ABT-869 on MV-4-11 cells. To minimize the unnatural state of a flank leukemia tumor, the bone marrow engraftment model with MV-4-11 cells was evaluated with ABT-869. ABT-869 effectively increased survival in this orthotopic model of leukemia. Disease in these animals was evident when human CD45-positive cells were observed in the bone marrow and correlated with disease progression. At 10 mg/kg/day, the survival of these animals was prolonged almost 20 weeks, demonstrating that ABT-869 is a potent and potentially useful therapeutic for AML in this xenograft model of disease. Preliminary analysis of MV-4-11 cells from the blood of these animals demonstrated inhibition of STAT5 and ERK phosphorylation, consistent with mechanism observed in vitro. These results are consistent with the direct effect of ABT-869 on leukemia cell growth but do not exclude an antangiogenic component in both models.
The ex vivo AML model is another model for the evaluation of ABT-869. The IC50 of approximately 100 nM for ABT-869 against FLT3 phosphorylation is approximately 100-fold greater than its IC50 estimated in MV-4-11 cells in the absence of protein (IC50 approximately1 nM) and 25-fold greater than its effect on in vitro proliferation in the presence of 10% fetal bovine serum. These results correlate well with the fact that ABT-869 is highly protein bound, more than 99%, in human plasma,15 suggesting that this model can be potentially used preclinically to define plasma drug concentrations. sunitinib, CEP-701, PKC412, and MLN 518 are currently in clinical studies as FLT3 inhibitors in patients with AML.1,10,19,25,26 These compounds also demonstrate a 100- to 150-fold shift in their respective IC50 values in similar normal blood models spiked with different AML cell lines or engineered cell lines.10,29
In conclusion, ABT-869 has potent activity against AML cell lines with mutated kinases, while being less effective against leukemia cell lines with wt-FLT3 at doses that do not affect normal bone marrow progenitor cells. Enzyme data would suggest variable activity against different activation loop mutations of FLT3. ABT-869 also has potent in vivo activity in MV-4-11 xenograft and engraftment tumor models demonstrating tumor regressions, tumor cell apoptosis and a decrease in proliferation index, and a 20-week prolongation of survival in the engraftment model. These attributes provide the basis for the clinical development of ABT-869 in AML. The in vitro activity of ABT-869 against AML patient samples with the FLT3-ITD and wt-FLT3 adds value to the development of ABT-869 in the treatment of AML.
Authorship
Contribution: D.B.S. and J.L. designed research, performed research, collected data, analyzed data, and wrote the paper; P.T., J.O.M., and L.J.P. performed research and analyzed data; Y.D. contributed vital new reagents; R.-Q.W. performed research and analyzed data; D.H.A. analyzed data and wrote the paper; J.B. performed research and analyzed data; D.O., J.G., P.A.M., and E.F.J. performed research and analyzed data; N.S. performed research; K.H. contributed vital new reagents; M.M. contributed vital new reagents and analyzed data; S.K.D. analyzed data and contributed to the organization and editing of manuscript; S.P., J.C., and K.R. performed research and analyzed data; N.S. and T.M. contributed to study design; and K.M.S. and K.B.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.L., P.T., J.O.M., L.J.P., Y.D., R.-Q.W., D.H.A., J.J.B., D.J.O., J.G., P.A.M., E.F.J., N.S., K.H., M.R.M., S.K.D., and K.B.G. are all employees of Abbott Laboratories. No other authors received financial support from Abbott Laboratories for the studies presented herein.
D.B.S. and J.L. contributed equally to the content and preparation of this manuscript. K.M.S. and K.B.G. were co-senior authors of this manuscript.
Correspondence: Keith B. Glaser, Cancer Research, R47J-AP9, Abbott Laboratories, Abbott Park, IL 60064-6121; e-mail: keith.glaser@abbott.com.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Hamburger endowment from the UCLA Jonsson Comprehensive Cancer Center (D.B.S.). K.M.S. is a Scholar of the Lymphoma and Leukemia Society and is supported by the National Institutes of Health (CA108545, HL75826, and RHL083077A), the Department of Defense, and the Diamond-Blackfan Anemia Foundation. Both T.B.M. and K.M.S. are funded by the UCLA Jonsson Comprehensive Cancer Center.

![Figure 1. Chemical structure of ABT-869 (N-[4-(3-amino-1H-indazol-4-yl)phenyl]-N′-(2-fluoro-5-methylphenyl)urea).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-06-029579/4/m_zh80080711440001.jpeg?Expires=1764018524&Signature=viuNUAoqNALtpsdJbgAII9fMe1cXPl8n44CaTRibBITwbQb0wnmi8r2hEePaOOxYhKHGZqtfp9YxePaHaUbjjw5hUHfSQ78ufCtNWYssEX7K-mD2To43OTuUxD-V-JqK3ujeVf2hDRaYZ0wi18r9s0iDIfognO~4Udm~5LSPnbLoxjSwyYrXlIstB~guEnF286V4d42AZd8jjCGLALwSB--0CSf~Xh0NrylMbVjWRL-nrdfpCW1nVmKa9YtaIBcJKI7t8AqPQAhxy8pocLwcIRKHT3Hu85drmnQmwAswT-hm2udrARLTHuhEV4l0BGX2T5h2zEFVN1KZb68WNtj4cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)